Abstract
A catalase monolayer adsorbed on a layer of arachidic acid deposited on a solid support was irradiated with 100 keV electrons simulating the conditions of electron microscopic imaging. Effective doses were calculated taking into account the angular and energy distribution of backscattered electrons. Enzymatic inactivation was chosen as the criterion for damage and was monitored by a rapid and quantifiable but nevertheless sensitive assay. Dose-response curves revealed that inactivation is a one-hit-multiple-target phenomenon, which is consistent with biochemical evidence for a cooperative function of subunits. The experimentally determined target size coincides fairly well with both calculated cross sections for inelastic interactions based on the atomic composition of catalase and with calculated cross sections for ionizing events based on the chemical bonds involved. This legitimates both types of calculations even for complex biomolecules.
Full text
PDF
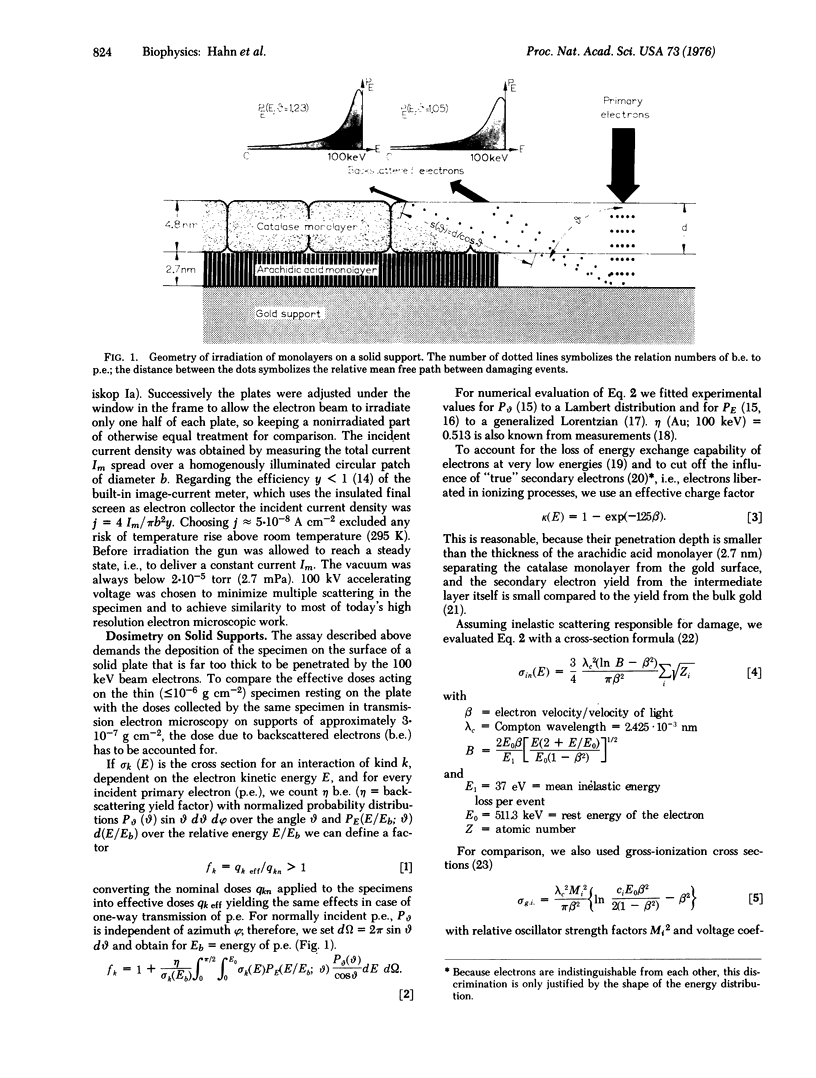
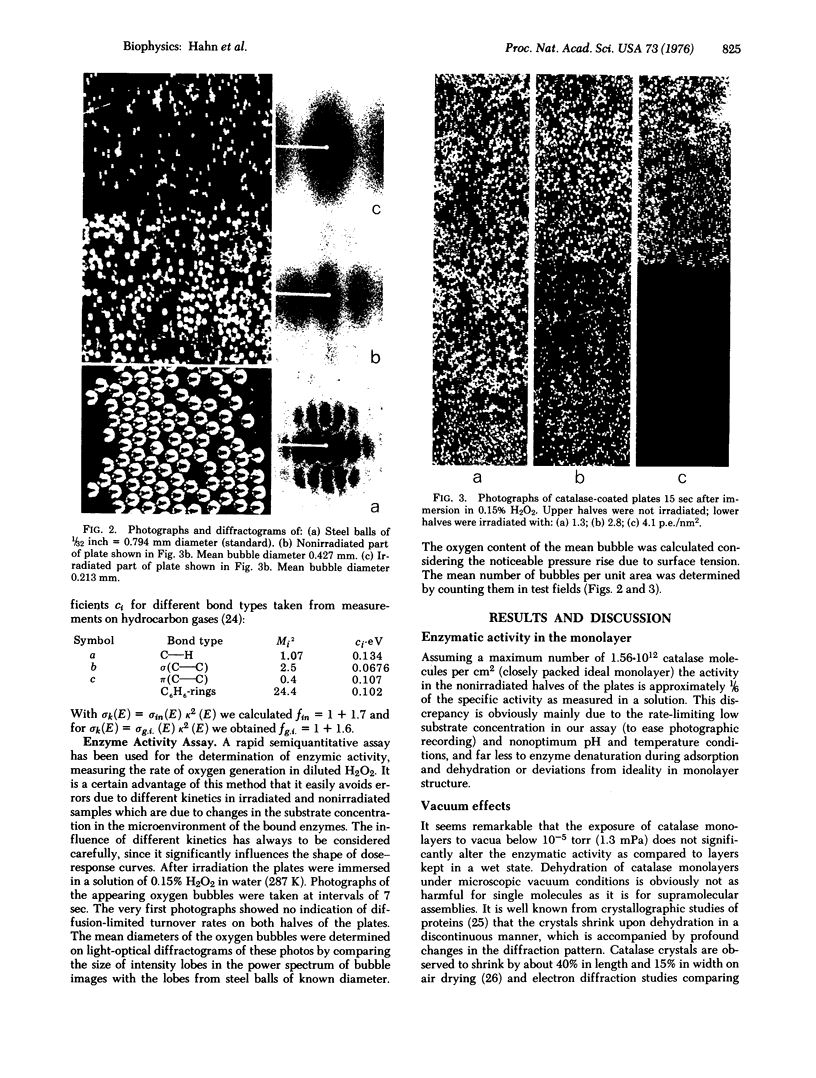
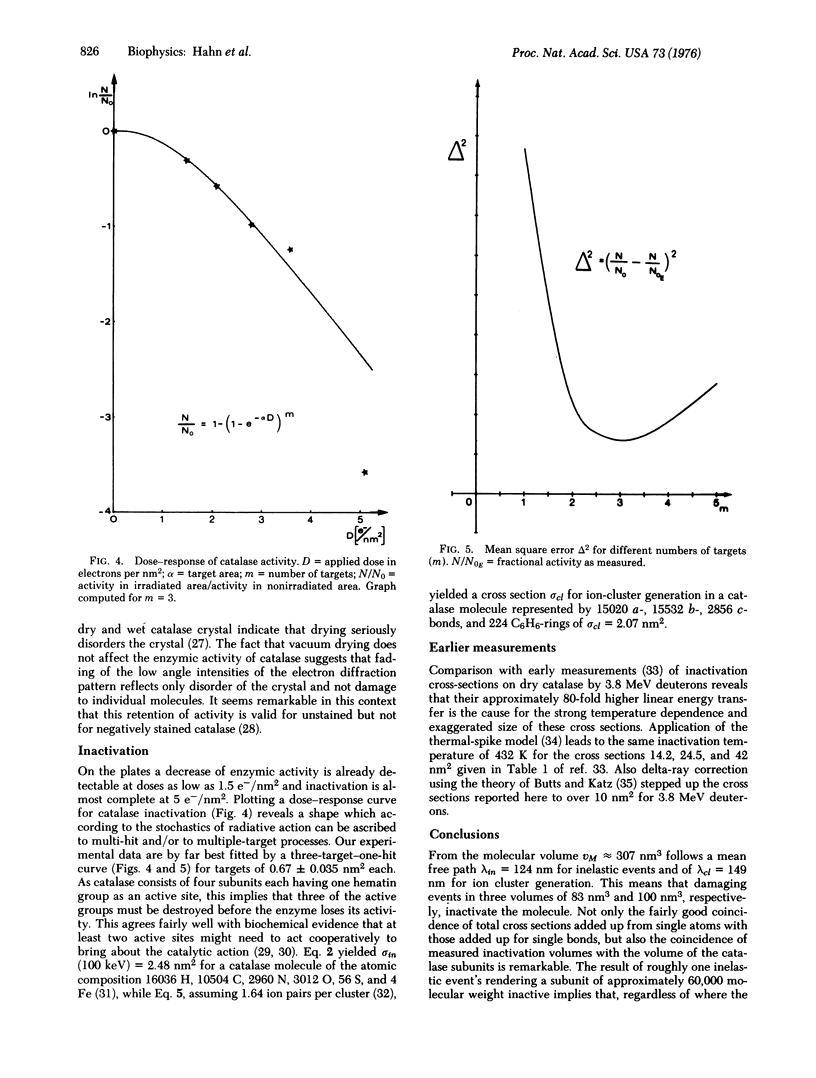

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumeister W., Hahn M. Relevance of three-dimensional reconstructions of stain distributions for structural analysis of biomolecules. Hoppe Seylers Z Physiol Chem. 1975 Aug;356(8):1313–1316. doi: 10.1515/bchm2.1975.356.2.1313. [DOI] [PubMed] [Google Scholar]
- Breedlove J. R., Jr, Trammell G. T. Molecular microscopy: fundamental limitations. Science. 1970 Dec 18;170(3964):1310–1313. doi: 10.1126/science.170.3964.1310. [DOI] [PubMed] [Google Scholar]
- Butts J. J., Katz R. Theory of RBE for heavy ion bombardment of dry enzymes and viruses. Radiat Res. 1967 Apr;30(4):855–871. [PubMed] [Google Scholar]
- Deisseroth A. B., Dounce A. L. Comparison of the catalytic and physical properties of the components of lyophilized beef erythrocyte catalase with those of lyophilized beef liver catalase components. Arch Biochem Biophys. 1969 Apr;131(1):30–48. doi: 10.1016/0003-9861(69)90102-7. [DOI] [PubMed] [Google Scholar]
- Fromherz P. A new technique for investigating lipid protein films. Biochim Biophys Acta. 1971 Feb 2;225(2):382–387. doi: 10.1016/0005-2736(71)90235-5. [DOI] [PubMed] [Google Scholar]
- Glaeser R. M. Limitations to significant information in biological electron microscopy as a result of radiation damage. J Ultrastruct Res. 1971 Aug;36(3):466–482. doi: 10.1016/s0022-5320(71)80118-1. [DOI] [PubMed] [Google Scholar]
- Grubb D. T. The calibration of beam measurement devices in various electron microscopes, using an efficient Faraday cup. J Phys E. 1971 Mar;4(3):222–224. doi: 10.1088/0022-3735/4/3/015. [DOI] [PubMed] [Google Scholar]
- Kim Y. K. Energy distribution of secondary electrons. I. Consistency of experimental data. Radiat Res. 1975 Jan;61(1):21–35. [PubMed] [Google Scholar]
- LUSE R. A. BASIC MECHANISMS IN THE RADIATION CHEMISTRY OF PROTEINS AND THE NUCLEIC ACIDS. Radiat Res. 1964;Suppl 4:SUPPL 4–4:192+. [PubMed] [Google Scholar]
- Longley W. The crystal structure of bovine liver catalase: a combined study by x-ray diffraction and electron microscopy. J Mol Biol. 1967 Dec 14;30(2):323–327. doi: 10.1016/s0022-2836(67)80042-1. [DOI] [PubMed] [Google Scholar]
- Matricardi V. R., Moretz R. C., Parsons D. F. Electron diffraction of wet proteins: catalase. Science. 1972 Jul 21;177(4045):268–270. doi: 10.1126/science.177.4045.268. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. R., Shelton J. B., Robberson B., Apell G. The amino acid sequence of bovine liver catalase: a preliminary report. Arch Biochem Biophys. 1969 May;131(2):653–655. doi: 10.1016/0003-9861(69)90441-x. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. The Radiation Sensitivity of Catalase as a Function of Temperature. Proc Natl Acad Sci U S A. 1952 Mar;38(3):166–172. doi: 10.1073/pnas.38.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenn K., Bahr G. F. Specimen damage caused by the beam of the transmission electron microscope, a correlative reconsideration. J Ultrastruct Res. 1970 Jun;31(5-6):526–550. doi: 10.1016/s0022-5320(70)90167-x. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Fisher H. W. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970 Aug 28;52(1):121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]