Abstract
Introduction
The amount of myocardial perfusion required for successful defibrillation after cardiac arrest is unknown. Coronary perfusion pressure (CPP) is a surrogate for myocardial perfusion. One limited clinical study identifies a threshold of 15 mmHg required for return of spontaneous circulation (ROSC). Our exploration of threshold and dose models of CPP during the initial bout of CPR indicates higher levels than previously demonstrated are required. CPP required for shock success throughout on-going resuscitation is unknown and other conceptual models of CPP have not been explored.
Hypothesis
An array of conceptual models of CPP is associated with and predicts defibrillation success throughout resuscitation.
Methods
Data from 6 porcine cardiac arrest studies were pooled. Mean and area under the curve (AUC) CPP were derived for 30-second epochs. Five conceptual models of CPP were analyzed: threshold, delta, cumulative delta, dose, and cumulative dose. Comparative statistics were performed with one-way ANOVA and two-tailed t-test. Regression models assessed CPP trends and prediction of ROSC.
Results
For 316 defibrillation attempts in 124 animals, those resulting in ROSC (n=75) had significantly higher threshold, delta, cumulative delta, dose, and cumulative dose CPP than those without. All conceptual models except delta CPP had significantly different values across successive defibrillation attempts and all five models were significant predictors of ROSC, along with experimental design.
Conclusions
Threshold, delta, cumulative delta, dose, and cumulative dose CPP predict individual defibrillation success throughout resuscitation.
Keywords: coronary perfusion pressure, heart arrest, ventricular fibrillation, cardiopulmonary resuscitation
Introduction
After more than 3–4 minutes of cardiac arrest, myocardial reperfusion is necessary prior to defibrillation to achieve successful resuscitation. (1–4) Prior animal and human studies demonstrate that chest compressions and/or vasoactive medications are needed to achieve adequate myocardial reperfusion, as indicated by a coronary perfusion pressure (CPP) of at least 15–25 mmHg). (5–15). However, some published animal studies (6–14) simulated brief untreated arrest duration (0–5 minutes) that may not reflect the most current clinically realistic model of out-of-hospital cardiac arrest with longer untreated durations before resuscitation. (16–23)The foremost human data published in 1990 by Paradis, et al. describe a necessary CPP of at least 15 mmHg for return of spontaneous circulation (ROSC) in out-of-hospital cardiac arrest (OHCA). (15) By nature of the steps needed for data collection, these were gathered very late during resuscitation (8–42 minutes after loss of pulses) and may not reflect the state of the myocardium during early therapies. As the understanding and modeling of time intervals in OHCA evolves to reflect the most recent literature, it is necessary to re-examine CPP required for defibrillation success and ROSC.
We have previously reported a “threshold” and “dose” model of coronary perfusion pressure required for ROSC. (24) The “threshold” model reflects the CPP achieved just prior to defibrillation. The “dose” model is a more longitudinal measure and reflects the total amount of CPP the myocardium has been exposed to before defibrillation. In those two models, we limited our investigation to the initial bout of compressions and medications given prior to the first rescue shock. In this study, we enlarged the pool of animals, expanded our analysis of CPP, and report CPP required for success of consecutive defibrillation attempts throughout the entire resuscitation. We report three different conceptual models of CPP (threshold, delta, and dose) and differentiate between the ‘short-term’ CPP between defibrillation attempts and ‘long-term’ cumulative CPP from the start of resuscitation. We hypothesized that animals with ROSC had a higher CPP irrespective of the conceptual model analyzed, and that the conceptual CPP models had differential predictive ability of individual defibrillation attempts throughout resuscitation.
Methods
The care and handling of the animals were in accord with NIH guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. We conducted a retrospective analysis of six experiments performed in our laboratory using mixed-breed domestic swine of either sex, including animals that received cardiopulmonary resuscitation (CPR), medications, and rescue shocks.
Swine were orally intubated with a 5–0 cuffed endotracheal tube. Animals were ventilated by a volume-cycled ventilator (Harvard Apparatus, South Natick, MA) with room air (tidal volume of 15–20 cc/kg, ventilatory rate of 12 breaths per minute and an inspiration: expiration ratio of 40%). Ventilation rate and tidal volume were adjusted to maintain an end-tidal CO2 between 35 and 45 mmHg measured by side-stream capnometry. Three limb-lead electrodes were secured in place to correspond to a standard lead II electrocardiogram (ECG), which was monitored continuously throughout the experiments (LifePak 12 Monitor-defibrillator, Medtronic Emergency Response Systems, Redmond WA).
Arterial and venous pressure transducers were inserted via right femoral cut-down. The arterial transducer (Mikro-Tip transducer model SPC 3705, Millar Instruments, Houston, TX) was advanced into the ascending aorta and the venous transducer was inserted into the right atrium. Correct positioning of the catheters was confirmed by interpretation of the pressure tracings. Heart rate and arterial and venous pressure were monitored continuously throughout the remaining procedures. All data were acquired digitally at a sampling rate of 1000 points/sec with a commercially available software package (Chart, AD Instruments, Castle Hill, Australia).
Arterial blood gases were obtained with a portable clinical analyzer (I-Stat, Heska Corporation, Waukesha, WI) after arterial access was established, any time ventilator settings were changed, and just prior to the induction of VF. Anesthesia time was defined as the time from the initial bolus of alpha-chloralose until the time VF was induced. The interval of anesthesia was standardized by initiating VF as close to 40 minutes of anesthesia time as possible. In all studies we induced VF by delivering a three second, 60 Hz, 100mA AC current externally across the thorax. VF was left untreated for 5–10 minutes depending on experimental study.
Depending on experimental protocol, chest compressions were delivered either manually or mechanically with a traditional, high-impulse, or continuous device. Manual compressions were delivered by one investigator (JJM), using the sound of the mechanical device as a metronome. Mechanical chest compressions were applied using an oxygen-driven resuscitation device (Thumper, Michigan Instruments, Grand Rapids, MI). High-impulse compressions were accomplished using a high-impulse Thumper (Model 1007, Michigan Instruments, Grand Rapids, MI). Continuous compressions with intermittent ventilations were performed using the active compression mode of the LUCAS device (Jolife, Lund, Sweden). All compressions were performed in an anterior-posterior direction at a rate of 100 per minute with a compression depth of 2.0 inches and a duty cycle of 50%. Compression to ventilation ratios of 5:1, 15:1, or 30:2 were dictated by experimental protocol. Ventilation was performed with 100% FiO2 for all experiments at a tidal volume of approximately 400 cc.
CPR was performed for 90 seconds to 5 minutes before delivery of the first rescue shock. All animals received drugs after 2 minutes of compressions. All animals received high-dose epinephrine (0.1 mg/kg), propanolol (1 mg), vasopressin (40 IU), and bicarbonate (1 mEq/kg) as an initial drug cocktail. Thereafter, the animals may have received additional doses of epinephrine (0.015 mg/kg) every 3 minutes as the resuscitation continued and pulses were not restored. If pulses were restored, sodium bicarbonate was administered for acidemia as necessary. Dosing of sodium bicarbonate was based on an arterial blood gas (NaHCO3 = 0.3 mEq × weight in kg × base deficit).
We used an impedance-compensating, truncated exponential biphasic defibrillation waveform (LifePak 12, 3-D, Medtronic Physio-Control, Redmond WA) with a fixed energy dose of 150 J for all rescue shocks. All countershocks were delivered by one investigator using defibrillation paddles (JJM) to eliminate inter-user variability.
Shock success (ROSC) was defined as an organized electrical rhythm with a systolic blood pressure of at least 80 mmHg for at least 1 minute continuously at any time during the resuscitation effort. We consider 1 minute of sustained ROSC to be an electrophysiologic success after defibrillation, while more prolonged maintenance of pulses can depend on many other variables. After a period of short-term survival (20–120 minutes), animals were euthanized with 40 mEq of potassium chloride. Resuscitation efforts consisting of continued CPR, further rescue shocks, standard dose epinephrine (0.015 mg/kg), and sodium bicarbonate were continued for 20 minutes in those animals that did not experience ROSC. Twenty minutes of failed resuscitation indicated no-ROSC.
Coronary Perfusion Pressure
Coronary perfusion pressure (CPP) was defined as aortic diastolic pressure minus right atrial diastolic pressure and was determined at the time point immediately prior to the compression upsurge in pressure (ie: end-relaxation). Custom MatLab (Mathworks, Natick, Massachusetts) code was utilized to identify, isolate, and analyze segments of the coronary perfusion pressure tracing corresponding to individual chest compressions. Pressure tracings were also validated manually to confirm the accuracy in detection (JCR and DDS). Both investigators were blinded as to outcome. Mean, standard deviation, minimum, and maximum CPP were derived for successive 30-second epochs throughout the entire resuscitation. Area under the curve (AUC) was estimated by the trapezoidal approximation method for each 30-second epoch.
“Threshold CPP” was defined as the mean CPP for the last 30-second epoch just prior to each defibrillation. “Delta CPP” was defined as the change in mean CPP from the first to the last epoch between defibrillation attempts. “Dose CPP” was defined as the AUC between defibrillation attempts. “Cumulative delta CPP” was defined as the change in CPP from the beginning of chest compressions to the last time epoch prior to each defibrillation attempt. “Cumulative dose CPP” was defined as the AUC from the beginning of chest compressions to each defibrillation attempt.
Data Analysis
Demographic data, including experimental study, resuscitation protocol, sex, weight, CPR ratio (compressions: ventilations), pre-resuscitation anesthesia duration, VF duration, and ROSC were abstracted from the experimental records. We used Microsoft Excel 2011 (Microsoft Corporation, Redmond, WA) and STATA (StataCorp, College Station, TX) to analyze the data. Comparative statistics were performed using one-way ANOVA and a two-tailed t-test. A random-effects generalized least squares regression model was used to compare CPP calculations over successive defibrillation attempts. We then constructed multiple logistic regression models to assess the relationship between ROSC and key predictors. Hosmer-Lemeshow test was used to assess goodness of fit. Univariate logistic regression was used to screen predictors with a p < 0.1 threshold for entrance into the multivariate model. Candidate predictors included sex, weight, duration of anesthesia during instrumentation, duration of untreated VF, ratio of compressions to ventilations, experimental study, threshold CPP, delta CPP, cumulative delta CPP, dose CPP, and cumulative dose CPP. Experimental study was used to account for variation in the method of chest compressions, drug cocktail utilized, year, and hypothermia. To further explore the relationship between cumulative dose CPP and ROSC, we modeled the competing effects of cumulative dose CPP and elapsed time to shock during the resuscitation. An alpha of 0.05 was used for both comparative and predictive statistics. Receiver operator curves were generated from the univariate models for threshold CPP, delta CPP, cumulative delta CPP, dose CPP, and cumulative dose CPP.
Results
In total, 316 rescue shocks were delivered to 124 animals. The total number of successful shocks resulting in ROSC was 75/316 (23.7%). The total number of animals achieving ROSC was 67/124 (52.8%). Some baseline demographics did vary by study (Table 1), notably sex, weight, CPR ratio, mean anesthesia time, and untreated VF duration.
Table 1.
Animal demographics from included experiments.
| Experimental Study (n) [Reference] | % Male* | Mean Weight ± SD (kg)* | CPR Ratio | Mean Anesthesia Time ± SD (min)* | Mean Untreated VF duration ± SD (min)* | % ROSC * |
|---|---|---|---|---|---|---|
| 1 (n = 27) [25] | 37.0 | 25.6 ± 1.9 | 15:1 | 34.2 ± 6.8 | 8.0 ± 0 | 59.3 |
| 2 (n = 11) [26] | 78.6 | 27.7 ± 2.4 | 5:1 | 30.5 ± 4.5 | 8.0 ± 0 | 50.0 |
| 3 (n = 43) [27] | 46.7 | 26.8 ± 2.4 | 15:1 | 34.4 ± 6.5 | 6.2 ± 1.5 | 24.4 |
| 4 (n = 17) [28] | 70.1 | 26.2 ± 1.7 | 30:2 | 39.8 ± 8.0 | 8.0 ± 0 | 100.0 |
| 5 (n = 13) [##] | 0.0 | 26.8 ± 1.4 | 30:2 | 50.9 ± 8.2 | 10.0 ± 0 | 76.9 |
| 6 (n = 13) [29] | 69.2 | 25.9 ± 1.2 | 15:1 | 37.7 ± 8.2 | 8.0 ± 0 | 53.8 |
SD: standard deviation. VF: ventricular fibrillation. ROSC: return of spontaneous circulation.
p-value < 0.05 across experimental studies.
data being analyzed prior to publication.
For all conceptual models of CPP (threshold, delta, cumulative delta, dose, and cumulative dose), CPP was higher in animals with ROSC than those without ROSC (Table 2). Results are reported as mean ± standard error of the mean.
Table 2.
CPP (coronary perfusion pressure) calculations for defibrillation attempts with and without ROSC (return of spontaneous circulation). CPP given as mean ± SEM
| Defibrillation Attempt | p-value | ||
|---|---|---|---|
| ROSC (n = 75) | No ROSC (n = 241) | ||
| Threshold CPP (mmHg) | 31.0 ± 1.5 | 13.7 ± 1.1 | < 0.001 |
| Delta CPP (mmHg) | 17.3 ± 1.9 | 5.5 ± 0.8 | < 0.001 |
| Cumulative Delta CPP (mmHg) | 26.6 ± 1.5 | 10.7 ± 1.0 | < 0.001 |
| Dose CPP (mmHg^2) | 5,974.0 ± 472.3 | 2,219.6 ± 248.7 | < 0.001 |
| Cumulative Dose CPP (mmHg^2) | 9,731.0 ± 638.2 | 5700.7 ± 389.9 | < 0.001 |
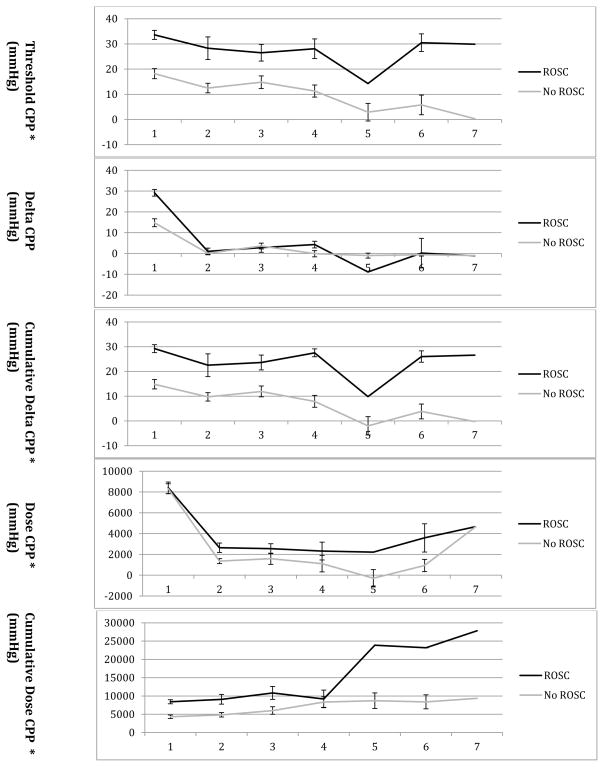
CPP measurements for animals achieving ROSC were compared over successive defibrillation attempts throughout the ongoing resuscitation (Table 3 and Figure 1). Threshold CPP (coefficient −2.48; 95%CI −4.45, −0.52; p = 0.01), cumulative delta CPP (coefficient −2.37; 95%CI −4.32, −0.43; p = 0.02), dose CPP (coefficient −1.90; 95%CI −2.52, − 1.28; p < 0.001), and cumulative dose CPP (coefficient 2.11; 95%CI 1.46, 2.75; p < 0.001) varied by defibrillation attempt.
Table 3.
Coronary perfusion pressure (CPP) calculations for successive defibrillations attempts with and without ROSC (return of spontaneous circulation). CPP given as mean ± SEM.
| 1st Attempt (n = 123) | 2nd Attempt (n = 81) | 3rd Attempt (n = 51) | 4th Attempt (n = 33) | 5th Attempt (n = 19) | 6th Attempt (n = 7) | 7th Attempt (n = 2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROSC (n =43) | No ROSC (n = 80) | ROSC (n = 64) | No ROSC (n = 17) | ROSC (n = 42) | No ROSC (n = 9) | ROSC (n = 2) | No ROSC (n = 31) | ROSC (n = 1) | No ROSC (n = 18) | ROSC (n = 2) | No ROSC (n = 5) | ROSC (n = 1) | No ROSC (n = 1) | |
| Threshold CPP (mmHg)* | 33.6 ± 1.8 | 18.2 ± 2.0 | 28.3 ± 4.5 | 12.5 ± 1.9 | 26.5 ± 3.3 | 14.8 ± 2.5 | 28.1 ± 3.9 | 11.3 ± 2.4 | 14.3# | 2.9 ± 3.5 | 30.5 ± 3.5 | 5.8 ± 3.9 | 29.9# | 0.3# |
| Delta CPP (mmHg) | 29.2 ± 1.6 | 14.8 ± 1.9 | 1.0 ± 1.6 | 0.1 ± 0.5 | 2.8 ± 2.2 | 3.6 ± 1.4 | 4.3 ± 1.6 | −0.1 ± 1.5 | −8.9# | −1.0 ± 1.2 | 0.2 ± 7.1 | −0.6 ± 0.6 | −1.1# | −1.0# |
| Cumulative Delta CPP (mmHg)* | 29.2 ± 1.6 | 14.8 ± 1.9 | 22.5 ± 4.6 | 9.7 ± 1.7 | 23.6 ± 3.0 | 11.9 ± 2.2 | 27.5 ± 1.6 | 7.9 ± 2.4 | 9.5# | −2.0 ± 3.7 | 26.0 ± 2.3 | 3.8 ± 3.0 | 26.6# | −0.3# |
| Dose CPP (mmHg)* | 8,405.5 ± 555.9 | 4,315.9 ± 480.5 | 2,642.3 ± 454.3 | 1,360.5 ± 243.3 | 2,553.1 ± 476.0 | 1,581.8 ± 539.1 | 2,325.7 ± 857.3 | 1,113.7 ± 794.1 | 2,220.8# | −287.6 ± 831.3 | 3,588.5 ± 1360.0 | 935.5 ± 588.3 | 4,671.4# | 1,494.9# |
| Cumulative Dose CPP (mmHg)* | 8,405.5 ± 555.9 | 4,315.9 ± 480.5 | 9,086.8 ± 1333.1 | 4,845.6 ± 608.8 | 10,830.5 ± 1763.6 | 6,016.8 ± 1023.1 | 9,193.0 ± 2408.1 | 8,324.5 ± 1443.4 | 23,889.5# | 8,688.3 ± 2136.4 | 23,173.8 ± 25.8 | 8,393.1 ± 1914.9 | 27,819.4# | 9,364.8# |
p < 0.05 for variation of CPP by defibrillation attempt for animals with ROSC.
n = 1, so unable to calculate SEM.
Figure 1.
Coronary perfusion pressure (CPP) calculations for successive defibrillation attempts with and without ROSC (return of spontaneous circulation). CPP given as mean ± SEM. * p < 0.05 for variation of CPP by defibrillation attempt for animals with ROSC. For attempts 5 and 7 n=1, so unable to calculate SEM.
Based on univariate logistic regression, study protocol (p < 0.001), shock number (OR 0.68; 95% CI 0.54, 0.86; p = 0.001), threshold CPP (OR 1.07; 495% CI 1.05, 1.09; p < 0.0001), delta CPP (OR 1.05; 95%CI 1.03, 1.07; p < 0.0001), cumulative delta CPP (OR 1.07; 95% CI 1.05, 1.09; p < 0.001), dose CPP in 1000 mmHg^2 (OR 1.27; 95% CI 1.18, 1.37; p < 0.001), and cumulative dose CPP in 1000 mmHg^2 (OR 1.12; 95% CI 1.07, 1.17; p < 0.001) were candidate variables for multiple variable analysis. Non-significant univariate predictors included sex, weight, compression to ventilation ratio, and anesthesia time.
The array of myocardial perfusion measurements that we explored (threshold CPP, delta CPP, cumulative delta CPP, dose CPP, and cumulative dose CPP) was extremely co-linear in a single regression model. Because of this extreme co-linearity and the five different conceptual models of CPP measurement represented, we established five separate multiple variable regression models to elucidate each measure’s predictive power of ROSC (Table 4). Interaction terms for shock number, experimental design, and each of the five conceptual models of CPP were non-significant predictors.
Table 4.
Predictive Value of CPP (coronary perfusion pressure) for ROSC (return of spontaneous circulation) based on multiple variable logistic regression modeling using five different conceptual models (threshold, delta, cumulative delta, dose, and cumulative dose).
| ROSC | |||
|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-value | |
| Threshold | |||
| Threshold CPP (mmHg) | 1.06 | 1.04, 1.08 | < 0.001 |
| Shock number | 0.86 | 0.67, 1.12 | 0.28 |
| Experimental Design* | |||
| 1 | 0.14 | 0.05, 0.36 | < 0.001 |
| 2 | 0.53 | 0.13, 2.13 | 0.37 |
| 3 | 0.54 | 0.23, 1.27 | 0.16 |
| 4 | 4.80 | 1.73, 13.34 | 0.01 |
| 5 | 0.40 | 0.13, 1.18 | 0.10 |
| 6 | 0.97 | 0.34, 2.74 | 0.95 |
| Delta | |||
| Delta CPP (mmHg) | 1.04 | 1.01, 1.06 | 0.01 |
| Shock number | 0.96 | 0.73, 1.26 | 0.77 |
| Experimental Design* | |||
| 1 | 0.28 | 0.11, 0.71 | 0.01 |
| 2 | 0.71 | 0.18, 2.88 | 0.64 |
| 3 | 0.35 | 0.16, 0.79 | 0.01 |
| 4 | 5.26 | 1.93, 14.37 | 0.01 |
| 5 | 0.60 | 0.21, 1.66 | 0.21 |
| 6 | 1.43 | 0.54, 3.82 | 0.47 |
| Cumulative Delta | |||
| Cumulative Delta CPP (mmHg) | 1.06 | 1.04, 1.09 | < 0.001 |
| Shock number | 0.85 | 0.66, 1.10 | 0.23 |
| Experimental Design* | |||
| 1 | 0.16 | 0.06, 0.41 | < 0.001 |
| 2 | 0.43 | 0.10, 1.80 | 0.25 |
| 3 | 0.59 | 0.25, 1.39 | 0.23 |
| 4 | 4.33 | 1.56, 12.00 | 0.01 |
| 5 | 0.40 | 0.13, 1.19 | 0.10 |
| 6 | 1.22 | 0.44, 3.39 | 0.71 |
| Dose | |||
| Dose CPP (1000 mmHg^2) | 1.22 | 1.12, 1.34 | < 0.001 |
| Shock number | 0.02 | 0.79, 1.33 | 0.88 |
| Experimental Design* | |||
| 1 | 0.15 | 0.06, 0.40 | < 0.001 |
| 2 | 1.31 | 0.37, 4.69 | 0.68 |
| 3 | 0.42 | 0.18, 0.95 | 0.04 |
| 4 | 6.55 | 2.36, 18.24 | < 0.001 |
| 5 | 0.66 | 0.23, 1.87 | 0.43 |
| 6 | 1.05 | 0.37, 2.96 | 0.93 |
| Cumulative Dose | |||
| Cumulative Dose CPP (1000 mmHg^2) | 1.18 | 1.10, 1.27 | < 0.001 |
| Shock number | 0.55 | 0.40, 0.75 | < 0.001 |
| Experimental Design* | |||
| 1 | 0.29 | 0.13, 0.65 | 0.01 |
| 2 | 1.58 | 0.45, 5.58 | 0.48 |
| 3 | 0.56 | 0.24, 1.32 | 0.18 |
| 4 | 6.33 | 2.27, 17.66 | < 0.001 |
| 5 | 0.70 | 0.25, 1.98 | 0.50 |
| 6 | 0.88 | 0.31, 2.49 | 0.81 |
denotes p < 0.05.
Multiple variable logistic modeling of cumulative dose CPP in 1000 mmHg^2 (OR 1.17; 95%CI 1.10, 1.24; p < 0.001) and elapsed time to shock in minutes (OR 0.81; 95%CI 0.71, 0.93; p = 0.002) yielded competing effects on successful defibrillation. (Table 5)
Table 5.
Competing effects of cumulative dose CPP (coronary perfusion pressure) and elapsed time to shock on ROSC (return of spontaneous circulation).
| ROSC | |||
|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-value | |
| Cumulative Dose CPP (1000 mmHg^2) | 1.17 | 0.11, 1.24 | < 0.001 |
| Elapsed time to shock (minutes) | 0.81 | 0.71, 0.93 | 0.002 |
Receiver operating characteristic curves were generated for the five different models of CPP. Threshold CPP (AUC 0.81), cumulative delta CPP (AUC 0.81), and dose CPP (AUC 0.78) had the highest area under the curve (Figure 2). Delta CPP and cumulative dose CPP had an AUC of 0.70.
Discussion
A primary objective of CPR and medication delivery is to generate an adequate CPP to reperfuse the myocardium before defibrillation. (30) For all five conceptual models of CPP, animals with ROSC achieved higher CPP than animals without ROSC.
Our discussion of intra-resuscitation hemodynamics is limited to coronary perfusion pressure, which is a product of coronary blood flow and vascular resistance. We utilize young, presumably healthy swine in our model, which do not have any known coronary artery disease that would independently limit myocardial blood flow. Changing CPP values/requirements ought to reflect changing vascular resistance that impacts blood flow.
We have previously shown that resuscitated animals achieve higher CPP from chest compressions alone compared with unresuscitated animals. Furthermore, resuscitated animals exhibit a greater response in CPP to exogenous catecholamines than unresuscitated animals. (24) We have postulated that animals with ROSC have higher residual levels of endogenous catecholamines, upregulated catecholamine receptors, or increased receptor sensitivity, and our laboratory is currently exploring pharmacogenomic differences in gene expression between animals with and without ROSC (data being analyzed prior to publication).
CPP requirements for ROSC appear to change over successive defibrillation attempts in an ongoing resuscitation. The necessary threshold, cumulative delta, and dose CPP decreases over successive defibrillation attempts in animals with ROSC. This may be because animals that receive repeated rescue shocks are also receiving ongoing chest compressions and myocardial perfusion, as reflected by the cumulative dose CPP, which increases over successive defibrillation attempts in animals with ROSC. The myocardium may be “partially” reperfused in earlier unsuccessful rescue shocks, and then be “adequately” reperfused during later successful rescue shocks with a smaller amount of subsequent reperfusion. This is reflected in the delta CPP measure, which increases by 29.2 ± 1.6 mmHg for the first rescue shock, and then varies only by 0.2 to 8.9 mmHg at a time between successive rescue shocks. (Table 3)
A more dynamic CPP measure, such as cumulative dose, may be needed to account for ongoing perfusion. This measure may be more illuminating than traditional static measures computed at a single point in time because it better reflects the ongoing state of the myocardium and cumulative oxygen debt during ongoing resuscitation. Further exploration of this cumulative dose shows that it is tempered by elapsed time-to-defibrillation-attempt. (Table 5).
The five conceptual models of CPP had “good” to “fair” discrimination between animals with and without ROSC. Threshold CPP and cumulative delta CPP had “good” discrimination, while dose CPP and cumulative dose CPP had “fair” discrimination between animals with and without ROSC.
Initial CPP required for ROSC in our porcine model of OHCA is higher than the often-quoted necessary threshold CPP of 15–25 mmHg for ROSC derived from prior animal and human data. (5–14) This needs to be externally validated by another laboratory. One reason for the threshold discrepancy between prior animal models and our data may be the timing of the experimental protocol. Previous studies were conducted with briefer untreated arrest intervals (0–5 minutes). These studies modeled cardiac arrest that rarely extended beyond the electrical phase. (5) Our studies were conducted with an untreated VF duration of 5 – 10 minutes (circulatory and metabolic phases). Our longer duration of “no -flow” may necessitate higher initial CPP requirements. Prior human data were collected in persons suffering 8–12 minutes of untreated cardiac arrest (circulatory and metabolic phase), yet there is still a significant difference in the necessary threshold CPP for ROSC. One reason for this discrepancy may be the timing of data collection. Human data collection began very late into cardiac arrest (24–25 minutes), whereas our data were collected immediately at the onset of resuscitation (8–11 minutes). The decreasing CPP requirements we demonstrated over the course of resuscitation are supported by the lower CPP values obtained during human data collection.
Future work in the field may benefit from a more comprehensive analysis and presentation of coronary perfusion pressure, taking into account different conceptual models and time-dependent effects on this important hemodynamic variable. If future work corroborates our analysis, then the existing Utstein style for reporting animal studies may require modification to more robustly describe coronary perfusion pressure.
Limitations
There were several limitations to our study. First, this was a retrospective analysis and there were some discrepancies in demographics between experiments. Second, we analyzed young healthy swine whose physiology may not reflect that of typical cardiac arrest patients. Third, there may be a species difference between swine and human cardiopulmonary physiologies. Fourth, our particular swine model utilizes early endotracheal intubation, ventilation with 100% FiO2 oxygen, and an optimized drug cocktail that may not be representative of typical clinical care rendered. Fifth, we analyzed CPP, not myocardial blood flow directly. Typical cardiac arrest patients have a high burden of cardiovascular disease with increased coronary vascular resistance, adversely affecting the translation of pressure into flow. Sixth, VF was electrically induced in all of these cases, not induced by ischemia. Finally, we examined ROSC, not long-term survival or neurologic outcomes.
Conclusions
Animals with ROSC have higher threshold, delta, cumulative delta, dose, and cumulative dose CPP than animals without ROSC. These five conceptual models of CPP predict ROSC with “good” to “fair” discrimination. CPP requirements for successful defibrillation change throughout the resuscitation and the competing effects of cumulative dose CPP with time-elapsed-to-shock may be a more sophisticated method of measuring this.
Footnotes
Will be presented at the National Association of EMS Physicians Annual Meeting Tucson, AZ, January 12, 2012 by Joshua C. Reynolds
Disclosures: Dr. Menegazzi is a co-inventor of a patented quantitative method of ECG analysis (the scaling exponent), which has been licensed to Medtronic Physio-Control, from which he receives royalties. Neither of the other authors have anything to disclose.
Conflict of Interest Statement
Support: Dr. Menegazzi receives support by contract RO1 HL080483, from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288:3035–8. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 2.Cobb LA, Fahrenbruch CE, Walsh TR, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–8. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 3.Wik L, Hansen TB, Fylling F, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–95. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 4.Berg RA, Hilwig RW, Ewy GA, Kern KB. Precoutnershock cardiopulmonary resuscitation improves initial response to defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Critical Care Medicine. 2004;32:1352–7. doi: 10.1097/01.ccm.0000127780.01362.e5. [DOI] [PubMed] [Google Scholar]
- 5.Babbs CF. New versus old theories of blood flow during CPR. Critical Care Medicine. 1980;8:191–5. doi: 10.1097/00003246-198003000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–50. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 7.Redding JS, Pearson JW. Evaluation of drugs for cardiac resuscitation. Anesthesiology. 1963;24:203–7. doi: 10.1097/00000542-196303000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Ditchey RV, Winkler JV, Rhodes CA. Relative lack of coronary blood flow during closed-chest resuscitation in dogs. Circulation. 1982;66:297–302. doi: 10.1161/01.cir.66.2.297. [DOI] [PubMed] [Google Scholar]
- 9.Sanders AB, Ewy GA, Alferness CA, Taft T, Zimmerman M. Failure of one method of simultaneous chest compressions, ventilation, and abdominal binding during CPR. Critical Care medicine. 1982;10:509–13. doi: 10.1097/00003246-198208000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Michael JR, Guerci AD, Koehler RC, Shi AY, Tsitlik J, Chandra N, Niedermeyer E, Rogers MC, Traystmann RJ, Weisfeldt ML. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation. 1984;69:822–35. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 11.Niemann JT, Rosborough JP, Niskanen RA, Alferness CA, Criley JM. Mechanical “cough” cardiopulmonary resuscitation during cardiac arrest in dogs. American Journal of Cardiology. 1985;55:199–204. doi: 10.1016/0002-9149(85)90328-5. [DOI] [PubMed] [Google Scholar]
- 12.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Annals of Emergency Medicine. 1985;14:521–8. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]
- 13.Sanders AB, Kern KB, Atlas M, Bragg S, Ewy GA. Importance of the duration of inadequate coronary perfusion pressure on resuscitation from cardiac arrest. Journal of the American College of Cardiology. 1985;6:113–8. doi: 10.1016/s0735-1097(85)80261-8. [DOI] [PubMed] [Google Scholar]
- 14.Niemann JT, Cruz B, Garner D, Lewis RJ. Immediate countershock versus cardiopulmonary resuscitation before countershock in a 5-minute swine model of ventricular fibrillation arrest. Annals of Emergency Medicine. 2000;36:543–6. doi: 10.1067/mem.2000.109441. [DOI] [PubMed] [Google Scholar]
- 15.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–13. [PubMed] [Google Scholar]
- 16.Mader TJ. Prolonged cardiac arrest: A revised model of porcine ventricular fibrillation. Resuscitation. 2008;76:481–4. doi: 10.1016/j.resuscitation.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) Study. Journal of the American Medical Association. 1994;271:678–83. [PubMed] [Google Scholar]
- 18.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New England Journal of Medicine. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 19.Fan KL, Leung LP. Prognosis of patients with ventricular fibrillation in out-of-hospital cardiac arrest in Hong Kong: prospective study. Hong Kong Medical Journal. 2002;8:318–21. [PubMed] [Google Scholar]
- 20.Herlitz J, Engdahl J, Svensson L, Angquist KA, Young M, Holmberg S. Factors associated with an increased chance of survival among patients suffering from an out-of-hospital cardiac arrest in a national perspective in Sweden. American Heart Journal. 2005;149:61–6. doi: 10.1016/j.ahj.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Stiell IG, Wells GA, DeMaio VJ, et al. Modifiable factors associated with improved cardiac arrest survival in a multicenter basic life support/defibrillation system: OPALS Study Phase I results. Ontario Prehospital Advanced Life Support. Annals of Emergency Medicine. 1999;33:44–50. doi: 10.1016/s0196-0644(99)70415-4. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds JC, Rittenberger JC, Menegazzi JJ. Drug administration in animal studies of cardiac arrest does not reflect human clinical experience. Resuscitation. 2007;74:13–26. doi: 10.1016/j.resuscitation.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2010 International consensus on cardiopulmonary resuscitation and cardiovascular care science with treatment recommendations. Circulation. 2010;122(Suppl 2) doi: 10.1161/CIRCULATIONAHA.110.971010. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehospital Emergency Care. 2010;14:78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menegazzi JJ, Rittenberger JC, Suffoletto BP, Logue ES, Salcido DD, Reynolds JC, Sherman LD. Effects of pre-arrest and intra-arrest hypothermia on ventricular fibrillation and resuscitation. Resuscitation. 2009;80:126–32. doi: 10.1016/j.resuscitation.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betz AE, Menegazzi JJ, Logue ES, Callaway CW, Wang HE. A randomized comparison of manual, mechanical, and high-impulse chest compression in a porcine model of prolonged ventricular fibrillation. Resuscitation. 2006;69:495–501. doi: 10.1016/j.resuscitation.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Rittenberger JC, Suffoletto BP, Salcido DD, Logue ES, Menegazzi JJ. Increasing CPR duration prior to first defibrillation does not improve return of spontaneous circulation or survival in a swine model of prolonged ventricular fibrillation. Resuscitation. 2008;79:155–60. doi: 10.1016/j.resuscitation.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suffoletto BP, Salcido DD, Logue ES, Caprio TW, Menegazzi JJ. Ethyl pyruvate enhances intra-resuscitation hemodynamics in prolonged ventricular fibrillation arrest. Resuscitation. 2009;80(12):1411–6. doi: 10.1016/j.resuscitation.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Mader TJ, Menegazzi JJ, Rittenberger JC, Suffoletto BS, Callaway CW, Salcido DD, Logue ES, Sherman DD. The effect of adenosine A1 receptor antagonism on return of spontaneous circulation and short-term survival in prolonged ventricular fibrillation. Prehospital Emergency Care. 2008;12(3):352–8. doi: 10.1080/10903120802101223. [DOI] [PubMed] [Google Scholar]
- 30.Frenneaux M. Cardiopulmonary resuscitation—some physiologic considerations. Resuscitation. 2003;58:259–265. doi: 10.1016/s0300-9572(03)00266-1. [DOI] [PubMed] [Google Scholar]