Abstract
In animal models of addiction, reducing glutamate stimulation of the metabotropic glutamate receptor 5 (mGluR5) inhibits drug-seeking. The present study used the reinstatement model of cocaine-seeking to show that blockade of mGluR5 directly in the core subcompartment of the nucleus accumbens (NAcore) prevented both conditioned cue- and cocaine-reinstated drug seeking. Consistent with this finding, microinjection of the mGluR5 agonist CHPG into the NAcore produced modest reinstatement of lever pressing when given alone, and significantly potentiated cue-induced reinstatement. Homer proteins are contained in the postsynaptic density and regulate mGluR5 intracellular signaling and trafficking to the membrane. Microinjecting a membrane permeable peptide antagonist of Homer binding to mGluR5 into the NAcore also inhibited cue- and cocaine-reinstated lever pressing. However, this peptide did not change the surface expression of mGluR5, indicating that the peptide inhibitor did not alter the surface trafficking of mGluR5. Taken together, these data show that mGluR5 inhibition and stimulation in the NAcore can regulate cocaine-seeking, and demonstrate that one mechanism for this effect is via interactions with Homer proteins.
Keywords: reinstatement, accumbens, cocaine, mGluR5, Homer
INTRODUCTION
Over the last decade, substantial effort has been made to discover the neuronal mechanisms of addiction by characterizing the enduring drug-induced changes in glutamate transmission and synaptic plasticity in corticostriatal projections, notably in the projection from the prefrontal cortex to the nucleus accumbens (Bowers et al., 2011; Kauer & Malenka, 2007; Gass & Olive, 2008; Kalivas, 2009). In animal models of cocaine addiction employing daily noncontingent cocaine injections, such as locomotor sensitization or conditioned place preference, the genetic deletion of one of the group I metabotropic glutamate receptors, mGluR5, antagonizes both cocaine sensitization and reward (Chiamulera et al., 2001). Although the initial finding with cocaine sensitization has been recently disputed (Bird et al., 2011), these original observations in mGluR5 knock-out mice heralded a series of experiments over the next decade employing pharmacological antagonism of mGluR5. These studies consistently show that systemic blockade of mGluR5 inhibits cocaine seeking measured either after noncontingent cocaine in a conditioned place preference paradigm, or by reinstating operant behavior in animals trained to self-administer cocaine (McGeehan & Olive, 2003; Lee et al., 2005; Backstrom & Hyytia, 2006; Martin-Fardon et al., 2009; Moussawi et al., 2009). The majority of these studies employed negative allosteric modulators of mGluR5 (NAMs), and this class of drugs has been proposed as a potential pharmacotherapy for cocaine addiction (Carroll, 2008).
Over the last decade, an alteration in glutamate transmission in the projection from the prelimibic prefrontal cortex to the core subcompartment of the nucleus accumbens (NAcore) has been identified as an important substrate underlying reinstated cocaine-seeking (Kalivas, 2009). However, it is not known whether systemically administered mGluR5 NAMs act directly in the NAcore to reduce cocaine-seeking. There are two studies investigating involvement of the accumbens. One study focused entirely on the shell subcompartment (NAshell), and found mGluR5 blockade inhibited reinstated cocaine-seeking (Kumaresan et al., 2009). The other study concluded that while inhibiting mGluR5 in the NAcore reduced cocaine-seeking that was reinstated by a conditioned cue, a control vehicle microinjection also reduced responding, which made the finding inconclusive (Backstrom & Hyytia, 2007).
The Homer family proteins are located largely in the postsynaptic density, bind to proline-rich sequences of mGluR5 via an EVH1 domain, and contribute to mGluR5 signaling and trafficking.(Tu et al., 1998; Xiao et al., 1998; Ango et al., 2000; Fagni et al., 2002). The mGluR5-Homer interaction facilitates the coupling of mGluR5 with IP3 receptors (Tu et al., 1998), mGluR5-dependent long term depression (Ronesi & Huber, 2008), activation of ERK signaling pathway (Mao et al., 2005), and the signaling of mGluR5 to N-type Ca2+ and M-type K+channels (Kammermeier et al., 2000). Taken together with the effects of mGluR5 on cocaine behavior described above, disrupting the mGluR5-Homer interaction might affect cocaine–seeking behavior.
In the present study, we conducted three experiments to demonstrate involvement of mGluR5 and Homer signaling in the NAcore in reinstated cocaine-seeking. We first showed that antagonism of mGluR5 in the NAcore inhibited both cue- and cocaine-induced reinstatement. Subsequently we demonstrated that stimulation of mGluR5 in the NAcore augmented cue-reinstated cocaine seeking. Finally, we found that reinstated cocaine-seeking was inhibited by disrupting the mGluR5-Homer interaction in the NAcore. Taken together, these data show that reinstated cocaine-seeking can be reduced or potentiated by mGluR5 inhibition or stimulation, respectively.
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (Charles River Laboratories, Indianapolis, IN), weighing 225–250, were individually housed in a temperature-controlled colony room under a reversed 12-h light/dark cycle. Rats were allowed to acclimate in the colony for at least 1 week prior to surgeries, with ad libitum access to water and food. All behavioral training and testing was done during the dark cycle. All experiments were performed in accordance with the guidelines of National Institutes of Health (NIH) for care of laboratory animals and approved by the Medical University of South Carolina Institutional Animal Care and Use Committee.
Surgery
Rats were anesthetized by intramuscular injection of ketamine HCl (100 mg/kg, Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (0.2 ml of a 10 mg/kg, AnaSed, LLOYD Laboratories, Shenandoah, IA). Ketorolac (0.3 ml of a 2 mg/ml, Sigma-Aldrich, St. Louis, MO) was injected intraperitoneally to provide analgesia. For catheter implantation, the right jugular vein was isolated and the one end of one silicone tubing (inner diameter 0.51 mm, outer diameter 0.94 mm, Dow Corning, Midland, MI) was inserted 3 cm into the vein and then sutured securely to the vein. The other end of the tubing was threaded subcutaneously to exit between the scapulae and connected to an infusion harness (Instech, Plymouth Meeting, PA). Catheters were sealed with plastic caps when not in use. Following catheter surgery, the rats were placed in a stereotaxic apparatus. Bilateral guide cannula (26 gauge, Plastics One, Wallingford, CT) were implanted in the medial NAcore (+1.8mm AP, 1.6 mm ML, and −5.5 mm DV from the surface of the skull) (Paxinos & Watson, 1986), and were attached to the skull via 3 surgical screws with dental acrylic cement. Stainless steel obturators were inserted into the guide cannula to prevent blockage. After surgery, the animals were returned to home cages. Catheters were daily flushed with cefazolin (0.2 ml of a 0.1 g/ml, Novation, Irving, TX) and heparin (0.2 ml of a 100 IU/ml, Hospira, Lake Forest, IL) for 1 week after surgery and then heparin alone through behavior training. Catheter patency was also tested periodically by injection of sodium methohexital (0.1–0.2 ml of a 10 mg/ml, JHP Pharmaceuticals, Parsippany, NJ).
Cocaine self-administration, extinction and reinstatement
During behavioral training and testing, food was restricted to 4 chow pellets per day. Water was always available ad libitum. Rats were trained to self-administer cocaine (NIH, Bethesda, MD) in standard operant chambers, each of which had 2 retractable levers, a house light, 2 cue lights and a tone generator. A fixed ratio (FR) 1 schedule was employed to self-administer cocaine. During daily 2-hr sessions, an active lever press resulted in an infusion of cocaine (0.2 mg in 0.05 ml) over 3 s, accompanied by a cue tone and a cue light for 5 s, and followed by a 20-s timeout during which active lever presses were recorded but did not result in further cocaine infusion. Inactive lever presses were of no scheduled consequence (no tone, light and cocaine infusion) but were recorded. Rats were trained for at least 2 weeks until the animals obtained 10 infusions or more per session for 10 consecutive days. After this criterion was met, extinction procedures began and lasted for at least 2 weeks. During daily 2-hr extinction sessions, active lever presses no longer resulted in cocaine infusions or a tone/light cue presentation. Extinction training continued until the active lever presses were less than 30% of those during self-administration training. Reinstatement testing began following extinction training. For cue reinstatement, active lever presses resulted in the presentation of the cues (both tone and light), but no cocaine infusions. For cocaine reinstatement, rats received a systemic cocaine injection (10 mg/kg, ip) and were immediately placed in the chambers. For cocaine+cue reinstatement, both a cocaine injection and the cues were given. Each reinstatement session was followed by at least 3 extinction sessions and the extinction criterion achieved. All drug and vehicle administrations were counterbalanced across reinstatement.
Sucrose self-administration procedures
To control for a nonspecific effect of microinjected TAT-mgluR5ct, one group of animals self-administered sucrose pellets for 12 days using an FR-1 schedule of reinforcement and a light/tone conditioned cue with the delivery of pellets. Sucrose self-administration was conducted in an identical manner to cocaine self-administration, with the exception that presses on the active lever delivered sucrose pellets (45 mg; Test Diet, Richmond, IL). Animals self-administered sucrose for 2 weeks, followed by a minimum of 10 days of extinction training. Once animals had met extinction criteria (less than 30% active lever pressing during self-administration), cue-primed reinstatement testing was conducted using a within-subjects design. All subjects were tested with both TAT-mglur5ct or control peptides in a counter-balanced manner. The timing and concentration of peptides administered was identical to the procedures used for reinstatement testing in cocaine self-administering animals described above.
Microinjection
The obturators were removed from the guide cannula. Two 33-gaugemicro injectors were bilaterally inserted into the NAcore and extended 2 mm beyond the tip of the guide cannula. The test drugs were infused in a volume of 0.5 µl over 1 min using an infusion pump (Harvard Apparatus, Holliston, MA). After the infusion was completed, the microinjectors were left in place for an additional 1 min to allow for diffusion of the drugs and then the obturators were replaced.
Drugs
MTEP hydrochloride, a NAM of mGluR5 (Carroll, 2008), and CHPG, a mGluR5 agonist (Schoepp et al., 1999), were purchased from Tocris (Ellisville, MO). MTEP hydrochloride was dissolved in aCSF at a concentration of 2 µg/µl. CHPG was dissolved in 1 M NaOH solution at a concentration of 20 µg/µl and diluted to working concentration with aCSF. TAT-mGluR5ct (YGRKKRRQRRR-ALTPPSPFR), a peptide inhibitor of the binding of mGluR5 to Homers (Ronesi & Huber, 2008; Mao et al., 2010; Tronson et al., 2010), and control peptide (YGRKKRRQRRR-ALTPLSPRR) were purchased from GenWay (San Diego, CA). Both peptides were dissolved in aCSF and stored at a concentration of 2 µg/µl. When used, these solutions were diluted into the required concentrations with aCSF. Cocaine hydrochloride was obtained from NIDA (Bethesda, MD) and dissolved in physiological saline.
Biotinylation assay and Western blotting
The method of biotinylation assay has been described previously (Knackstedt et al., 2010). The NAcore was dissected and sliced into sections (200 µm) with a McIlwain tissue chopper (Vibratome, St. Louis, MO). The tissue was incubated for 30 min in 300 µl PBS containing 1 mg/ml Sulfo-NHS-Biotin (Pierce, Rockford, IL) at 4 °C with gentle shaking. The reaction was quenched by adding ice-cold 100 mM glycine in PBS (PH=7.8). The tissues were washed twice with ice-cold 100 mM glycine in PBS, and then homogenized by sonication in 200 µL 1% SDS in RIPA buffer (Pierce, Rockford, IL) containing a phosphatase inhibitor (Roche, Indianapolis, IN) and protease inhibitor cocktail (Pierce, Rockford, IL). After incubating 30–60 min on ice, the samples were centrifuged at 10,000×g for 10 min at 4 °C and the supernatants were collected. The protein concentration of the samples was measured and adjusted to the same concentration of protein for each sample. A small portion of each sample was reserved to run as the total protein traction. The remainder of the samples were added with 100 µl NeuroAvidin Agarose Resin (Pierce, Rockford, IL) and incubated for 2 hr at 4 °C with gentle shaking. The samples were centrifuged at 10,000×g for 3 min at 4 °C and the supernatant was removed as the non-biotinylated fraction. The pellets were washed with ice-cold PBS and spun down twice at 10,000×g for 3 min. The biotinylated protein was eluted by 50 µL loading buffer (Invitrogen, Carlsbad, CA) with 100 mM DTT (Sigma-Aldrich, St. Louis, MO) and heated at 80°C for 5 min. Finally, samples were spun down at 10,000×g for 3 min and the supernatant was collected as the biotinylated subfraction. The total protein fraction was also mixed with loading buffer containing 100 mM DTT and heated at 80°C for 5 min. The total and biotinylated subfractions were stored at −80°C for Western blotting, as described by Knackstedt et al. (2010). Antibodies used included mGluR5 (1:300, Upstate, Lake Placid, NY) and homer1b/c (1:500), with α-Tublin (1:5000) used as an internal loading control for the whole cell measurements (Santa Cruz Biotechnology, Santa Cruz, CA). Band density was quantified by image J software (NIH, Bethesda, MD) and normalized to control subjects.
Experiment 1
For cocaine reinstatement, the mGluR5 NAM, MTEP, or an equivalent volume of vehicle (0.5 µl aCSF per side) was bilaterally microinjected into the NAcore, followed by cocaine injection (10 mg/kg, i.p.) 20–30 min later, and the rat placed into an operant chamber. For cued reinstatement, either 0.3 or 1 µg MTEP per side was microinjected into the NAcore, and the animals placed in the operant chambers 20–30 min after MTEP or aCSF microinjection. A subgroup of animals was also used to evaluate whether 1 µg MTEP alone could reinstate responding. The dose range of MTEP was based on previous findings with MPEP microinjection in other behavioral assays, and preliminary data indicating that a behaviorally active dose in reinstated cocaine-seeking was about a half log below previously published intracranial doses (Simonyi et al., 2009; Tronson et al., 2010).
Experiment 2
In this experiment, only cued reinstatement was performed. The rats received microinjections of CHPG. Doses of 3 or 6 µg per side were employed based on the intrancranial dosage range capable of influencing locomotor activity by a chemically related, less selective agonist of group I mGluRs; (Swanson & Kalivas, 2000)) or vehicle (aCSF). Rats were placed in the operant chambers 10 min after completing the microinjection. Also, CHPG (6 µg/side) alone was tested during an extinction trial for the capacity to reinstate lever pressing.
Experiment 3
For cued reinstatement, TAT-mgluR5ct or control peptides were microinjected bilaterally (0.15 µg/side) into the NAcore and the rats were placed in the operant chambers 20–30 min later. The dose was derived from previous in vivo and in vitro studies that also found a 30 min or greater delay between peptide administration and experimental measurements produced physiological (Mao et al., 2005; Mameli et al., 2009; Tronson et al., 2010). For cocaine+cue-induced reinstatement, the same doses of TAT-mgluR5ct and control were used. Cocaine (10 mg/kg, i.p.) was administrated immediately before putting the rats into the operant chambers. Also, TAT-mgluR5ct alone was tested during an extinction trial for its ability to induce reinstated lever pressing. Finally, the capacity of TAT-mGlur5ct to affect reinstated lever pressing for sucrose pellets was examined. The procedure was identical to that described for cue and cocaine+cue-induced reinstatement, except that reinstatement was initiated by presenting 2 food pellets (see above).
To evaluate the potential effects of disrupting mGluR5-Homer binding induced by the TAT-mgluR5ct peptide on the surface expression of mGluR5, a behaviorally effective dose of TAT-mgluR5ct (0.15 µg/side) or control peptidewas bilaterally microinjected into the NAcore of drug naïve rats. The animals were decapitated 1 hr later and the NAcore processed for biotinylation and Western blotting (see above).
Histology and statistical analysis
Rats were overdosed with pentobarbital (100 mg/kg, i.p.) and perfused transcardially with 0.9% saline followed by 10% formalin. Brains were removed and stored in 10% formalin solution. Coronal sections (150 µm) were cut through guide cannula tract using a vibratome (Technical Products International, St. Louis, MO), mounted on gelatin-coated slides and were stained with 1% cresyl violet. All data are presented as mean ± SEM. Both SPSS 11.0 (SPSS, Chicago, IL) and Graphpad (Graphpad Software, San Diego, CA) software were used for the data analysis. Behavioral data and Western blot density were analyzed with a one-way repeated analysis of variance (ANOVA) or paired t test if not indicated otherwise. A Bonferroni post-hoc adjustment was used for multiple comparisons.
RESULTS
Experiment 1: Inhibition of mGluR5 in the NAcore inhibits cue and cocaine-induced reinstatement
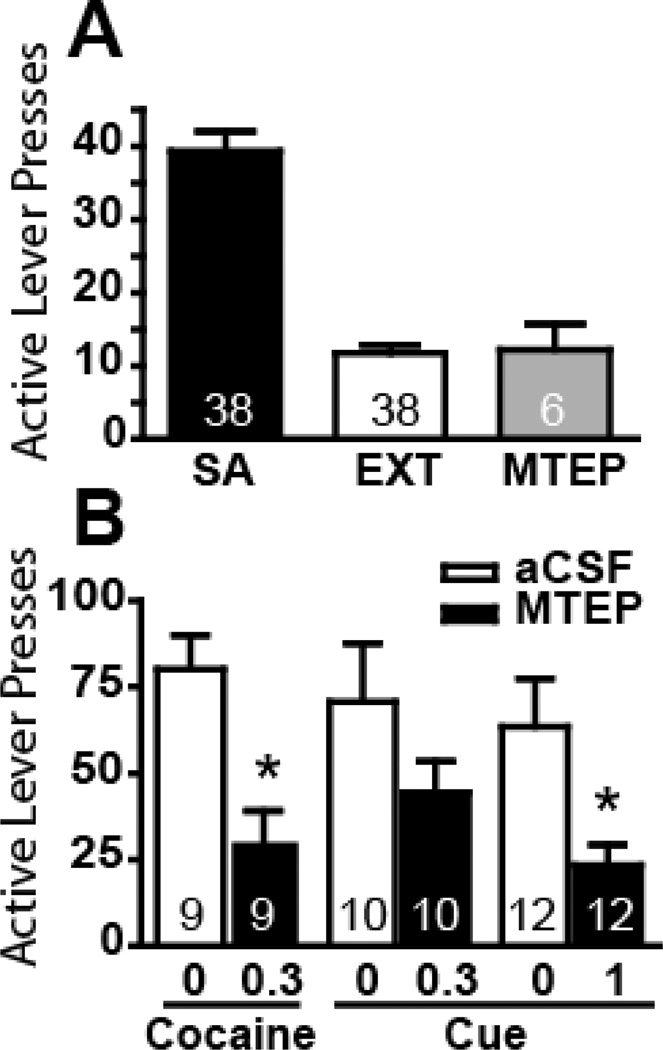
Fig. 1A shows the average number of active lever presses over the last 3 sessions of self-administration (39.4 ± 2.7) and extinction (11.8 ± 1.1), and also shows that MTEP alone (1 µg/side) did not reinstate the active lever pressing. The effects of MTEP on cue or cocaine-induced reinstatement of cocaine-seeking are depicted in Fig. 1B. There was a significant inhibitory effect of MTEP (0.3 µg/side) on cocaine-primed reinstatement [t(8)= 6.75, p < 0.001], but not for cue-induced reinstatement, even though there was a trend towards inhibition. However, the higher dose of MTEP (1 µg/side) significantly attenuated cue-induced reinstatement [t(11)= 3.14, p= 0.009].
Figure 1.
MTEP infusion into the NAcore inhibited cue- and cocaine-reinstated drug seeking. (A) Number of active lever presses within last 3 day self-administration training (SA) and within last 3 day extinction training (EXT), as well as after the administration of MTEP (1 µg/side). (B) Attenuation of cocaine-primed reinstatement by MTEP (0.3 µg/side) and of cue-induced reinstatement by MTEP (1 µg/side). *p < 0.05, compared with aCSF treatment. Animal number for each treatment is indicated in the bar.
Experiment 2: Stimulation of mGluR5 potentiates cue-induced reinstatement
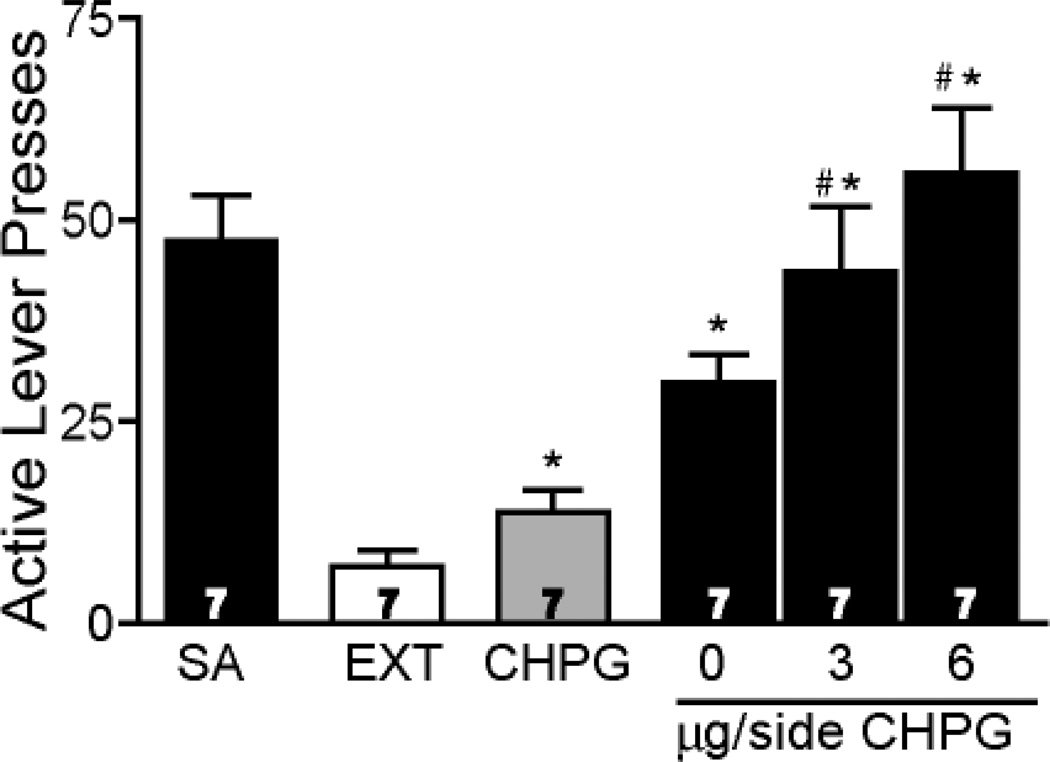
The average number of active lever presses over the last 3 sessions of self-administration (47.4 ± 5.7) and extinction pressing (7.0± 2.0) are shown in Fig. 2. A one-way repeated measures ANOVA [F(4,34)= 11.93, p < 0.001] revealed a significant increase reinstated lever pressing after microinjecting the mGluR5 agonist CHPG into the NAcore compared to extinction training. Moreover, when animals were pretreated with a CHPG microinjection prior to a cue-induced reinstatement trial, a dose-dependent augmentation of lever pressing was measured.
Figure 2.
Microinjection of CHPG into the NAcore potentiated cue-induced reinstatement of cocaine-seeking. SA and EXT represent averaged number of active lever presses within last 3 day self-administration training and within last 3 day extinction training, respectively. CHPG (6 µg/side) alone increased the reinstatement of cocaine-seeking and also potentiated cue-induced reinstatement. *p < 0.05, compared with the EXT using a Bonferroni multiple comparisons. #p < 0.05, compared with aCSF injection (0 µg/side). Animal number for each treatment is indicated in the bar.
Experiment 3: Inhibiting mGluR5 interactions with Homer reduces cue or cue+cocaine-induced reinstated drug seeking
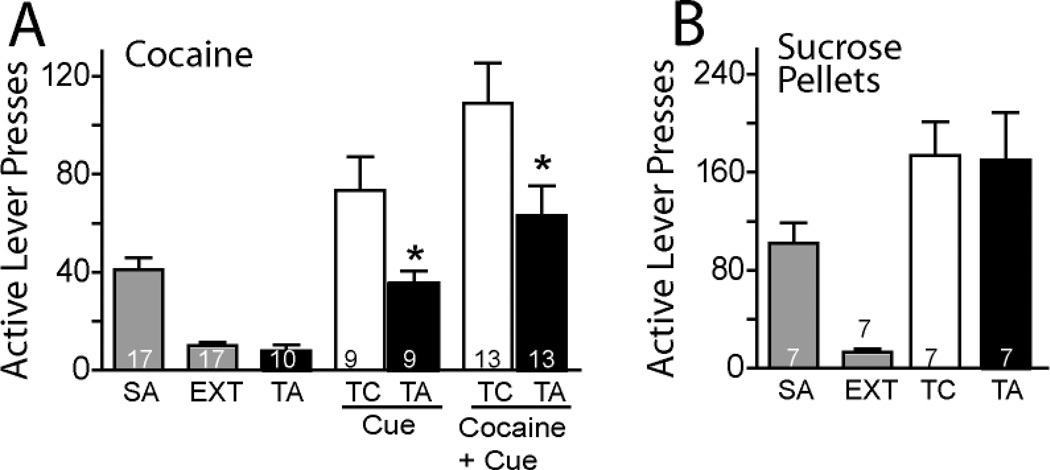
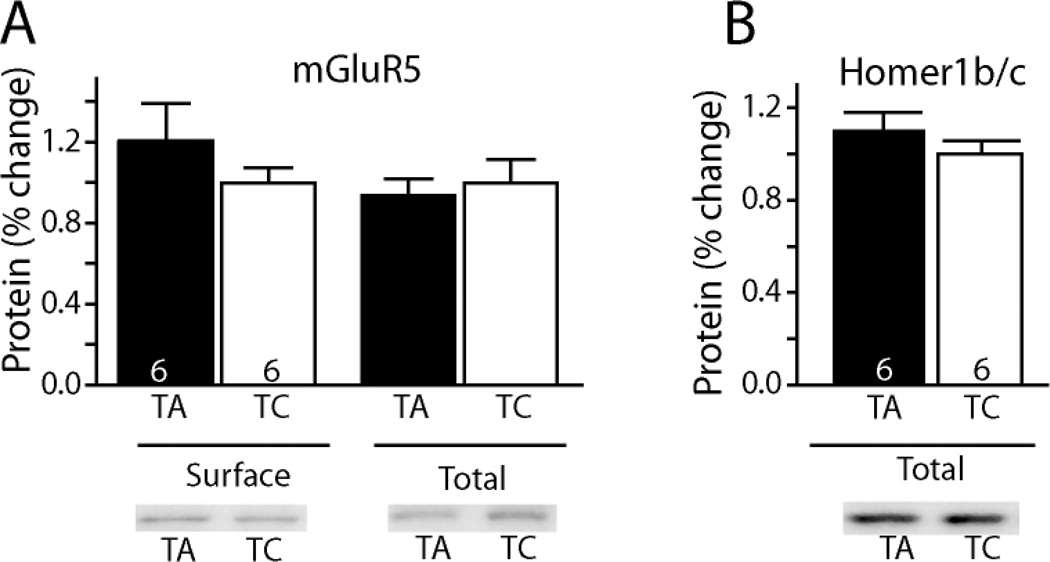
Fig. 3A shows the average number of active lever presses over the last 3 sessions of self-administration (41.5 ± 5.3) and extinction (9.2 ± 1.5), and also shows that TAT-GluR5ct alone did not reinstate active lever pressing. There was a significant inhibitory effect of TAT-mGluR5ct (0.15 µg/side) on cue-induced reinstatement [t(8) = 2.83, p= 0.022]. Further, the same dose of TAT-mGluR5ct attenuated cue+cocaine-induced reinstatement when compared to the control peptide [t(12) = 3.00, p= 0.011]. To determine if the effect of TAT-GluR5ct would generalize to reinstated responding for a natural reinforcer, rats were trained to self-administer, extinguished and reinstated to sucrose pellets. Fig 3B shows that while sucrose-seeking was successfully reinstated compared to extinction levels of active lever pressing, the reinstated lever pressing was equivalent between TAT-GluR5ct and control peptide[F(2,23) = 18.53, p < 0.001]. A biotinylation strategy was used to estimate surface levels of mGluR5 in the NAcore. At 60 min after microinjecting TAT-GluR5ct no change in surface mGluR5 was measured (Fig. 4). TAT-GluR5ct also did not affect whole cell levels of mGluR5. Similarly, TAT-GluR5ct microinjection did not alter whole cell levels of Homer1b/c.
Figure 3.
Microinjection of TAT-mgluR5ct (TA; 0.15 µg/side) into the NAcore inhibited the reinstatement of cocaine-seeking. (A) SA and EXT represent the average number of active lever presses over last 3 days of cocaine self-administration training and over last 3 days extinction training, respectively. TA alone had no effect on the number of active lever presses, but significantly reduced reinstated cocaine seeking by a cue or cue+cocaine prime compared to control peptide (TC).(B) TA had no effect on reinstated sucrose-seeking compared with TC microinjections into the NAcore. *p < 0.05, comparing TA with TC. Animal number for each treatment is indicated in the bar.
Figure 4.
Microinjection of TAT-mglur5ct (0.15 µg/side) into the NAcore did not change the surface expression of mGluR5. TA and TC represent TAT-mglur5ct and control peptides, respectively. Representative blots are shown below each panel. (A) Expression of mGur5 in biotinylated (surface) and whole cell fractions. (B) Expression of Homer1b/c in total fraction. Animal number for both treatments is indicated in the bar.
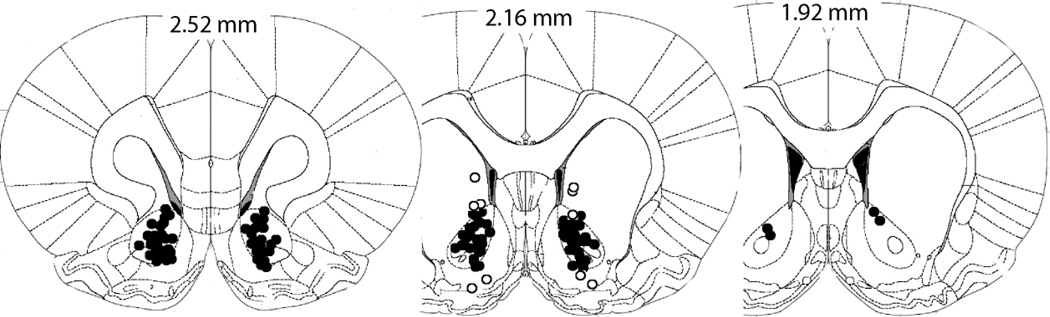
Histology
Fig. 5 illustrates the tip of the cannula tracks terminating in the NAcore. The majority of injection sites were targeted for the medial NAcore, and animals with one or both cannula tracks outside of the NAcore were excluded from data analysis.
Figure 5.
Illustration of the location of cannula tips where injections were made into the NAcore (closed circles) according to the atlas of Paxinos and Watson (1986). Animals with one or the both injection sites outside of the NAcore were not used in the study (open circles).
DISCUSSION
The data presented in this study show that intra-NAcore administration of MTEP reduced cue- and cocaine-reinstated drug seeking. The mGluR5 agonist CHPG microinjected into the NAcore produced a small increase in reinstated cocaine seeking, and markedly augmented cue-induced cocaine seeking. Finally, by pharmacologically inhibiting mGluR5 signaling through Homer proteins in the NAcore, reinstated cocaine seeking was significantly reduced.
In general, these findings are consistent with a body of behavioral pharmacology literature showing that systemically administered mGluR5 NAMs reduce reinstated cocaine seeking (Lee et al., 2005; Backstrom & Hyytia, 2006; Iso et al., 2006; Kumaresan et al., 2009; Moussawi et al., 2009). A previous study did not observe a significant reduction in cue-induced cocaine seeking by microinjecting mGluR5 NAMs into the NAcore (Backstrom & Hyytia, 2007). However, these data were deemed inconclusive by the authors since the intra-NAcore vehicle injection also inhibited cocaine seeking. Fig. 1 clearly shows that in the present study MTEP reduced both cue- and cocaine-reinstated drug seeking compared to aCSF vehicle using a within subjects comparison. Possible explanations for this difference include the use of a saline vehicle in the previous study rather than aCSF, and the use of a different NAM (MPEP instead of MTEP) that may have led to under-dosing.
The present findings with MTEP are consistent with recent findings that antagonism of mGluR5 in the NAshell inhibited cocaine-reinstated cocaine-seeking (Kumaresan et al., 2009). The efficacy of mGluR5 antagonism to inhibit reinstated behavior was approximately the same as in the present study, although Kumaresan et al. employed MPEP rather than MTEP. Interestingly, in the NAcore we observed that MTEP was a somewhat more effective antagonist of cocaine compared to cue. The circuitry regulating cue- versus cocaine-reinstated behavior differs (See, 2002), and could contribute to the different inhibitory efficacy of mGluR5 antagonism. For example, while cue-induced reinstatement is prevented by inactivating the basolateral amygdala, cocaine-induced reinstatement is not affected by amygdala inactivation (See, 2002). Although the basolateral amygdala projects to both the NAshell and NAcore, the density of the projection is greater to the NAshell (Kelley et al., 1982). Thus, it is possible that MTEP may more potently inhibit cue-induced reinstatement after microinjection into the NAshell than NAcore, but this possibility was not evaluated. However, given that many microinjections were made into the medial NAcore, diffusion of MTEP into the NAshell cannot be ruled out.
The reduction in cocaine-seeking after blockade of mGluR5 demonstrates that tone on mGluR5 during reinstatement is supporting drug-seeking behavior. mGluR5s are located largely outside of the synaptic cleft in the postsynaptic membrane (Mitrano & Smith, 2007), and during a reinstatement event there is substantial overflow of synaptic glutamate into the nonsynaptic extracellular compartment (McFarland et al., 2003; Berglind et al., 2009). Thus, these data are consistent with a hypothesis that stimulating mGluR5 by reinstatement-induced increases in synaptic glutamate overflow is critical for cocaine-seeking since antagonizing mGluR5 inhibits the behavior. Although the efficacy of NAMs to inhibit reinstated behavior indicates that stimulating these mGluRs is necessary, the overflow of glutamate onto mGluR5 may not be sufficient for initiating cocaine seeking. Thus, directly stimulating mGluR5 with CHPG induced only very modest reinstatement. Nonetheless, mGluR5 stimulation was consequential in regulating reinstated behavior since CHPG potentiated cue-induced reinstatement. This is similar to an observation made following systemic administration of a positive allosteric modulator of mGluR5 (CDPPB) (Moussawi et al., 2009). While CDPPB alone did not reinstate cocaine-seeking, when the levels of glutamate were elevated in the accumbens by systemic pretreatment with N-acetylcysteine to activate cystine-glutamate exchange (Baker et al., 2003), CDPPB potentiated cocaine-induced reinstatement.
Homer binding is an important intracellular signaling mechanism underlying mGluR5 receptor stimulation (Tu et al., 1998; Xiao et al., 1998; Ango et al., 2000; Fagni et al., 2002), Correspondingly, pharmacologically disrupting the capacity of mGluR5 to bind to Homers with a membrane permeable peptide antagonist reduced reinstated cocaine-seeking akin to blocking mGluR5. Similar to a previous in vitro study (Mao et al., 2005), we found that TAT-GluR5ct administration did not reduce the surface expression of mGluR5. In apparent contrast with these observations, genetic regulation of Homer expression in vitro reveals both increases and decreases in the surface expression of mGluR5 (Fagni et al., 2002; Kammermeier, 2006). Also, in our earlier in vivo study, extinction training after cocaine self-administration resulted in parallel increases in Homer 1b/c and reductions in surface mGluR5 in the accumbens (Knackstedt et al., 2010). Moreover, we found that further increases in Homer1c in the accumbens using a viral overexpression strategy inhibited reinstated cocaine-seeking, causing us to speculate that down-regulated surface expression of mGluR5 may be an important compensatory mechanism for inhibiting cocaine-seeking (Knackstedt et al., 2010). The lack of effect by TAT-GluR5ct on mGluR5 surface expression in the present study could be seen as contradictory to the postulated role for homeostatic regulation of mGluR5 surface expression in cocaine-extinguished animals. However, the Homer viral overexpression studies involved extended periods of time in the presence of elevated Homer (~3 wks), whereas the TAT-GluR5ct studies utilized acute disruption of mGluR5 – Homer signaling. Thus, while an enduring homeostatic change to over-expressed Homer involves mGluR5 surface expression, the primary short-term effect of TAT-GluR5ct on cocaine-seeking most likely arose from disrupting signaling through Homer proteins, and did not require changes in mGluR5 surface expression.
The disruption of mGluR5 signaling by MTEP or TAT-GluR5ct could have occured in either glia or neurons. In astroglia mGluR5 stimulation leads to Gq mediated increases in intracellular calcium that provoke glial release of glutamate (D'Ascenzo et al., 2007; D'Ascenzo et al., 2009). In seeming contrast, mGluR5-induced increases in intracellular neuronal calcium result in a NMDA- and endocannabinoid-dependent long-term depression (LTD), and can also regulate NMDA-dependent long-term potentiation depending on experimental conditions (Lafourcade et al., 2007; Neyman & Manahan-Vaughan, 2008; Ronesi & Huber, 2008; Ireland & Abraham, 2009; Moussawi et al., 2009). Importantly, both mGluR5 induced LTD and glutamate release in the NAcore are blunted by repeated cocaine administration or Homer gene deletion (Swanson et al., 2001; Szumlinski et al., 2004; Moussawi et al., 2009), and at least the loss of LTD is restored after returning glutamatergic tone to mGluR5 by activating cystine-glutamate exchange (Moussawi et al., 2009). Taken together these data cannot clearly distinguish a role for glial versus neuronal mGluR5 in the regulation of cocaine-seeking behavior, and viral over-expression studies are underway using an astroglia-specific promoter to selectively regulate astroglial expression of proteins involved in mGluR5 signaling.
In summary, our data demonstrate that mGluR5 in the NAcore can promote or inhibit reinstated cocaine-seeking. The capacity of an mGluR5 NAM to inhibit cocaine-seeking was mimicked by an inhibitor of the binding between mGluR5 and Homer proteins, indicating that this interaction is important. However, the inhibition appears to result from disrupting the intracellular signaling mediated by Homers, rather than the capacity of Homers to regulate mGluR5 surface expression. The consistency in the literature indicating that mGluR5 NAMs reduce not just cocaine, but also alcohol, methamphetamine and opioid seeking (Popik & Wrobel, 2002; Backstrom et al., 2004; Gass et al., 2009), poses this class of drugs as a potential pharmacotherapy in ameliorating drug addiction.
Acknowledgements
This research was supported in part by NIH grants DA003906, DA012513 and DA015369.
Footnotes
Conflict of Interest: None
REFERENCES
- Ango F, Pin J, Tu J, Xiao B, Worley P, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. Journal of Neuroscience. 2000;20:8710–9716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Reid CA, Chen F, Tan HO, Petrou S, Lawrence AJ. Cocaine-mediated synaptic potentiation is absent in VTA neurons from mGlu5-deficient mice. Int J Neuropsychopharmacol. 13:133–141. doi: 10.1017/S1461145709990162. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI. Antagonists at metabotropic glutamate receptor subtype 5: structure activity relationships and therapeutic potential for addiction. Ann N Y Acad Sci. 2008;1141:221–232. doi: 10.1196/annals.1441.015. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan M, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquiet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nature Neuroscience. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ascenzo M, Podda MV, Fellin T, Azzena GB, Haydon P, Grassi C. Activation of mGluR5 induces spike afterdepolarization and enhanced excitability in medium spiny neurons of the nucleus accumbens by modulating persistent Na+ currents. J Physiol. 2009;587:3233–3250. doi: 10.1113/jphysiol.2009.172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Worley PF, Ango F. Homer as both a scaffold and transduction molecule. Sci STKE. 2002;2002:RE8. doi: 10.1126/stke.2002.137.re8. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland DR, Abraham WC. Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral-CA1 synapses. J Neurophysiol. 2009;101:1375–1385. doi: 10.1152/jn.90643.2008. [DOI] [PubMed] [Google Scholar]
- Iso Y, Grajkowska E, Wroblewski JT, Davis J, Goeders NE, Johnson KM, Sanker S, Roth BL, Tueckmantel W, Kozikowski AP. Synthesis and structure-activity relationships of 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine analogues as potent, noncompetitive metabotropic glutamate receptor subtype 5 antagonists; search for cocaine medications. J Med Chem. 2006;49:1080–1100. doi: 10.1021/jm050570f. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kammermeier P, Xiao B, Tu J, Worley P, Ikeda S. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. Journal of Neuroscience. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier PJ. Surface clustering of metabotropic glutamate receptor 1 induced by long Homer proteins. BMC Neurosci. 2006;7:1. doi: 10.1186/1471-2202-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJH. The amygdalostriatal projection in the rat-an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. The Journal of Pharmacology and Experimental Therapeutics. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–242. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. 2008;27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–1217. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Serfozo P, Parker KE, Ramsey AK, Schachtman TR. Metabotropic glutamate receptor 5 in conditioned taste aversion learning. Neurobiology of Learning and Memory. 2009;92:460–463. doi: 10.1016/j.nlm.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson C, Baker D, Carson D, Worley P, Kalivas P. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: A potential role for Homer 1b/c. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area1. J. Pharmacol. Exp. Ther. 2000;292:406–414. [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Guzman YF, Guedea AL, Huh KH, Gao C, Schwarz MK, Radulovic J. Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear. Biological Psychiatry. 2010;68:1007–1015. doi: 10.1016/j.biopsych.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]