Abstract
AIM: To investigate the risk factors affecting the liver metastasis (LM) of pancreatic ductal adenocarcinoma (PDAC) after resection.
METHODS: We retrospectively analyzed 101 PDAC patients who underwent surgical resection at the Samsung Medical Center between January 2000 and December 2004. Forty one patients with LM were analyzed for the time of metastasis, prognostic factors affecting LM, and survival.
RESULTS: LM was found in 40.6%. The median time of the LM (n = 41) was 6.0 ± 4.6 mo and most LM occurred within 1 year. In univariate analysis, tumor size, preoperative carbohydrate antigen 19-9, and perineural invasion were factors affecting LM after resection. In multivariate analysis, tumor size was the most important factor for LM. In univariate analysis, tumor cell differentiation was significant to LM in low-risk groups.
CONCLUSION: LM after resection of PDAC occurs early and shows poor survival. Tumor size is the key indicator for LM after resection.
Keywords: Pancreatic ductal adenocarcinoma, Liver metastasis, Recurrence
INTRODUCTION
Pancreatic cancer is the fourth leading cause of death from cancer in the United States, 2006 and the fifth in South Korea[1,2]. Anatomical features including retroperitoneal location with proximity to the portal vein, celiac trunk, and superior mesenteric artery are associated with aggressive behavior of pancreatic ductal adenocarcinoma (PDAC). In many cases, patients present with PDAC which is already at an advanced stage at the time of diagnosis and unresectable. Pancreatectomy offers the only chance for long-term survival and is the single most important factor affecting patient outcome[3,4]. Even after curative radical surgery, the recurrence rate of PDAC is very high and high-volume centers report 5-year survival rates of only 10%-20%[3,5-7]. Postoperative adjuvant therapy, with the purpose of reducing hepatic metastasis and local recurrence, can influence survival gain[5]. Post-operative recurrence is categorized mainly by liver metastasis (LM), peripancreatic or retroperitoneal recurrence, peritoneal seeding, and distant other organ metastasis. In this study, we analyzed LM after resection for PDAC.
MATERIALS AND METHODS
Between January 2000 and December 2004, 106 patients with PDAC underwent pancreatic resection with curative intent in the Department of Surgery, Samsung Medical Center, Seoul. Excluding for five patients who dropped out, 101 patients were enrolled. The clinical features 41 patients with LM and 60 patients without LM were compared. The average age was 58.6 years (range, 31-79 years) and the median follow-up period was 15.5 ± 17.4 mo (range, 3.3-81.4 mo). Before surgery, we evaluated the radiological tumor status using abdominal computed tomography (CT) with or without magnetic resonance imaging, but we did not performed positron emission tomography scans routinely.
Patients with cancer in the head, neck and uncinate process of the pancreas underwent pancraticoduodenectomy or pylorus-preserving pancreaticoduodenectomy (n = 70), and patients with cancer in the body or tail underwent distal pancreatectomy (n = 19). Total pancreatectomy was performed in twelve patients with severe pancreatitis combined with cancer or with tumors extending beyond the neck of the pancreas, delineated by the left border of the superior mesenteric vessels and into the body of the gland. Peripancreatic lymph nodes, hepatoduodenal nodes as well as the celiac axis and superior mesenteric lymph nodes were cleared in patients with head, neck and uncinate process cancer while aortocaval nodes were dissected in cases of enlargement. Follow-up study included routine laboratory tests, serum carbohydrate antigen 19-9 (CA 19-9), and abdominal CT in first month after surgery and every 3 mo thereafter. The time of recurrence or metastasis was defined initial occurrence time in CT and the site of recurrence or metastasis was defined from CT findings. We categorized the type of recurrence into LM, locoregional recurrence defined as a tumor confined at retroperitoneal margin and lymph nodes. Peritoneal dissemination and distant metastasis were also categorized. Tumor stage was defined according to the American Joint Cancer Committee (AJCC) criteria. Medical records were retrospectively reviewed to investigate radiological findings, pathological findings with T stage, tumor differentiation, lymph node or perineural invasion.
There was a lack of consensus on the indications and effectiveness of adjuvant therapy for resected PDAC, and a standard chemoradiation protocol has not been developed at our institute. The decision on whether adjuvant therapy was undertaken was made giving consideration to the age, compliance, economic status, and social activity of the patient. However, the majority of patients received adjuvant therapy protocols that consisted of 4000 to 5000 cGy of external beam radiation and gemcitabine or capecitabine based chemotherapy[6]. In this study, forty patients underwent concurrent chemoradiation therapy and ten patients underwent the alternatives of chemotherapy or radiation. For evaluating the clinical, pathological characteristics and survival with LM group, the patients were divided into two groups based on the occurrence of LM. Chi-square and Fisher exact tests were used for comparisons among the categorical variables. Survival analysis was performed using the Kaplan-Meier method. Univariate differences in survival among the subgroups were compared using the log-rank test. P < 0.05 was considered significant. SPSS 12.0 for Windows was used for all statistical analysis.
RESULTS
Analysis for patients with metastasis
Forty-one patients with LM comprised 28 solely with LM, and 13 patients who had additional metastases: retroperitoneal node and soft tissue metastases or peritoneal dissemination or lung or bone metastases (Table 1). Among 60 without LM, 40 patients showed various types of metastasis with locoregional recurrence, peritoneal dissemination, lung and bone metastasis, while 20 showed no evidence of recurrence or metastasis.
Table 1.
Recurrence patterns of patients with liver metastasis (n = 41)
| Recurrence patterns | n (%) |
| Liver metastasis only | 28 (68.3) |
| Mixed | 13 (31.7) |
| + locoregional | 6 (14.6) |
| + peritoneal seeding | 4 (9.8) |
| + locoregional + peritoneal seeding | 1 (2.4) |
| + locoregional + seeding + lung1 + bone2 | 1 (2.4) |
| + lung1 | 1 (2.4) |
Lung = lung metastasis;
Bone = bone metastasis.
Timing of LM
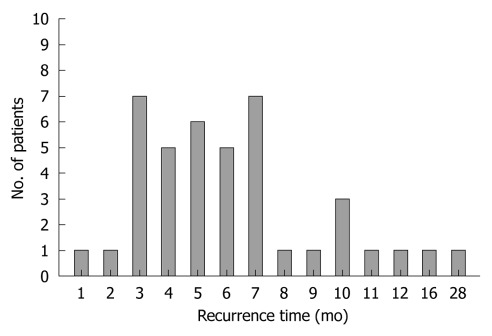
The timing of LM after pancreatectomy was as follows; within 2 mo - 2 patients (4.9%), between 3 and 4 mo - 12 patients (29.3%), between 5 and 6 mo - 11 patients (26.8%), between 7 and 12 mo - 14 patients (34.1%), beyond 1 year - two patients had metastasis. LM occurred within 6 mo LM in 60.9 % of patients and within 1 year in 95.1% (39 patients). The median LM time was 6.0 ± 4.6 mo (Figure 1).
Figure 1.
Time of liver metastasis in resected pancreatic ductal adenocarcinoma. The median time of the llive metastasis (n = 41) was 6.0 ± 4.6 mo. Almost liver metastasis occurred within 1 year.
Factors affecting LM after pancreatic resection
Analysis using the via χ2 test indicated that preoperative high level of CA 19-9, tumor size above 3cm, and perineural invasion of the tumor were significant clinical and pathological factors favouring LM in univariate analysis. Tumor location, cell differentiation, pancreatic resection margin involvement of the tumor, T stage (AJCC 6th), and lymph node involvement were not significant. Post operative adjuvant therapy including radiation did not influence LM (Table 2). In multivariate analysis, preoperative CA 19-9 and tumor size were significant, but perineural invasion was not significant (Table 3).
Table 2.
Factors influencing liver metastasis after pancreatectomy for pancreatic ductal adenocarcinoma
| Liver metastasis (n = 41) | Non-liver metastasis (n = 60) | P-value | |
| Location | |||
| Head | 30 | 52 | 0.09 |
| Body and tail | 11 | 8 | |
| Size (cm) | |||
| < 3 | 23 | 45 | 0.04 |
| ≥ 3 | 18 | 15 | |
| Differentiation | |||
| Well, moderate | 29 | 49 | 0.08 |
| Poor | 12 | 11 | |
| Perineural invasion | |||
| Yes | 28 | 30 | 0.04 |
| No | 13 | 30 | |
| T-stage (AJCC, 6th) | |||
| 1-2 | 1 | 5 | 0.21 |
| 3-4 | 40 | 55 | |
| N-stage | |||
| N0 | 23 | 24 | 0.13 |
| N1 | 18 | 36 | |
| Resection margin | |||
| Positive | 3 | 6 | 0.46 |
| Negative | 38 | 54 | |
| CA 19-9 (IU/mL) | |||
| < 37 | 4 | 20 | 0.001 |
| ≥ 37 | 28 | 20 | |
| Adjuvant CTx | |||
| Yes | 14 | 26 | 0.41 |
| No | 27 | 34 | |
| Adjuvant RTx | |||
| Yes | 16 | 29 | 0.32 |
| No | 25 | 31 |
CTx: Chemotherapy; RTx: Radiotherapy; AJCC: American Joint Cancer Committee; CA 19-9: Carbohydrate antigen 19-9.
Table 3.
Factors influencing liver metastasis after pancreatectomy for pancreatic ductal adenocarcinoma: multivariate analysis
|
95% CI for Exp (B) |
||||
| P-value | Odds ratio | Lower | Upper | |
| Size (> 3 cm) | 0.046 | 1.416 | 0.176 | 0.986 |
| CA 19-9 | 0.013 | 0.204 | 0.058 | 0.713 |
| Perineural invasion | 0.059 | 2.228 | 0.969 | 5.123 |
CA 19-9: Carbohydrate antigen 19-9.
Survival of patients with LM
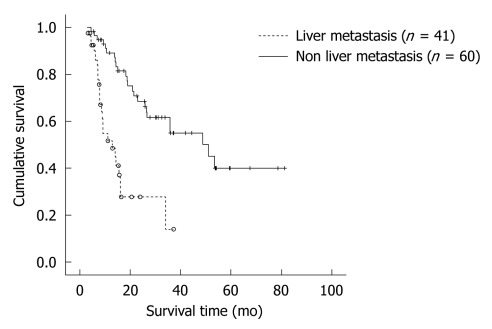
The survival of 41 patients with LM and 60 without LM were compared. The median survival time with LM patients was 12.9 ± 3.2 mo, and the cumulative 1- and 3-year survival rates were 51.7% and 13.8%, respectively. In patients without LM, the median survival time was 48.8 ± 9.8 mo, the cumulative 1-, 3-, 5-year survival rates were 89.1%, 55.0% and 40.0% (P < 0.001) (Figure 2).
Figure 2.
Comparison of overall survival according to liver metastasis. In patients without liver metastasis (n = 60), overall survival is better than those with liver metastasis (n = 41) (P < 0.001).
Pattern of LM with low risk patients
We investigated 22 patients with the low risk of LM (tumor size < 3 cm, preoperative normal CA 19-9 level); four patients with LM and eighteen without LM. Comparing clinical and pathological factors, univariate analysis indicated that poorly differentiated pancreatic tumors were more common in the LM group (Table 4).
Table 4.
Factors influencing liver metastasis after pancreatectomy for pancreatic ductal adenocarcinoma in patients without risk factors for liver metastasis
| Liver metastasis (n = 4) | Non-liver metastasis (n = 18) | P-value | |
| Location | |||
| Head | 3 | 15 | 0.49 |
| Body and tail | 1 | 3 | |
| Size (cm) | |||
| < 2 | 3 | 6 | 0.19 |
| ≥ 2 | 1 | 12 | |
| Differentiation | |||
| Clear, moderate | 0 | 14 | 0.01 |
| Poor | 4 | 4 | |
| Perineural invasion | |||
| Yes | 2 | 13 | 0.38 |
| No | 2 | 5 | |
| T-stage (AJCC, 6th) | |||
| 1-2 | 0 | 2 | 0.23 |
| 3-4 | 4 | 16 | |
| N-stage | |||
| N0 | 4 | 11 | 0.19 |
| N1 | 0 | 7 | |
| Resection margin | |||
| Positive | 0 | 0 | NA |
| Negative | 4 | 18 |
NA: Not available; AJCC: American Joint Cancer Committee.
DISCUSSION
Until recently, few clinical studies had been conducted on the recurrence of PDAC after pancreatic resection. The infrequency of study is influenced by the higher rate of recurrence and poorer survival rate after curative resection compared with another gastrointestinal cancers, and by the limited evidence of survival improvement after adjuvant treatment for PDAC. Surgical resection for PDAC is a unique treatment modality which is expected to curative. The investigation of surgical resection and research into adjuvant therapy are essential to achieve improvement in survival. For this reason, the evaluation of recurrence and metastasis of PDAC after resection is important.
Sperti et al[8] reported 89% patients with PDAC recurrence after surgical resection. Local recurrence was 72%, and hepatic metastasis was 62%, over 22 years of study. Nitecki et al[9] reported 25% local recurrence and 37.5% hepatic metastasis. In a Korean study of PDAC recurrence in a single institute[10], 69% of recurrences occurred during the 12 mo after surgical resection. A 74.4% local recurrence included 51.2% of hepatic metastasis, while independent local recurrence and hepatic metastasis were 41% and 18.6%, respectively. In our investigation, over 16 mo of median follow up, 34.6% local recurrence and 34.6% independent LM occurred. Total LM occurred in 51 patients (50.6%). The recurrence mostly consisted of local recurrence and LM, with similar distribution of both recurrence patterns. Mixed LM with another type of recurrence pattern was dominant over independent LM. Several studies[11-13] have revealed that the prognosis in those with local recurrence is superior to those with distant metastasis including LM. Shibata et al[14] reported that mean survival time and actuarial 5-year disease-specific survival were significantly lower in cases of hepatic metastasis (13 mo, 0%) than in cases of local retroperitoneal recurrence (30 mo, 21%). In our report, the median survival time of 41 patients with hepatic metastasis was 12.9 mo and compared with 26.4 mo for patients without hepatic metastasis. These results are similar to those of a previous Japanese study. In particular, hepatic metastasis occur early after surgical resection and appear to have very poor prognosis. Sperti et al[8] reported that the median survival time with independent hepatic metastasis was 9 mo and with combined hepatic metastasis and local recurrence was no more than 6 mo.
Another report[11] showed that the median survival time of patients with hepatic metastasis was 6 mo and when hepatic metastasis in combined with local recurrence only 4 mo, Various investigations have shown few patients with over 1 year survival. Poor survival and prognoisis were influenced by very early recurrence within a year despite radical resection for PDAC[15,16].
In particular, Hishinuma et al[17] demonstrated that local recurrence occurs frequently, but is rarely a direct cause of death, and most patients died of metastatic disease according to 27 patients autopsies. Our series revealed that 60.9% of LM occur within 6 mo, and 95.1% LM within a year. It is not too much to say that LM of PDAC will almost certainly arise within a year.
Amikura suggested that the early development of liver metastases within 3 mo after pancreatic resection supports the hypothesis that occult microscopic liver metastases are frequently present at the time of resection[18]. A recent Japanese study reported that undifferentiated PDAC is independently associated with hepatic metastasis after pancreatic resection[14]. Our data showed no difference in terms of tumor cell differentiation between LM and other type of recurrence. In groups at low risk for LM, cell differentiation is a meaningful predictor for LM. In our study, tumor size and CA 19-9 levels are significant predictors for LM, Takamori et al[19] revealed the positive correlation between the expression of CA 19-9 and the hepatic metastatic potential of pancreatic cancer. The depth of portal vein wall invasion significantly alters survival after curative pancreatic resection combined with portal vein resection[20]. Previous studies have suggested that several molecules, including epidermal growth factor receptor, E-cadherin, and laminin g-chain, that are expressed at high levels in undifferentiated PDAC are associated with postoperative hepatic metastasis[21-23]. Such molecular changes may enhance the ability of pancreatic ductal carcinoma to metastasize to the liver[14]. Niedergethmann et al[24] reported that CTSB and CTSL rather than UICC stage, TNM classification, or tumor grading, are strong and independent prognostic markers in resectable pancreatic adenocarcinoma. Furthermore, CTSB is a predictor for early recurrence after curative resection. Seo et al[25] suggested that vascular endothelial growth factor expression seems to be an important predictor for both LM and poor prognosis in ductal pancreatic adenocarcinoma. Other study suggested that fibrotic focus reduced membranous β-catenin expression, and reduced cytoplasmic β-catenin expression were significantly associated with shorter LM-free survival[26,27]. These investigations suggest that cancer differentiation relating factors influence the hepatic metastasis of PDAC, and predict poor prognosis. We support cautiously the hypothesis that selective adjuvant chemotherapy may be possible using post operative LM prediction.
Although no standard post pancreatectomy adjuvant chemotherapy for PDAC has been established, several gemcitabine-based adjuvant therapies have been investigated since 2000. In one European ramdomized controlled prospective study, postoperative gemcitabine significantly delayed the development of recurrent disease after complete resection of pancreatic cancer[28]. A Japanese study[29] reported similar results for patients with PDAC and LM. A number of papers have revealed that in patients with advanced PDAC, intra-arterial chemotherapy or chemotherapy via portal vein with systemic chemotherapy appeared to be effective against PDAC and LM[15,30-33]. Hepatectomy was applied in patients with LM in Germany, with between 9 and 24 mo of survival after hepatectomy reported[34]. Not only adjuvant local chemotherapy but also liver resection for LM were successful in achieving survival improvement after pancreatectomy with PDAC. The enthusiastic efforts of several researchers searching for molecular factors predicting LM may result in selective adjuvant therapy and improvement of survival in future.
In conclusion, LM after resection of PDAC occurs early and shows poor survival. Tumor size is the clearest indicator for LM after resection.
COMMENTS
Background
The prognosis of pancreatic ductal adenocarcinoma (PDAC) with liver metastasis (LM) is dismal even after curative pancreatectomy. However, prognostic factors for LM after PDAC resection are not well established.
Research frontiers
The authors retrospectively analyzed 101 PDAC patients who underwent surgical resection at the Samsung Medical Center between January 2000 and December 2004.
Innovations and breakthroughs
In univariate analysis, tumor size, preoperative carbohydrate antigen 19-9, and perineural invasion were factors affecting LM after resection. In multivariate analysis, tumor size was the most important factor for LM. In univariate analysis, tumor cell differentiation was significant to LM in low-risk groups. LM after resection of PDAC occurs early and shows poor survival. Tumor size is the key indicator for LM after resection.
Applications
To investigate prognostic factors for LM, the authors compared and analyzed the clinical and pathological factors between two groups, segmented according to LM.
Peer review
This report deals with very important problems and supports the understanding of the physician treating pancreatic cancer.
Footnotes
Peer reviewer: Yo-ichi Yamashita, MD, PhD, Department of Surgery, Hiroshima Red Cross Hospital and Atomic Bomb Survivors Hospital, Senda-machi 1-9-6, Naka-ku, Hiroshima 730-8619, Japan
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Ministry Health and Welfare. 2002 Annual report of Korea Central Cancer Registry. 2003. Available from: http://www.ncc.re.kr. [Google Scholar]
- 3.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 5.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731; discussion 731-733. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37–43. doi: 10.1097/01.mpa.0000194609.24606.4b. [DOI] [PubMed] [Google Scholar]
- 7.Magistrelli P, Antinori A, Crucitti A, La Greca A, Masetti R, Coppola R, Nuzzo G, Picciocchi A. Prognostic factors after surgical resection for pancreatic carcinoma. J Surg Oncol. 2000;74:36–40. doi: 10.1002/1096-9098(200005)74:1<36::aid-jso9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 9.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving. Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JI, Kim SC, Kim IH, Lee SG, Lee YJ, Park KM, Hwang S, Kim KH, Han DJ. Analysis on Recurrence of an Invasive Ductal Adenocarcinoma of the Pancreas. Korean J Hepatobiliary Pancreat Surg. 2005;9:171–178. [Google Scholar]
- 11.Westerdahl J, Andrén-Sandberg A, Ihse I. Recurrence of exocrine pancreatic cancer--local or hepatic. Hepatogastroenterology. 1993;40:384–387. [PubMed] [Google Scholar]
- 12.Menke-Pluymers MB, Klinkenbijl JH, Tjioe M, Jeekel J. Treatment of locoregional recurrence after intentional curative resection of pancreatic cancer. Hepatogastroenterology. 1992;39:429–432. [PubMed] [Google Scholar]
- 13.Sunamura M, Egawa S, Shibuya K, Shimamura H, Takeda K, Kobari M, Matsuno S. [Therapeutic strategy for the recurrence of pancreatic cancer following pancreatectomy] Nihon Geka Gakkai Zasshi. 1999;100:200–205. [PubMed] [Google Scholar]
- 14.Shibata K, Matsumoto T, Yada K, Sasaki A, Ohta M, Kitano S. Factors predicting recurrence after resection of pancreatic ductal carcinoma. Pancreas. 2005;31:69–73. doi: 10.1097/01.mpa.0000166998.04266.88. [DOI] [PubMed] [Google Scholar]
- 15.Yamaue H, Tani M, Onishi H, Kinoshita H, Nakamori M, Yokoyama S, Iwahashi M, Uchiyama K. Locoregional chemotherapy for patients with pancreatic cancer intra-arterial adjuvant chemotherapy after pancreatectomy with portal vein resection. Pancreas. 2002;25:366–372. doi: 10.1097/00006676-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–329. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 17.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10:511–518. doi: 10.1016/j.gassur.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Amikura K, Kobari M, Matsuno S. The time of occurrence of liver metastasis in carcinoma of the pancreas. Int J Pancreatol. 1995;17:139–146. doi: 10.1007/BF02788531. [DOI] [PubMed] [Google Scholar]
- 19.Takamori H, Hiraoka T, Kanemitsu K, Tsuji T. Pancreatic liver metastases after curative resection combined with intraoperative radiation for pancreatic cancer. Hepatogastroenterology. 2004;51:1500–1503. [PubMed] [Google Scholar]
- 20.Fukuda S, Oussoultzoglou E, Bachellier P, Rosso E, Nakano H, Audet M, Jaeck D. Significance of the depth of portal vein wall invasion after curative resection for pancreatic adenocarcinoma. Arch Surg. 2007;142:172–179; discussion 180. doi: 10.1001/archsurg.142.2.172. [DOI] [PubMed] [Google Scholar]
- 21.Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, Yamazaki H, Nakamura M, Ueyama Y, Tanaka M, et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med. 2003;11:305–309. [PubMed] [Google Scholar]
- 22.Li YJ, Meng YX, Ji XR. Relationship between expressions of E-cadherin and alpha-catenin and biological behaviors of human pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2003;2:471–477. [PubMed] [Google Scholar]
- 23.Takahashi S, Hasebe T, Oda T, Sasaki S, Kinoshita T, Konishi M, Ochiai T, Ochiai A. Cytoplasmic expression of laminin gamma2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer. 2002;94:1894–1901. doi: 10.1002/cncr.10395. [DOI] [PubMed] [Google Scholar]
- 24.Niedergethmann M, Wostbrock B, Sturm JW, Willeke F, Post S, Hildenbrand R. Prognostic impact of cysteine proteases cathepsin B and cathepsin L in pancreatic adenocarcinoma. Pancreas. 2004;29:204–211. doi: 10.1097/00006676-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239–2245. doi: 10.1002/(sici)1097-0142(20000515)88:10<2239::aid-cncr6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Nakatsura T, Hasebe T, Tsubono Y, Ryu M, Kinoshita T, Kawano N, Konishi M, Kosuge T, Kanai Y, Mukai K. Histological prognostic parameters for adenocarcinoma of the pancreatic head. Proposal for a scoring system for prediction of outcome. J Hepatobiliary Pancreat Surg. 1997;4:441–448. [Google Scholar]
- 27.Watanabe I, Hasebe T, Sasaki S, Konishi M, Inoue K, Nakagohri T, Oda T, Mukai K, Kinoshita T. Advanced pancreatic ductal cancer: fibrotic focus and beta-catenin expression correlate with outcome. Pancreas. 2003;26:326–333. doi: 10.1097/00006676-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 29.Horiuchi H, Uchida S, Hisaka T, Ishikawa H, Sakai T, Kawahara R, Kinoshita H, Shirouzu K. [A study of recurrent pancreatic cancer with metastatic liver tumors after pancreatectomy] Gan To Kagaku Ryoho. 2005;32:1685–1687. [PubMed] [Google Scholar]
- 30.Beger HG, Gansauge F, Büchler MW, Link KH. Intraarterial adjuvant chemotherapy after pancreaticoduodenectomy for pancreatic cancer: significant reduction in occurrence of liver metastasis. World J Surg. 1999;23:946–949. doi: 10.1007/s002689900604. [DOI] [PubMed] [Google Scholar]
- 31.Link KH, Formentini A, Gansauge F, Papachristov E, Beger HG. Regional celiac artery infusion as adjuvant treatment after pancreatic cancer resection. Digestion. 1997;58:529–532. doi: 10.1159/000201496. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa O, Ohhigashi H, Sasaki Y, Furukawa H, Imaoka S. Extended pancreatectomy and liver perfusion chemotherapy for resectable adenocarcinoma of the pancreas. Digestion. 1999;60 Suppl 1:135–138. doi: 10.1159/000051470. [DOI] [PubMed] [Google Scholar]
- 33.Papachristou E, Link KH, Schoenberg MH. Regional celiac artery infusion in the adjuvant treatment of pancreatic cancer. Anticancer Res. 2003;23:831–834. [PubMed] [Google Scholar]
- 34.Kleeff J, Reiser C, Hinz U, Bachmann J, Debus J, Jaeger D, Friess H, Büchler MW. Surgery for recurrent pancreatic ductal adenocarcinoma. Ann Surg. 2007;245:566–572. doi: 10.1097/01.sla.0000245845.06772.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]