Abstract
We previously reported that melatonin modulates the Plasmodium falciparum erythrocytic cycle by increasing schizont stage population as well as diminishing ring stage population. In addition, the importance of calcium and cAMP in melatonin signaling pathway in P. falciparum was also demonstrated. Nevertheless the molecular effectors of the indoleamine signaling pathway remain elusive. We now demonstrate by real time PCR that melatonin treatment up-regulates genes related to ubiquitin/proteasome system (UPS) components and that luzindole, a melatonin receptor antagonist, inhibits UPS transcription modulation. We also show that protein kinase PfPK7, a P. falciparum orphan kinase plays a crucial role in the melatonin transduction pathway, since following melatonin treatment of P. falciparum parasites where pfpk7 gene is disrupted (pfpk7− parasites) (i) the ratio of asexual stages remain unchanged, (ii) the increase in cytoplasmatic calcium in response to melatonin was strongly diminished and (iii) up-regulation of UPS genes did not occur. The wild type melatonin-induced alterations in cell cycle features, calcium rise and UPS gene transcription were restored by re-introduction of a functional copy of the pfpk7 gene in the pfpk7− parasites.
Keywords: kinase, malaria, melatonin, signaling, ubiquitin
INTRODUCTION
Malaria remains a major infectious disease in the developing world. Despite all effort to control the disease, drug resistance and antigenic variations are important obstacles. Signaling pathways that control the development of the pathogenic asexual intraerythrocytic forms through ring, trophozoite and schizont stages of Plasmodium falciparum, the etiological agent of the most severe form of human malaria, are essentially uncharacterized. Thus advances in this area might identify novel targets for intervention [1].
Our group has shown that asexual blood stage P. falciparum parasites respond to melatonin (and its derivatives, tryptamine, serotonin N-acetyl serotonin and N1-acetyl-N2-formyl-5-metoxykynuramine) by modulating their proliferation cycle in vitro. The melatonin treatment causes increase in the schizont population, the proliferative stage, as well as restoring synchrony of P. chabaudi in infected pinealectomized mice [2–4]. Interestingly, melatonin does not cause any change in intraerythrocytic stages of P. berghei and P. yoelii, asynchronous parasites [5].
It is known that melatonin-induced signaling pathway involves a crosstalk between calcium and cAMP signaling [6–7] whose role in Plasmodium development has been the subject of numerous studies [8–17], for review please see [18]. Furthermore, the importance of phosphorylation in melatonin signaling was determined through protein kinase A activity and its inhibition assays [6, 19].
In this paper we tested whether other protein kinases could be involved in melatonin-induced cycle modulation. Plasmodium falciparum protein kinase 7 (PfPK7) is a kinase with no orthologues in mammalian cells. Its C-terminal region has highest sequence similarity to mitogen-activated protein kinase kinases (MAPKKs ou MEK) while its N-terminal segment is more similar to fungal protein kinase A [20]. Interestingly, the 3D structure of PfPK7 displays maximal similarity to TAO2 kinase, a MAPKKK [21]. However, neither PKA or MEK inhibitors block PfPK7 activity [20].
Dorin-Semblat et al. [22] reported a slower parasite growth caused by a reduction in the number of merozoites produced by individual schizonts, and a block in oocyst formation, in parasites with disrupted pfpk7 gene. In view of the phenotype of pfpk7− that involves reduced invasion efficiency we tested the effect following melatonin treatment in these parasite, by investigated whether this atypical kinase is involved in the melatonin response pathway. To confirm that the absence of the PfPK7 is really the cause of the phenotypes found in the knockout parasites, we tested a complemented line where pfpk7 is introduced back to the pfpk7− parasites via an episome [22]. As negative controls, we monitored a possible cell cycle phenotype in P. falciparum parasites with disrupted pfnek-2 [23] or pfnek-3 genes, both of which are expressed predominantly in gametocytes.
Similar to phosphorylation, ubiquitin/proteasome system (UPS) plays important roles in fundamental processes of eukaryotic cells, including cell cycle regulation, signal transduction and transcription regulation [24]. Ubiquitination occurs basically through three enzymatic steps in which ubiquitin moiety are transferred to substrate. Briefly, E1 activating enzyme activates ubiquitin in an ATP-dependent process. Activated ubiquitin is then transferred to an E2 conjugating enzyme that in turn transfers it to an E3 ubiquitin ligase that recognizes specifically the substrate. Specific attachment of ubiquitin molecules leads the substrate to proteasomal degradation or to other fate [25].
Transcriptome analysis of P. falciparum showed that genes encoding proteasome components are activated in schizont stage [26]. Reverse transcriptase PCR assay showed that steady-state levels of mRNA from the polyubiquitin gene is upregulated in late trophozoite to schizont, when protein ubiquitination dramatically increases [27] suggesting the participation of UPS in parasite growth and development. This is consistent with the observation that proteasome inhibitors such as MLN-273 and bortezomib blocks Plasmodium development [28–29].
MATERIAL AND METHODS
Parasites
The 3D7 clone of P. falciparum was cultivated in flasks with RPMI 1640 medium (GIBCO, Billings, USA) supplemented with 0.04% of gentamicin sulfate, 0.05% of hypoxanthine and 10% human serum (blood group A+) and 0.23% sodium bicarbonate, as previously described [30]. For pfpk7− parasite, the same medium was supplemented with 2.5 μg ml−1 blasticidin.
Distribution of developmental stages in response to melatonin treatment
After incubation for 24 h with 0.1 μM melatonin (or solvent: ethanol 0.00005%), an aliquot of cells was removed and parasitemia was assessed by counting 2,000 cells on Giemsa-stained slides. Vehicle blanks (no drug) were analyzed as controls. Proportions of the different parasite forms were determined by analyzing 2,000 cells at the same conditions for parasitemia. Triplicate smears were prepared for each drug concentration and for controls and at least three isolated experiments were carried out. All determinations were carried out in double-blind conditions. Results are means from three separate cell preparations. Data were analyzed in GraphPad Prism 4 using ANOVA and Newman-Keuls Multiple Comparison Test.
Real Time PCR
Parasite cultures were synchronized by sorbitol treatment [31] and trophozoites were treated with 0.1 μM melatonin (SIGMA-Aldrich, St. Louis, USA) or luzindole 250 μM (Tocris Bioscience, Bristol, UK) approximately 24–30 hours post invasion. The RNA was extracted 5 hours post treatment with Trizol (Invitrogen, Carlsbad, USA) and subjected to DNAse treatment. cDNA synthesis was carried out using random primers and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. SYBR green incorporation was measured during PCR amplification performed on the 7300 Real Time PCR System (Applied Biosystems, Foster City, USA). We performed the statistical analysis applying Student’s t test on the log2 values of relative expression (2−ΔΔCT) of the genes (normalized with 18S ribosomal housekeeping gene). The experiments were performed independently at least three times and each experiment was analysed in triplicate by Real time PCR.
Calcium spectrofluorimetric determinations
108 infected red blood cells were lysed with 10 mg ml−1 saponin (in PBS). Parasite pellets were obtained by centrifugation (8700 g for 10 min at 4°C), washed twice in buffer A (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgS04, 5.5 mM D-glucose, 50 mM MOPS and 2 mM CaCl2, pH 7.2), and resupended in the same buffer as described previously (Farias et al, 2005). For cytoplasmic calcium staining we incubated parasites in the presence of Fluo4AM 5 mM and probenecid 40 μM for 50 min at 37 °C. In each experiment an aliquot of 100 μl (105 cells) was placed in a thermostated cuvette equipped with magnetic stirring. The experiments were performed independently three times. Spectrofluorometric measurements were performed by using the RF-5301PC (Shimadzu) with excitation and emission wavelengths of 488 nm and 505–530 nm, respectively.
RESULTS
Melatonin is able to modulate P. falciparum cell cycle [2, 32] and treatment of cultures with the indoleamine results in synchronization by increasing the proportions of schizont while diminishing ring stage proportion. However, when similar experiments were performed with the pfpk7− (clone B9) [22], we did not observe any change in the distribution of parasite development stages as was seen for wild type parasites (Fig. 1 and Supplementary Table 1).
Figure 1.
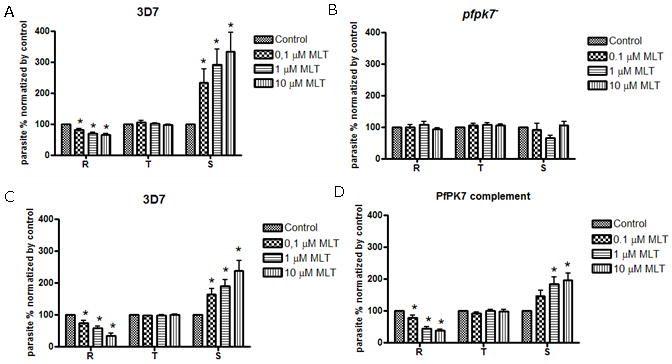
Melatonin does not affect the cell cycle of pfpk7− parasites. Parasite culture was treated with melatonin (MLT) (or vehicle) for 24 h. Proportions of the different parasite forms (ring, trophozoite and schizont) were determined by analyzing 2,000 cells in triplicates. The data show the average of three independent experiments. Addition of melatonin in Plasmodium falciparum 3D7 causes a modulation in the distribution of developmental stages in the intraerythrocytic cycle (A and C). However, this modulation is lost in pfpk7− parasites (clone B9) (B). Upon complementation of the KO parasite with an extra copy of the pfpk7 gene, the effect of melatonin is restored (D). Statistical analysis by ANOVA and Newman-Keuls Multiple Comparison Test. * p<0.05.
To confirm that the absence of the effect of melatonin in pfpk7− parasites was indeed due to the lack of the PfPK7 enzyme, we repeated the experiment with a complemented line where PfPK7 is introduced back to the pfpk7− parasites via an episome [22]. Fig. 1D shows that the changes in the distribution of developmental stages in response to 0.1 μM melatonin treatment. The wild type phenotype is restored in the complemented clone resulting in an increase in the schizont population with a corresponding decrease in ring population compared to the control.
In contrast, transgenic parasite clones unable to express the gametocyte-specific (stage not found in our parasite culture) NIMA-related kinases PfNek-2 [23] or PfNek-3 behaved as wild-type parasites in response to melatonin, confirming that the defect in the melatonin response in pfpk7− parasites was not due to non-specific effects (Fig. 2).
Figure 2.
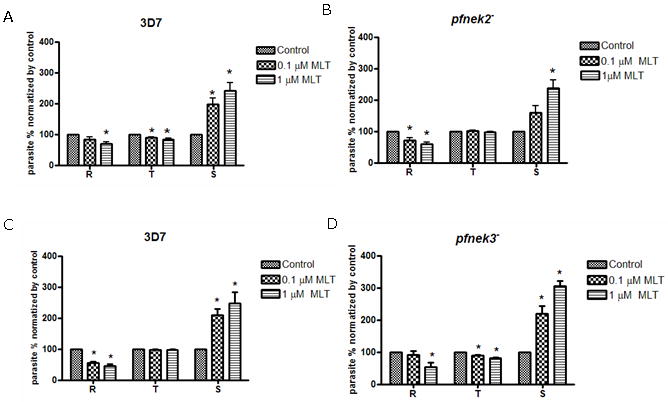
Knockout of pfnek-2 and 3 does not affect melatonin signaling. Parasite culture was treated with melatonin (MLT) (or vehicle) for 24 h. Proportions of the different parasite forms (ring, trophozoite and schizont) were determined by analyzing 2,000 cells in triplicates. The data show the average of three independent experiments. Addition of melatonin in Plasmodium falciparum 3D7 causes a modulation in the distribution of developmental stages in the intraerythrocytic cycle (A and C). The modulation persist in pfnek-2− (B) and pfnek-3− parasites (D). Statistical analysis by ANOVA and Newman-Keuls Multiple Comparison Test. * p<0.05.
Addition of either melatonin or tryptophan derivatives to the culture medium of P. falciparum trophozoites induces an increase in cytosolic calcium from intracellular storages and this response is blocked by a PLC inhibitor U73122 [2, 6–7]. The inhibition of calcium rise with PLC inhibitor U73122 also abolish melatonin-induced intraerythrocytic cycle modulation suggesting that the synchronization of cell cycle is modulated by a calcium-dependent pathway [2, 4, 6].
As pfpk7− parasites do not respond to melatonin–induced intraerythrocytic developmental cycle modulation, we investigate the ability of melatonin to induce calcium release in pfpk7− parasites. For this purpose, trophozoites were loaded with the calcium sensor Fluo-4 and fluorescence changes were measured upon hormone addition. The concentration of cytosolic calcium released upon addition of 0.1 μM melatonin was measured. We found that calcium rise in response to melatonin was diminished from approximately 50 nM in wild-type 3D7 to less than 20 nM in pfpk7− cells (Fig. 3). As expected, the phenotype similar to the wild-type parasites was restored, albeit partially in the complemented PfPK7 clone, where calcium release reaches approximately 40 nM. This confirms that the lack of response in the disrupted line is indeed due at least partly to the absence of the enzyme. We can hypothesize that possibly PK7 act in a way similar to the PKA modulation of calcium in mammalian cells [33].
Figure 3.
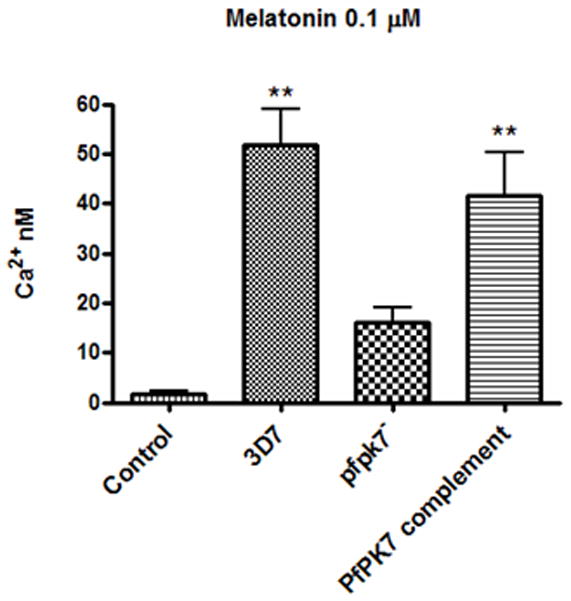
Calcium rise in response to melatonin in pfpk7− strain. Fluorimetric calcium measure in melatonin treated wild-type 3D7, pfpk7− parasites (clone B9), and in the pfpk7-complemented clone B9 (see Materials and Methods). The data show the average of three independent experiments.
To investigate whether the ubiquitin/proteasome system (UPS) could be affected by melatonin treatment since this system is involved in a broad range of cellular functions including signal transduction, cell cycle control and others. There are about 100 genes encoding UPS components in P. falciparum most of them comprising E3 ubiquitin ligases [34]. We selected 14 genes, about 14 % of UPS genes, representing E1, E2, E3, ubiquitin-like, deubiquitinase and proteosome sbunits to assess the profile of transcript level upon melatonin 0.1 μM treatment by real time PCR. We also included non-UPS genes (PFI1485c encoding a diacylglycerol kinase, PF13_0252 encoding a nucleoside transporter 1 and MAL13P1.216 encoding a DNA helicase) as control to ensure that gene expression modulation is specific rather than a general increase in transcript level. Our data show that 5 hours of 0.1 μM melatonin treatment of P. falciparum trophozoite-infected RBCs (approximately 24–30 h post-invasion) resulted in up-regulation of at least 2 times of a subset of UPS genes (Fig. 4 and Supplementary Table 2), including the E1 activating enzymes (PFL1790w and PFL1245w), an E2 conjugating enzyme (PFL0190w), two E3 ubiquitin ligases (MAL8P1.23 and PFC0845c), the deubiquitinating enzyme (PF11_0177) and two proteasome subunits (PF14_0025 and PFA0400c). Cullin-like proteins (PF08_0094 and PFF1445c), an E3 ubiquitin protein ligase RING finger (PFC0845c) that belongs to SCF complex (Skp1-Cullin-F-box), all of which are involved in UPS activity (reviewed in [34]), as well as two F-box domain containing protein (PFL1565c and PFF0960c) were also up-regulated.
Figure 4.
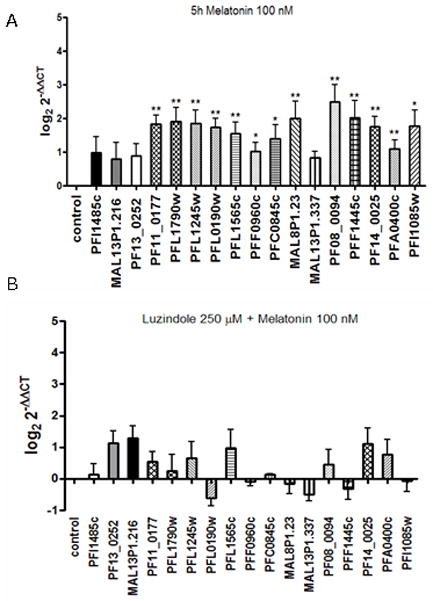
Plasmodium UPS genes are upregulated upon melatonin treatment. Differential transcription of UPS genes from parasites subjected to melatonin treatment compared to control was analyzed by real time PCR (A) and in the presence of luzindole, both using 18S ribosomal RNA for normalization. At least three independent experiments were performed (See Supplementary table 2). See experimental procedures for statistical treatment. * p value < 0.05, ** p value < 0.01. The criterion for altered gene expression was twofold (up or downregulation). The ID of the genes are available in supplemental material.
As observed in figure 4A the non-UPS genes did not show any statistically significant up-regulation in response to melatonin.
In order to evaluate whether melatonin-induced UPS transcription modulation could be blocked by luzindole, a melatonin receptor antagonist, [35] we treated parasites with melatonin in presence of luzindole.
Figure 4B shows that in presence of luzindole the UPS transcription modulation no longer occurs in response to melatonin implying that UPS transcript modulation is involved in melatonin signaling pathway in P. falciparum resulting in the changes in the intraerythrocytic stages.
We then assessed if the increase in UPS transcripts caused by melatonin treatment remains in parasites where PfPK7 is absent (pfpk7−), as these parasites are unable to respond to melatonin–induced variation of intraerythrocytic stages. We carried out real time PCR analysis of the pfpk7− clone as described above similar to the wild type parasites (Fig. 5A).
Figure 5.
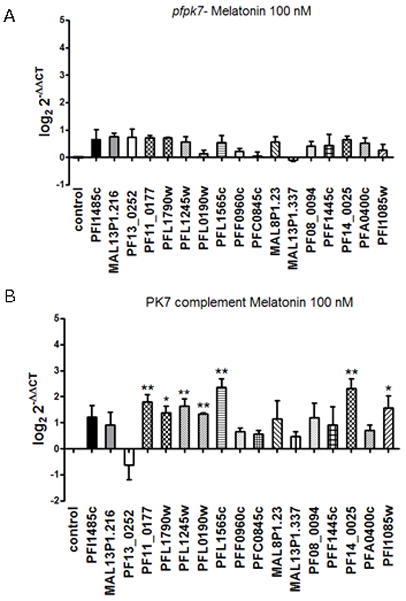
Melatonin-induced up-regulation of UPS gene transcript levels is mediated by PfPK7. The effect of 0.1 μM melatonin treatment on UPS gene transcript levels observed in wild-type 3D7 parasites (see Fig. 1) was assessed by real time PCR in pfpk7− parasites (clone B9 (A) and the pfpk7-complemented clone B9 (B) The effect of melatonin treatment is abolished in the knockout clone and partially restored in the complemented parasites (PfPK7 – complement) (B) * p<0.05 and ** p<0.01. The data show the average of at least three independent experiments.
We have observed that the response to 0.1 μM melatonin in up-regulating the UPS genes was abolished in the absence of PfPK7. In the pfpk7 complemented clone however the effect of the treatment is partially restored: Seven (PF11_0177, PFL1790w PFL1565c, PFL1245w, PF14_0025, PFI1085w and PF08_0094) of the eleven genes that are regulated in the wild type strain were also up-regulated in the complemented strain, while PFC0845c, MAL8P1.23, PFF1445c and PFL0190 remained unaffected. Unexpectedly, PFI1085w, an ubiquitin-like protein was up-regulated only in PfPK7 complemented parasites.
This data provide us evidence that PfPK7 participates in melatonin-induced UPS up-regulation.
DISCUSSION
Transcription of proteasome genes is activated at the schizont stage in P. falciparum [26], suggesting an important role of the UPS in parasites development. This is consistent with the observation that proteasome inhibitors such as MLN-273 and bortezomib block Plasmodium development [28–29]. It is interesting to note that some genes are co-expressed and also co-regulated as it is reported for proteasome genes, motifs as G-rich, CACA, and TGTATG occur with positional conservation [39].
Our data shed some interesting light on the mode of action of melatonin in P. falciparum. First, treatment with the indoleamine clearly affects the steady-state level of mRNA from selected genes. Also, it remains to be determined if the process that is affected by melatonin is transcription or RNA degradation (or both). It is important to note that the ability to promote changes in the expression of UPS genes by melatonin also occurs in mammalian cells [40–41].
Second, the atypical kinase PfPK7 plays a crucial role in the signaling events that connect hormone sensing (whose receptor remains to be identified) to the intraerythrocytic cell cycle modulation as this response is abolished in pfpk7−. The variation in UPS transcript level is abolished in pfpk7− parasites, which is restored in part upon complementation; the fact that restoration is only partial suggests that more than one pathway leading to gene expression control may be involved. Although it is to be noted that the complemented pfpk7 is driven by a promoter different from the endogenous one. Third, PfPK7 acts upstream of the calcium rise triggered by hormone treatment, as this rise is diminished in pfpk7− parasites and, like UPS mRNA level up-regulation, is restored upon complementation.
The importance of PfPK7 in merozoite production by schizonts underscores its role in cell cycle control in P. falciparum. Classical MAPK substrates include regulatory proteins such as cell cycle control proteins and transcription factors. Although PfPK7 share some similarity to MEK, a member of MAP kinases, it has been suggested that MAP kinase signaling does not occur through three component system (ERK1/2, p38 or JNK MAPKs) in P. falciparum [20]. Thus PfPK7 could modulate the parasite cell cycle in an atypical way from classical MAP kinases.
The classical melatonin receptors in vertebrates are membrane receptors from GPCR family (G-protein coupled receptor) MT1 and MT2 [42]. However, melatonin is not a MT1-MT2 exclusive ligand, but also can bind to nuclear receptors such as retinoic acid receptor family [43], or even to cytosolic proteins such as calmodulin [44].
Although we haven’t found a melatonin receptor MT1 or MT2 orthologues in Plasmodium genome even though luzindole, a competitive melatonin receptor antagonist [35] is able to block melatonin-induced intraerythrocytic cycle modulation in P. falciparum [3]. It is possible that in P. falciparum receptors binding to melatonin have weak primary sequence homology to MT1 or MT2. Furthermore, the present work demonstrates that melatonin-induced UPS mRNA level up-regulation is blocked by luzindole.
Thus, we identified components of the melatonin response pathway and effectors. This generates a new set of questions that we can now address to complete this emerging picture, such as the modalities of PfPK7 effect on calcium release, the global transcriptional response to melatonin, details of gene expression-regulating mechanisms involved, the ubiquitination targets and the pathway(s) through which UPS gene products mediate cell cycle and synchronization modulation.
Melatonin influences host-parasite relationships by a variety of mechanisms [48]. Disrupting malaria synchrony causes a 50 % drop in in-host replication and transmission stages [43]. The importance of synchrony in malaria therapy has been assessed by combining luzindole and chloroquine [44]. Besides, the application of melatonin as a malaria therapeutics has been proposed as melatonin acts as an antioxidant, protecting liver cells from apoptosis caused by sporozoites [45]. In addition, melatonin produced by Trypanossoma cruzi affects the differentiation of metacyclic forms [46] and UPS has been implicated in the transformation of trypomastigotes into amastigotes [47].
Supplementary Material
Acknowledgments
We thank Domique Dorin-Semblat, Luc Reininger and Christian Doerig (Inserm-EPFL Joint Laboratory, Switzerand) for providing the parasites knock-out clones for PfPK7, PfNek-2 and PfNek-3. We thank Fundação de Amparo à pesquisa de São Paulo (FAPESP), INCT-InBqmed and Pronex–Malaria for funding C.R.S.G., F.C.K. and JG received fellowship from FAPESP. RY received CAPES fellowship. D.C. laboratory is funded by a grant from the U.S. National Institutes of Health (AI73795).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no con ict of interest.
References
- 1.DOERIG C, BAKER D, BILLKER O, et al. Signalling in malaria parasites. The MALSIG Consortium Parasite (Paris, France) 2009;16:169–182. doi: 10.1051/parasite/2009163169. [DOI] [PubMed] [Google Scholar]
- 2.HOTTA CT, GAZARINI ML, BERALDO FH, et al. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nature Cell Biology. 2000;2:466–468. doi: 10.1038/35017112. [DOI] [PubMed] [Google Scholar]
- 3.BERALDO FH, GARCIA CR. Products of tryptophan catabolism induce Ca2+ release and modulate the cell cycle of Plasmodium falciparum malaria parasites. Journal of Pineal Research. 2005;39:224–230. doi: 10.1111/j.1600-079X.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 4.BUDU A, PERES R, BUENO VB, et al. N1-acetyl-N2-formyl-5-methoxykynuramine modulates the cell cycle of malaria parasites. Journal of Pineal Research. 2007;42:261–266. doi: 10.1111/j.1600-079X.2006.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.BAGNARESI P, ALVES E, DA SILVA EB, et al. Unlike the synchronous Plasmodium falciparum and P. chabaudi infection, the P. berghei and P.yoelii asynchronous infections are not affected by melatonin. International Journal of General Medicine. 2009;2:47–55. doi: 10.2147/ijgm.s3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BERALDO FH, ALMEIDA FM, DA SILVA AM, et al. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. The Journal of Cell Biology. 2005;170:551–557. doi: 10.1083/jcb.200505117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HOTTA CT, MARKUS RP, GARCIA CR. Melatonin and N-acetyl-serotonin cross the red blood cell membrane and evoke calcium mobilization in malarial parasites. Brazilian Journal of Medical and Biological Research. 2003;36:1583–1587. doi: 10.1590/s0100-879x2003001100016. [DOI] [PubMed] [Google Scholar]
- 8.BAKER DA, KELLY JM. Purine nucleotide cyclases in the malaria parasite. Trends in Parasitology. 2004;20:227–232. doi: 10.1016/j.pt.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 9.CALDAS ML, WASSERMAN M. Cytochemical localisation of calcium ATPase activity during the erythrocytic cell cycle of Plasmodium falciparum. International Journal for Parasitology. 2001;31:776–782. doi: 10.1016/s0020-7519(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 10.GARCIA CR, DE AZEVEDO MF, WUNDERLICH G, et al. Plasmodium in the postgenomic era: new insights into the molecular cell biology of malaria parasites. International Review of Cell and Molecular Biology. 2008;266:85–156. doi: 10.1016/S1937-6448(07)66003-1. [DOI] [PubMed] [Google Scholar]
- 11.MUHIA DK, SWALES CA, ECKSTEIN-LUDWIG U, et al. Multiple splice variants encode a novel adenylyl cyclase of possible plastid origin expressed in the sexual stage of the malaria parasite Plasmodium falciparum. The Journal of Biological Chemistry. 2003;278:22014–22022. doi: 10.1074/jbc.M301639200. [DOI] [PubMed] [Google Scholar]
- 12.NAGAMUNE K, SIBLEY LD. Comparative genomic and phylogenetic analyses of calcium ATPases and calcium-regulated proteins in the apicomplexa. Molecular Biology and Evolution. 2006;23:1613–1627. doi: 10.1093/molbev/msl026. [DOI] [PubMed] [Google Scholar]
- 13.ONO T, CABRITA-SANTOS L, LEITAO R, et al. Adenylyl cyclase alpha and cAMP signaling mediate Plasmodium sporozoite apical regulated exocytosis and hepatocyte infection. PLoS Pathogens. 2008;4:e1000008. doi: 10.1371/journal.ppat.1000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PASSOS AP, GARCIA CR. Characterization of Ca2+ transport activity associated with a non-mitochondrial calcium pool in the rodent malaria parasite P. chabaudi. Biochemistry and Molecular Biology International. 1997;42:919–925. doi: 10.1080/15216549700203361. [DOI] [PubMed] [Google Scholar]
- 15.VAID A, THOMAS DC, SHARMA P. Role of Ca2+/calmodulin-PfPKB signaling pathway in erythrocyte invasion by Plasmodium falciparum. The Journal of Biological Chemistry. 2008;283:5589–5597. doi: 10.1074/jbc.M708465200. [DOI] [PubMed] [Google Scholar]
- 16.VAROTTI FP, BERALDO FH, GAZARINI ML, et al. Plasmodium falciparum malaria parasites display a THG-sensitive Ca2+ pool. Cell Calcium. 2003;33:137–144. doi: 10.1016/s0143-4160(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 17.GAZARINI ML, GARCIA CR. The malaria parasite mitochondrion senses cytosolic Ca2+ fluctuations. Biochemical and Biophysical Research Communications. 2004;321:138–144. doi: 10.1016/j.bbrc.2004.06.141. [DOI] [PubMed] [Google Scholar]
- 18.KOYAMA FC, CHAKRABARTI D, GARCIA CR. Molecular machinery of signal transduction and cell cycle regulation in Plasmodium. Molecular and Biochemical Parasitology. 2009;165:1–7. doi: 10.1016/j.molbiopara.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GAZARINI ML, BERALDO FH, ALMEIDA FM, et al. Melatonin triggers PKA activation in the rodent malaria parasite Plasmodium chabaudi. Journal of Pineal Research. 2011;50:64–70. doi: 10.1111/j.1600-079X.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 20.DORIN D, SEMBLAT JP, POULLET P, et al. PfPK7, an atypical MEK-related protein kinase, reflects the absence of classical three-component MAPK pathways in the human malaria parasite Plasmodium falciparum. Molecular Microbiology. 2005;55:184–196. doi: 10.1111/j.1365-2958.2004.04393.x. [DOI] [PubMed] [Google Scholar]
- 21.MERCKX A, ECHALIER A, LANGFORD K, et al. Structures of P. falciparum protein kinase 7 identify an activation motif and leads for inhibitor design . Structure. 2008;16:228–238. doi: 10.1016/j.str.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 22.DORIN-SEMBLAT D, SICARD A, DOERIG C, et al. Disruption of the PfPK7 gene impairs schizogony and sporogony in the human malaria parasite Plasmodium falciparum. Eukaryotic Cell. 2008;7:279–285. doi: 10.1128/EC.00245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.REININGER L, TEWARI R, FENNELL C, et al. An essential role for the Plasmodium Nek-2 Nima-related protein kinase in the sexual development of malaria parasites. The Journal of Biological Chemistry. 2009;284:20858–20868. doi: 10.1074/jbc.M109.017988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HERSHKO A, CIECHANOVER A. The ubiquitin system. Annual Review of Biochemistry. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 25.CIECHANOVER A. The ubiquitin-proteasome pathway: on protein death and cell life. The EMBO Journal. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BOZDECH Z, LLINAS M, PULLIAM BL, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biology. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.HORROCKS P, NEWBOLD CI. Intraerythrocytic polyubiquitin expression in Plasmodium falciparum is subjected to developmental and heat-shock control. Molecular and Biochemical Parasitology. 2000;105:115–125. doi: 10.1016/s0166-6851(99)00174-7. [DOI] [PubMed] [Google Scholar]
- 28.LINDENTHAL C, WEICH N, CHIA YS, et al. The proteasome inhibitor MLN-273 blocks exoerythrocytic and erythrocytic development of Plasmodium parasites. Parasitology. 2005;131:37–44. doi: 10.1017/s003118200500747x. [DOI] [PubMed] [Google Scholar]
- 29.REYNOLDS JM, EL BISSATI K, BRANDENBURG J, et al. Antimalarial activity of the anticancer and proteasome inhibitor bortezomib and its analog ZL3B. BMC Clinical Pharmacology. 2007;7:13. doi: 10.1186/1472-6904-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.TRAGER W, JENSEN JB. Human malaria parasites in continuous culture. Science New York, NY. 1976;193:673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 31.LAMBROS C, VANDERBERG JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. The Journal of Parasitology. 1979;65:418–420. [PubMed] [Google Scholar]
- 32.GARCIA CR, MARKUS RP, MADEIRA L. Tertian and quartan fevers: temporal regulation in malarial infection. Journal of Biological Rythms. 2001;16:436–443. doi: 10.1177/074873001129002114. [DOI] [PubMed] [Google Scholar]
- 33.BRUCE JI, STRAUB SV, YULE DI. Crosstalk between cAMP and Ca2+ signaling in non-excitable cells. Cell Calcium. 2003;34:431–444. doi: 10.1016/s0143-4160(03)00150-7. [DOI] [PubMed] [Google Scholar]
- 34.PONTS N, YANG J, CHUNG DW, et al. Deciphering the ubiquitin-mediated pathway in apicomplexan parasites: a potential strategy to interfere with parasite virulence. PLoS ONE. 2008;3:e2386. doi: 10.1371/journal.pone.0002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DUBOCOVICH ML. Luzindole (N-0774): a novel melatonin receptor antagonist. The Journal of Pharmacology and Experimental Therapeutics. 1988;246:902–910. [PubMed] [Google Scholar]
- 36.O’DONNELL AJ, SCHNEIDER P, MCWATTERS HG, et al. Fitness costs of disrupting circadian rhythms in malaria parasites. Proceedings of Royal Society B. 2011 doi: 10.1098/rspb.2010.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.BAGNARESI P, MARKUS RP, HOTTA CT, POZZAN T, GARCIA CRS. Desynchronizing Plasmodium cell cycle by a melatonin antagonist: a potential therapeutic target in malaria? International Journal of General Medicine. 2008 [Google Scholar]
- 38.SRINIVASAN V, SPENCE DW, MOSCOVITCH A, et al. Malaria: therapeutic implications of melatonin. Journal of Pineal Research. 2010;48:1–8. doi: 10.1111/j.1600-079X.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- 39.IENGAR P, JOSHI NV. Identification of putative regulatory motifs in the upstream regions of co-expressed functional groups of genes in Plasmodium falciparum. BMC Genomics. 2009;10:18. doi: 10.1186/1471-2164-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CHO JW, KIM CW, LEE KS. Modification of gene expression by melatonin in UVB-irradiated HaCaT keratinocyte cell lines using a cDNA microarray. Oncology Reports. 2007;17:573–577. [PubMed] [Google Scholar]
- 41.SUNG JH, CHO EH, KIM MO, et al. Identification of proteins differentially expressed by melatonin treatment in cerebral ischemic injury - a proteomics approach. Journal of Pineal Research. 2009;46:300–306. doi: 10.1111/j.1600-079X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 42.DUBOCOVICH ML. Melatonin receptors: are there multiple subtypes? Trends in Pharmacological Sciences. 1995;16:50–56. doi: 10.1016/s0165-6147(00)88978-6. [DOI] [PubMed] [Google Scholar]
- 43.WIESENBERG I, MISSBACH M, CARLBERG C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restorative Neurology and Neuroscience. 1998;12:143–150. [PubMed] [Google Scholar]
- 44.BENITEZ-KING G, HUERTO-DELGADILLO L, ANTON-TAY F. Binding of 3H-melatonin to calmodulin. Life Sciences. 1993;53:201–207. doi: 10.1016/0024-3205(93)90670-x. [DOI] [PubMed] [Google Scholar]
- 45.SRINIVASAN V, SPENCE DW, MOSCOVITCH A, et al. Malaria: therapeutic implications of melatonin. Journal of Pineal Research. 2010;48:1–8. doi: 10.1111/j.1600-079X.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- 46.MACIAS M, RODRIGUEZ-CABEZAS MN, REITER RJ, et al. Presence and effects of melatonin in Trypanosoma cruzi. Journal of Pineal Research. 1999;27:86–94. doi: 10.1111/j.1600-079x.1999.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 47.DE DIEGO JL, KATZ JM, MARSHALL P, et al. The ubiquitin-proteasome pathway plays an essential role in proteolysis during Trypanosoma cruzi remodeling. Biochemistry. 2001;40:1053–1062. doi: 10.1021/bi001659k. [DOI] [PubMed] [Google Scholar]
- 48.BAGNARESI PIERO, NAKABASHI MYNA, THOMAS ANDREWP, et al. The role of melatonin in parasite biology. Molecular & Biochemical Parasitology. 2011 doi: 10.1016/j.molbiopara.2011.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


