Abstract
Introduction
It is generally accepted that at least two major mechanisms contribute to sinus node (SN) pacemaking: a membrane voltage (mainly If) clock and a calcium (Ca) clock (localized submembrane sarcoplasmic reticulum Ca2+ release during late diastolic depolarization). The aim of this study was to compare the contributions of each mechanism to pacemaker activity in SN and Purkinje fibers (PF) exhibiting normal or abnormal automaticity.
Methods and Results
Conventional microelectrodes were used to record action potentials in isolated spontaneously beating canine SN and free running PF in control and in the presence of 0.1 µM isoproterenol. Ryanodine (0.1–3 µM) and ivabradine (3µM) were used to inhibit sarcoplasmic reticulum Ca2+ release or If, respectively. To induce automaticity at low membrane potentials, PF were superfused with BaCl2. In SN, ivabradine reduced the rate whereas ryanodine had no effect. Isoproterenol significantly accelerated automatic rate which was decreased by ivabradine and ryanodine. In normally polarized PFs, ryanodine had no effects on the automatic rate in the absence or presence of isoproterenol whereas ivabradine inhibited both control and isoproterenol-accelerated automaticity. In PF depolarized with BaCl2, ivabradine decreased BaCl2-induced automatic rate while ryanodine had no effect.
Conclusion
In canine SN,If contributes to both basal automaticity and β-adrenergic-induced rate acceleration while the ryanodine-inhibited Ca clock appears more involved in β-adrenergic regulation of pacemaker rate. In PF, normal automaticity depends mainly on If. Inhibition of basal potassium conductance results in high automatic rates at depolarized membrane potentials with SN-like responses to inhibition of membrane and Ca clocks.
Keywords: Sinus node, Purkinje fiber, Pacemaker, β-Adrenergic receptor stimulation, Ivabradine, Ryanodine
Introduction
Ivabradine is the first selective inhibitor of pacemaker current (If) in the sinus node (SN) approved for clinical application.1 Clinical trials have demonstrated that ivabradine has anti-anginal and anti-ischemic efficacy at least as good as that of conventional therapies, but in contrast to β-adrenoreceptor blockers and calcium channel blockers, has no negative inotropic properties.1-3 Consequently, ivabradine-induced reduction in heart rate is accompanied by an improvement of ejection fraction.4, 5 Ivabradine decreases SN rate by a concentration-dependent inhibition of If.6 However, the effects of ivabradine on secondary pacemakers have not been studied, although ivabradine slows the rate of automaticity in rabbit pulmonary vein cardiomyocytes.7 Therefore, the first aim of this study was to compare the effects of ivabradine in the primary and secondary pacemakers of the same species – canine SN and Purkinje fibers (PF).
It is generally accepted that at least two major mechanisms contribute to SN pacemaking: a voltage (or membrane) clock and a Ca2+ clock.8 The voltage clock to which the pacemaker current If has been considered a major contributor, initiates diastolic depolarization.6 Studying ivabradine effects (the first aim) allows comparison of the contribution of the voltage clock to primary and secondary pacemaking. The Ca2+-clock depends on localized Ca2+ release from the sarcoplasmic reticulum during late diastole.9 Local Ca2+ releases activate the Na+/Ca2+ exchanger (NCX) current, causing an exponential increase in the rate of diastolic depolarization to drive the membrane potential to the threshold of the action potential upstroke.10 The contribution of the Ca2+ clock to spontaneous activity in secondary pacemakers is not very clear. Consequently, the second aim of our study was to compare the effects of ryanodine (inhibitor of Ca2+ release from sarcoplasmic reticulum) in canine SN and in PF. Because both clocks are responsive to β adrenergic receptor stimulation,11, 12 the experiments were performed in the absence and presence of β adrenergic activation. We hypothesized that the relative contributions of voltage and Ca2+ clocks to pacemaker activity are different in primary and secondary pacemakers.
Methods
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Heath (NIH Publication No. 85–23, revised 1996). All protocols were approved by the Columbia University Institutional Animal Care and Use Committee. Mongrel dogs weighing 22–25 kg were anesthetized with thiopental (17 mg/kg IV), intubated, and ventilated with isoflurane 9(1.5% to 3.0%). The hearts with at least 1 cm of each vena cava attached were removed through a left lateral thoracotomy and immersed in cold Tyrode’s solution equilibrated with 95% O2 - 5% CO2 and containing (in mmol/L): NaCl 131, NaHCO3 18, KCl 4, CaCl2 2.7, MgCl2 0.5, NaH2PO4 1.8, and dextrose 5.5. A piece of atrial tissue containing SN (~10×15 mm) was cut from the junction of the superior vena cava and free wall of the right atrium and placed in a tissue bath epicardial surface up.13 To expose the SN to solution and facilitate microelectrode impalements the epicardial tissue layer of 0.3–0.5 mm thickness was removed. The preparations beat spontaneously and multiple microelectrode impalements were made to localize the cells in the center of the nodal region (smooth transition between diastolic depolarization and action potential upstroke), the cells located at the periphery of the SN (more abrupt transition), and atrial cells. The atrial tissue was cut off and the final dimension of preparations was ~2–3×5 mm approximately corresponding to the central region of canine SN.14
Free-running Purkinje fibers (PFs) were dissected from both ventricles. All preparations were placed in a 4–mL chamber, superfused with Tyrode’s solution (37°C, ph 7.3 to 7.4) at 12 mL/min and allowed to beat spontaneously. Conventional microelectrode techniques were used to record transmembrane potentials.
Validation of SN preparation
Microelectrode recordings of transmembrane potentials from canine SN cells have previously been made using arterially-perfused right atrial preparations.15, 16 Obtaining stable recordings was hampered because it was difficult to penetrate the several layers of epicardial cells covering the SN with microelectrodes, and electrodes were often dislodged and/or recordings distorted by mechanical displacement of the microelectrode. As a result, few identical action potentials from the same cell could be recorded consecutively. At present, canine perfused right atrial preparations are being used for optical recordings of SN cell action potentials.14, 17 The method allows recording of the time course of transmembrane voltage changes but not the actual voltages. However, recordings of absolute values of transmembrane potential are important for understanding the mechanisms of pacemaking and for interpreting the effects of pharmacological interventions. In addition, the maximum diastolic potential (MDP) is one of the major parameters that differentiate between primary and secondary pacemakers. Therefore, in the present study, to have a stable and long-lasting transmembrane potential recording, we developed an isolated superfused canine SN preparation. Because this preparation has not been used previously, control series of 5 experiments were performed to test its stability. Transmembrane potentials were continuously recorded during 3.5 hours beginning at 30 minutes after isolation of preparations. There were no significant changes between the start and end of registration either in rate (111±5 vs 107±6 beats/min, respectively, P>0.05) or MDP (-61±2 vs –62±2 mV, respectively, P>0.05). These values of rate and MDP are similar to those recorded in perfused right atrial preparations.15, 16 The total durations of the protocols in the present study (<3 hours) were within the time frame during which the preparations were stable. It is well known that neural input and pulse within the node artery modulate the rate of SN.18 Interestingly, despite of the absence of these factors the control rate of isolated SN preparations used in this study varied between 80 and 151 beats/min which is very similar to heart rate in adult dogs which varies between 70 and 160 beats/min.19 This suggests that the preparations can be used for studying the basic mechanisms underlying SN pacemaking.
Automaticity in PF induced by BaCl2 depolarization
After 1 hour of equilibration in control solution, fibers were superfused for 20 minutes with solution containing 0.15 mmol/L of BaCl2. If automaticity at low membrane potentials did not appear, the BaCl2 concentration was increased in steps of 0.05 mmol/L and at 20 minute intervals until stable automaticity was evident. Such stability is characterized by a low maximum diastolic potential, phase 4 depolarization, and action potentials with slow upstrokes and low overshoots.20, 21
Isoproterenol and ryanodine were purchased from Sigma Chemical Co. (USA). Ivabradine was a gift from Servier. Ivabradine was used in a concentration of 3×10−6 mol/L (the maximum concentration of the compound having specificity for If.22 To functionally disable ryanodine receptors we used 10−7 and 3×10−6 mol/L ryanodine. These ryanodine concentrations lock ryanodine receptors in an open subconductance state, leading to depletion of SR Ca2+ content.23 The effects of both compounds are reported at 30 minutes of superfusion.
Data are expressed as mean+SEM. Continuous parameters were analyzed with one- or two-way ANOVA for repeated measures. P<0.05 was considered significant.
Results
Effects of ivabradine and ryanodine on automaticity at normal membrane potentials in the SN and PF
Figures 1A and 1B show representative action potential traces recorded respectively from one SN and one PF preparation in control and in the presence of ivabradine. In control, the SN preparation beating rate was 125 bpm and the shape of the action potential was typical of pacemaker cells. MDP was –62 mV and there was a smooth transition between phase 4 and phase 0. Thirty minutes of superfusion with ivabradine resulted in slowing of the rate to 110 bpm and no change in MDP. In contrast to the SN preparation, ivabradine completely terminated automaticity in PF. Summary data (Figure 1, C through F) show that ivabradine significantly decreased spontaneous rate in both types of preparations with more prominent effects on PF. No changes in MDP occurred.
Figure 1.
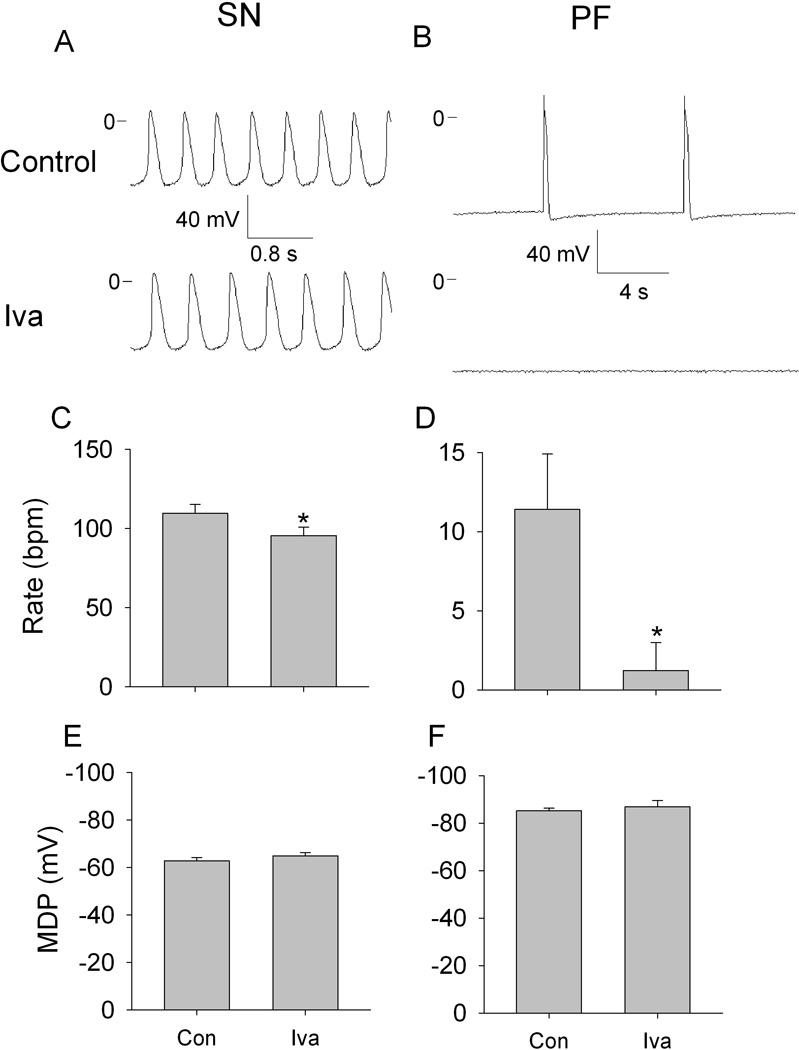
Effects of ivabradine (Iva) on automaticity in SN and PF. A and B, representative transmembrane potentials recorded from spontaneously beating SN preparation and free running PF in control (upper traces) and in the presence of 3 µmol/L ivabradine (lower traces). C through F, respective summary data showing ivabradine effects on rate and maximum diastolic potential (MDP) in SN (C and E) and in PF (D and F). N=10 for SN, n=6 for PF. *P<0.05 versus respective control.
The effects of ryanodine on SN and PF are shown in Figure 2. Neither ryanodine concentration altered SN automaticity: and there were no changes in rate or MDP (A, C and E). In PF, the low ryanodine concentration had no effects on automaticity and on MDP (B, middle trace, D and F), whereas 3×10−6 mol/L induced repetitive self-initiating and self-terminating episodes of impulse initiation at depolarized membrane potentials (B, bottom trace). The duration of episodes was about one minute. This pattern of activity started 12–15 minutes after beginning the ryanodine superfusion and lasted during the entire observation period.
Figure 2.
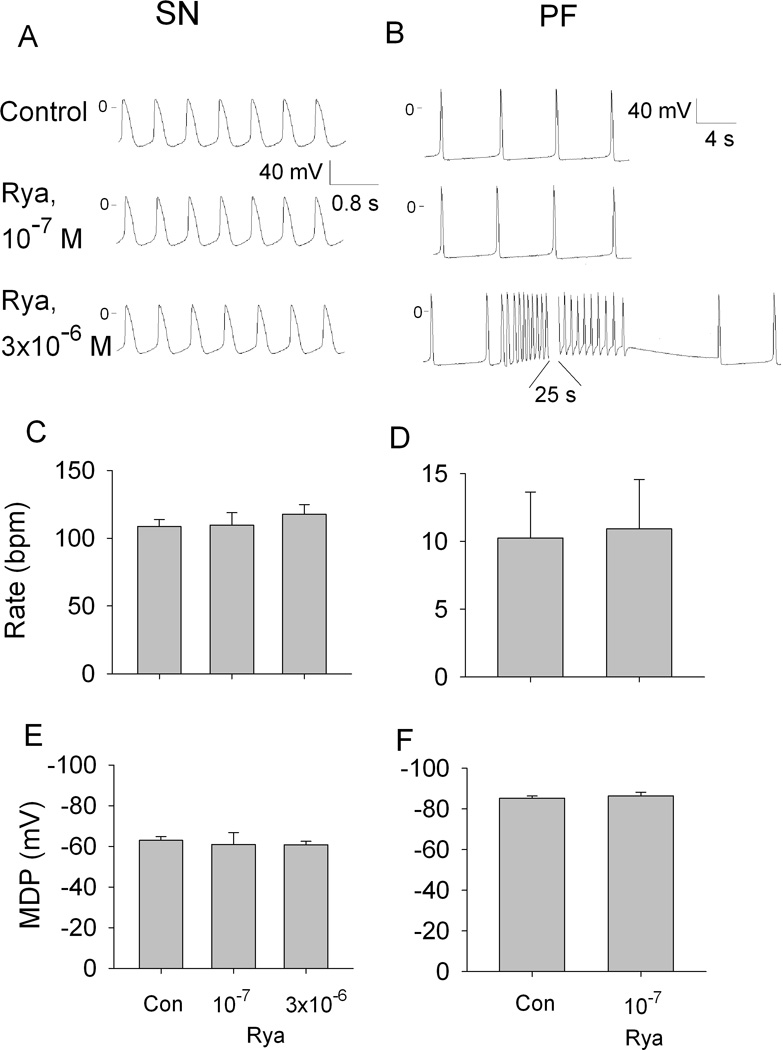
Effects of ryanodine (Rya) on automaticity in SN and PF. Representative transmembrane potentials recorded from SN preparation (A) and PF (B) in control and in the presence of 0.1 and 3 µmol/L ryanodine. C through F, summary data showing the effects of 0.1 and 3 µmol/L ryanodine on the rate and maximum diastolic potential (MDP) in SN (C and E) and 0.1 µmol/L ryanodine in PF (D and F). N=6 for SN and PF.
Effects of ivabradine and ryanodine on SN and PF automaticity in the presence of isoproterenol
Isoproterenol significantly accelerated rate and hyperpolarized MDP in SN preparations (Figure 3, A, C and E). Ivabradine applied in addition to isoproterenol decreased rate and further hyperpolarized MDP. Qualitatively similar effects of isoproterenol were seen in PF: automatic rate increased and MDP hyperpolarized (Figure 3, B, D and F). In the presence of isoproterenol, ivabradine almost completely eliminated the acceleration in rate and had no effects on MDP.
Figure 3.
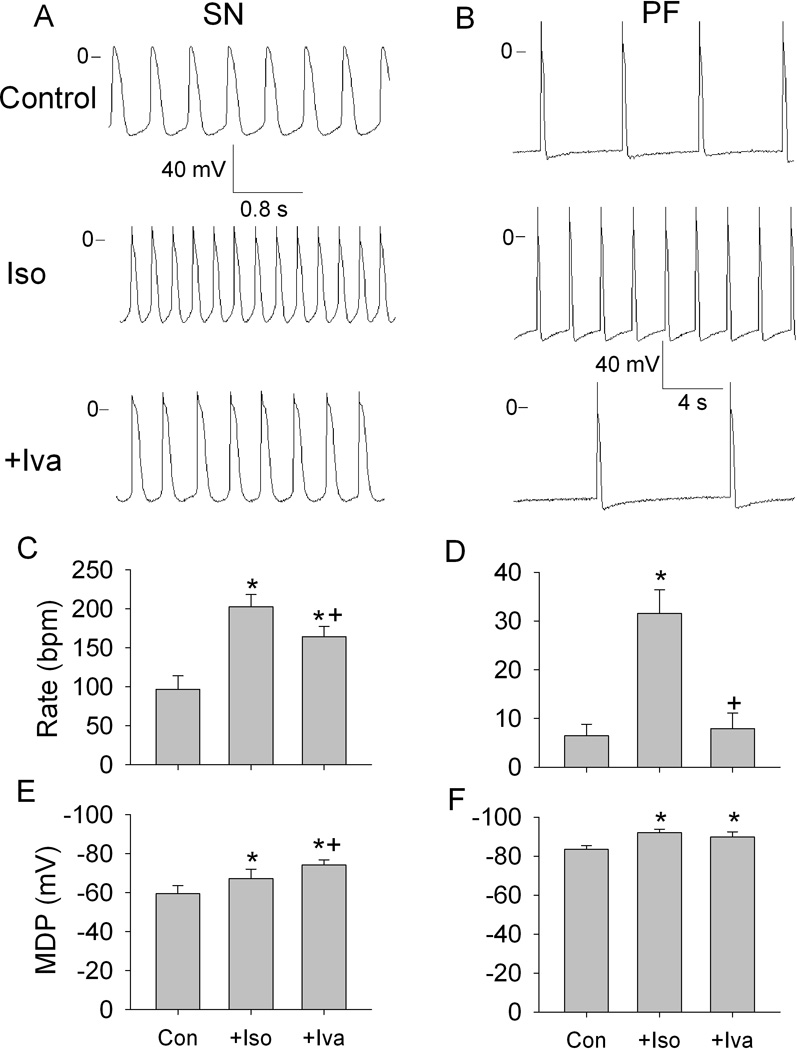
Effects of ivabradine (Iva) on automaticity in the presence of isoproterenol (Iso) in SN and PF. A and B, representative transmembrane potentials recorded from spontaneously beating SN preparation and free running PF in control (upper traces), in the presence of 0.1 µmol/L isoproterenol (middle traces) and in the presence of isoproterenol and 3 µmol/L ivabradine (lower traces). C through F, respective summary data showing the rate and maximum diastolic potential (MDP) in SN (C and E) and in PF (D and F). N=6 for SN and for PF. *P<0.05 versus respective control. +P<0.5 versus respective Iso.
Ryanodine significantly inhibited isoproterenol-accelerated SN automaticity while having no effect on isoproterenol-hyperpolarized MDP (Figure 4, A, C and E). Interestingly, the maximum ryanodine effect was achieved at the low (10−7 mol/L) concentration; increasing concentration to 3×10−6 mol/L did not further affect rate. In PF, in the presence of isoproterenol, the low ryanodine concentration did not change rate, whereas at 3×10−6 mol/L it induced repetitive self-initiating and self-terminating episodes of automaticity at depolarized membrane potentials (Figure 4, B D and F). These effects of ryanodine were similar to those seen in the absence of isoproterenol (compare bottom traces in panel B in Figures 2 and 4). In addition, the low ryanodine concentration eliminated the hyperpolarization of MDP induced by isoproterenol (Figure 4F).
Figure 4.
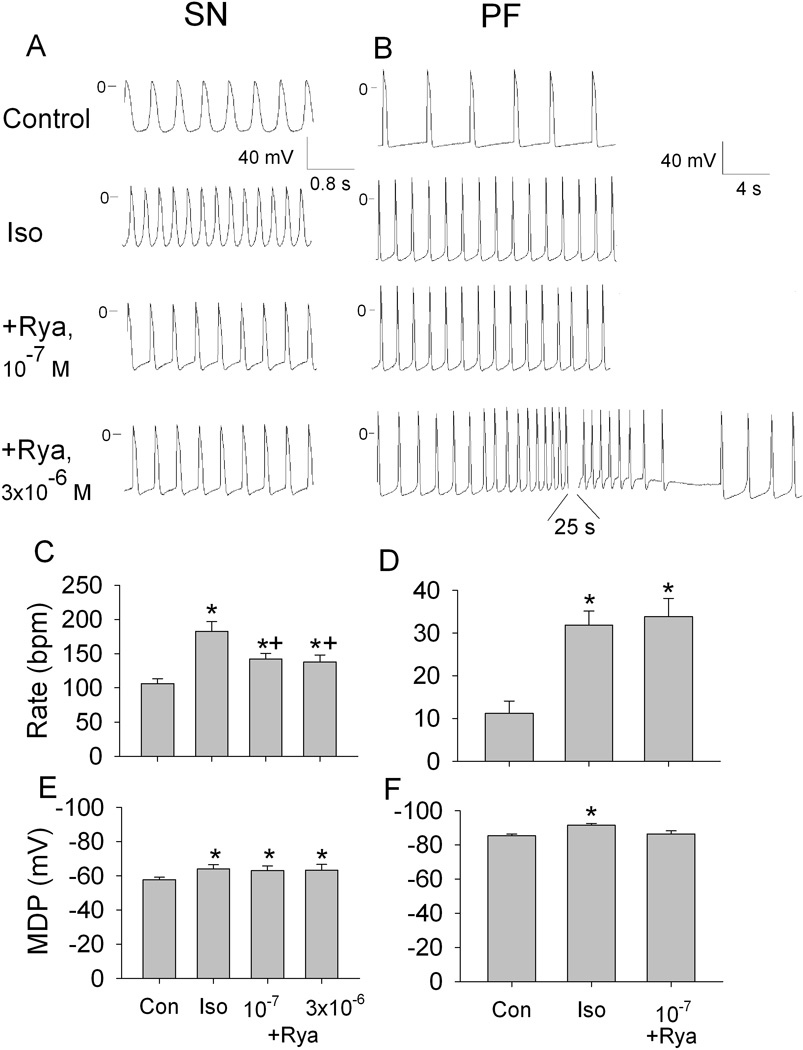
Effects of ryanodine (Rya) on automaticity in the presence of isoproterenol (Iso) in SN and PF. A and B, representative transmembrane potentials recorded from spontaneously beating SN preparation and free running PF in control, in the presence of 0.1 µmol/L isoproterenol, in the presence of isoproterenol plus 0.1 µmol/L ryanodine and in the presence of isoproterenol plus 3 µmol/L ryanodine. C through F, respective summary data showing the rate and maximum diastolic potential (MDP) in SN (C and E) and in PF (D and F). N=6 for SN and n=8 for PF. *P<0.05 versus respective control. +P<0.5 versus respective Iso.
Effects of ivabradine and ryanodine on abnormal automaticity in PF
Superfusion with BaCl2 led to a depolarization of PF and development of rapid automatic rhythms (Figure 5, A and B, middle panels). Ivabradine but not ryanodine slowed the rate of barium-induced automaticity (Figure 5C). Neither compound affected the depolarized MDP (Figure 5D).
Figure 5.
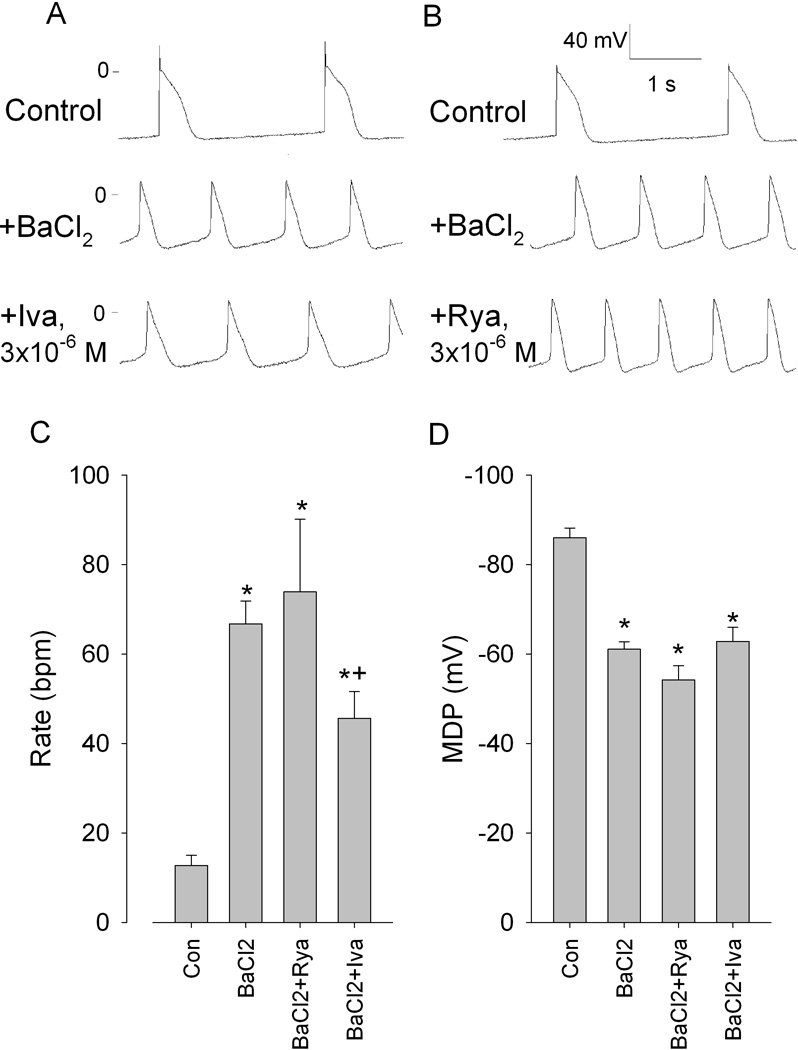
Effects of ivabradine (Iva) and ryanodine (Rya) on barium-induced automaticity at low membrane potentials in Purkinje fibers. A and B, representative transmembrane potentials in control, in the presence of 0.4 mmol/L BaCl2, and after adding Iva (3 µmol/L), or Rya (3 µmol/L). C and D, summary data showing the rate and maximum diastolic potential (MDP). N=5 for Iva and Rya. *P<0.05 versus respective control. +P<0.05 versus respective BaCl2.
Discussion
Isolated superfused SN preparation
In this study we developed a new canine SN preparation – an isolated multicellular slice of the SN region. The rates and MDPs in these preparations are similar to those recorded previously in canine arterially-perfused right atrial preparations.15, 16 In contrast to arterially-perfused preparations, the multicellular slices permit stable and long-lasting recordings of transmembrane potential. Control experiments demonstrated the stability of major parameters of pacemaker activity (the rate and MDP) during 3.5 hours. Therefore, we were able to test the effects of ivabradine and ryanodine on SN pacemaker activity.
Voltage clock in SN and PF
Our results allow us to estimate and compare the importance of voltage and calcium clocks for pacemaking in primary (SN) and secondary (PF) pacemakers. In the SN, ivabradine slowed basal pacemaking activity and partially decreased isoproterenol-induced rate acceleration. These results are in agreement with observations in disaggregated canine SN cells24 and show that If is an important contributor to basal SN automaticity and to the rate acceleration induced by β-adrenergic receptor stimulation. However, because If blockade does not eliminate automaticity or completely prevent rate increases in response to isoproterenol, our data suggest that If-independent mechanisms also contribute to canine SN pacemaking.
In PF, in contrast to SN, ivabradine almost completely terminated basal automaticity and abolished the isoproterenol-induced rate acceleration. Similar results have been demonstrated in situ: in dogs with complete atrioventricular block, intravenous administration of ivabradine totally suppressed idioventricular rhythm.25 These results point to If as a major determinant of automaticity in PF.
HCN2 and HCN4 are two major subunits of the HCN channels family that underlie If in pacemaker structures, with HCN4 being more expressed in the SN – along with HCN1 - and HCN2 in PF.26-28 Importantly, HCN2 and HCN4 show nearly equal sensitivity to inhibition by ivabradine.25, 29 Consequently, quantitatively different effects of ivabradine in the SN and PF likely do not result from a difference in sensitivity of If to the compound but reflect a different contribution of If to pacemaking in these structures.
Calcium clock in SN and PF
In the SN, ryanodine had no effects on basal pacemaker activity but significantly inhibited isoproterenol-accelerated SN automaticity. The effects of ryanodine on SN automaticity differ among various studies (from no effect to complete inhibition of automaticity) depending on species and experimental conditions.30 Hence, it is interesting to compare our results with those obtained in canine preparations with a lesser and higher degree of SN tissue isolation: arterially- perfused right atrium17 and single SN cells,24 respectively. Studies of both preparations showed that ryanodine only marginally (and statistically insignificantly) reduced basal automaticity but considerably impaired the rate acceleration induced by isoproterenol. These data are similar to our results and suggest that in canine SN, a Ca2+-dependent mechanism (Ca2+ clock) does not contribute substantially to basal automaticity but plays an important role in sinus acceleration during β-adrenergic stimulation. In PF, ryanodine at a low concentration had no effects on automaticity either in the absence or presence of isoproterenol. The latter effect is different from that in SN where a low ryanodine concentration maximally inhibited isoproterenol-induced rate acceleration. These data suggest that in PF, the Ca2+ clock does not contribute substantially to basal automaticity and to acceleration induced by β-adrenergic stimulation.
In PF, the high ryanodine concentration induced repetitive self-initiating and self-terminating episodes of automaticity at depolarized membrane potentials. The mechanism of this effect is unclear but the following explanation is plausible: block of SR Ca release induced by ryanodine inhibits calcium-dependent inactivation of calcium current.31, 32 As a result, the current through L-type channels decays slowly and more Ca2+ ions enter the cell at each excitation. Intracellular calcium elevation activates the forward mode of the electrogenic Na+/Ca2+ exchanger that generates an inward current.33 Increased inward current leads to membrane depolarization and generation of automaticity. The Na+/Ca2+ exchanger functions here to overload the cell with sodium, which activates the Na+/K+ pump.34 Enhanced outward pump current hyperpolarizes the membrane and inhibits abnormal automaticity. Then this sequence of events is repeated.
Voltage and calcium clocks in abnormal automaticity in PF
The response to ivabradine and ryanodine is clearly different in the SN and PF preparations. One of the major differences between these pacemakers is the value of MDP: PFs are significantly more hyperpolarized than SN cells. The more positive MDP in the SN results from the difference in the background K+ current (IK1) in these pacemaker structures IK1 is almost absent in central SN cells.35, 36 Therefore, we used Ba2+ to decrease IK1 in PF. Gradually increasing the Ba2+ concentration allowed us to depolarize MDP in PFs to values similar to those in the SN. In this MDP range, PFs exhibited automaticity comparable to that of SN. Thus, PFs depolarized with barium mimic the MDP and automaticity of the SN. Interestingly, the effects of ivabradine and ryanodine on this abnormal automaticity in PFs are comparable to those in SN: ivabradine moderately slows rate while ryanodine has no effect. The reduced ivabradine effects in depolarized PFs result, most likely, from a decreased availability of If at low membrane potentials. They also suggest that, as for SN, other (If-independent) mechanisms contribute to automaticity in PFs at depolarized level of membrane potentials. These results emphasize the role of the MDP in determining the mechanisms of automaticity in primary and secondary pacemakers.
Conclusions
Membrane and Ca clocks differentially contribute to canine SN pacemaking: If contributes to both basal automaticity and β-adrenergic-induced rate acceleration while the ryanodine-inhibited Ca clock appears more involved in β-adrenergic regulation of pacemaker rate. In PF, normal automaticity depends mainly on If. Inhibition of basal potassium conductance results in high automatic rate at depolarized membrane potentials with SN-like responses to inhibition of membrane and Ca clocks.
Acknowledgements
The authors express their gratitude to Servier Laboratories (France) for providing ivabradine to us.
We gratefully acknowledge Dr. Michael R. Rosen for his critical reading of the manuscript.
This work was financially supported by USPHS-NHLBI grants HL-67101 and HL-094410.
Footnotes
Ivabradine was a gift from Servier. No other disclosures.
References
- 1.Thollon C, Vilaine J-P. If Inhibition in cardiovascular diseases. Adv Pharmacol. 2010;59:53–92. doi: 10.1016/S1054-3589(10)59003-3. [DOI] [PubMed] [Google Scholar]
- 2.Fox K, Ford I, Steg PG, Tendera M, Ferrari R. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–816. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 3.Vilaine JP. The discovery of the selective If current inhibitor ivabradine: A new therapeutic approach to ischemic heart disease. Pharmacol Res. 2006;53:424–434. doi: 10.1016/j.phrs.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Bohora S, Lokhandwala Y, Parekh P, Vasavda A. Reversal of tachycardiomyopathy due to left atrial tachycardia by ivabradine. J Cardiovasc Electrophysiol. 2011;22:340–342. doi: 10.1111/j.1540-8167.2010.01860.x. [DOI] [PubMed] [Google Scholar]
- 5.Sette A, Martino A, Lioy E, Calo L. Efficacy of ivabradine in a case of inappropriate sinus tachycardia and ventricular dysfunction. J Cardiovasc Electrophysiol. 2010;21:815–817. doi: 10.1111/j.1540-8167.2009.01699.x. [DOI] [PubMed] [Google Scholar]
- 6.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–446. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 7.Suenari K, Cheng C-C, Chen Y-C, Lin Y-K, Nakano Y, Kihara Y, Chen S-A, Chen Y-J. Effects of ivabradine on the pulmonary vein electrical activity and modulation of pacemaker currents and calcium homeostasis. J Cardiovasc Electrophysiol. 2011 doi: 10.1111/j.1540-8167.2011.02173.x. [DOI] [PubMed] [Google Scholar]
- 8.Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca2+clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res. 2008;77:274–284. doi: 10.1093/cvr/cvm058. [DOI] [PubMed] [Google Scholar]
- 9.Lipsius SL, Hüser J, Blatter LA. Intracellular Ca2+release sparks atrial pacemaker activity. News Physiol Sci. 2001;16:101–106. doi: 10.1152/physiologyonline.2001.16.3.101. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanov KY, Maltsev VA, Vinogradova TM, Lyashkov AE, Spurgeon HA, Stern MD, Lakatta EG. Membrane potential fluctuations resulting from submembrane Ca2+ releases in rabbit sinoatrial nodal cells impart an exponential phase to the late diastolic depolarization that controls their chronotropic state. Circ Res. 2006;99:979–987. doi: 10.1161/01.RES.0000247933.66532.0b. [DOI] [PubMed] [Google Scholar]
- 11.DiFrancesco D. Funny channels in the control of cardiac rhythm and mode of action of selective blockers. Pharmacol Res. 2006;53:399–406. doi: 10.1016/j.phrs.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Vinogradova TM, Bogdanov KY, Lakatta EG. β-Adrenergic stimulation modulates ryanodine receptor Ca2+release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ Res. 2002;90:73–79. doi: 10.1161/hh0102.102271. [DOI] [PubMed] [Google Scholar]
- 13.James TN. Anatomy of the sinus node of the dog. Anat Rec. 1962;143:251–265. doi: 10.1002/ar.1091430310. [DOI] [PubMed] [Google Scholar]
- 14.Fedorov VV, Schuessler RB, Hemphill M, Ambrosi CM, Chang R, Voloshina AS, Brown K, Hucker WJ, Efimov IR. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ Res. 2009;104:915–923. doi: 10.1161/CIRCRESAHA.108.193193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods W, Urthaler F, James T. Spontaneous action potentials of cells in the canine sinus node. Circ Res. 1976;39:76–82. doi: 10.1161/01.res.39.1.76. [DOI] [PubMed] [Google Scholar]
- 16.Woods WT, Urthaler F, James TN. Electrical activity in canine sinus node cells during arrest produced by acetylcholine. J Mol Cell Cardiol. 1981;13:349–357. doi: 10.1016/0022-2828(81)90278-9. [DOI] [PubMed] [Google Scholar]
- 17.Joung B, Tang L, Maruyama M, Han S, Chen Z, Stucky M, Jones LR, Fishbein MC, Weiss JN, Chen P-S, Lin S-F. Intracellular calcium dynamics and acceleration of sinus rhythm by β-adrenergic stimulation. Circulation. 2009;119:788–796. doi: 10.1161/CIRCULATIONAHA.108.817379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James TN. The sinus node as a servomechanism. Circ Res. 1973;32:307–313. doi: 10.1161/01.res.32.3.307. [DOI] [PubMed] [Google Scholar]
- 19.Ettinger S, Suter P. Canine Cardiology. Philadelphia: WB: Saunders Company; 1970. p. 102. [Google Scholar]
- 20.Anyukhovsky EP, Rosen MR. Electrophysiologic effects of alprafenone on canine cardiac tissue. J Cardiovasc Pharmacol. 1994;24:411–419. doi: 10.1097/00005344-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Dangman KH, Hoffman BF. Effects of nifedipine on electrical activity of cardiac cells. Am J Cardiol. 1980;46:1059–1067. doi: 10.1016/0002-9149(80)90367-7. [DOI] [PubMed] [Google Scholar]
- 22.Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–1057. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- 24.Gao Z, Chen B, Joiner M-lA, Wu Y, Guan X, Koval OM, Chaudhary AK, Cunha SR, Mohler PJ, Martins JB, Song L-S, Anderson ME. If and SR Ca2+ release both contribute to pacemaker activity in canine sinoatrial node cells. J Mol Cell Cardiol. 2010;49:33–40. doi: 10.1016/j.yjmcc.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotnikov AN, Bucchi A, Shlapakova I, Danilo P, Brink PR, Robinson RB, Cohen IS, Rosen MR. HCN212-channel biological pacemakers manifesting ventricular tachyarrhythmias are responsive to treatment with If blockade. Heart rhythm. 2008;5:282–288. doi: 10.1016/j.hrthm.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han W, Bao W, Wang Z, Nattel S. Comparison of ion-channel subunit Expression in canine cardiac Purkinje fibers and ventricular muscle. Circ Res. 2002;91:790–797. doi: 10.1161/01.res.0000039534.18114.d9. [DOI] [PubMed] [Google Scholar]
- 27.Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- 28.Shi W, Wymore R, Yu H, Wu J, Wymore RT, Pan Z, Robinson RB, Dixon JE, McKinnon D, Cohen IS. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res. 1999;85:e1–e6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- 29.Bucchi A, Tognati A, Milanesi R, Baruscotti M, DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J Physiol (Lond) 2006;572:335–346. doi: 10.1113/jphysiol.2005.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinogradova TM, Lakatta EG. Regulation of basal and reserve cardiac pacemaker function by interactions of cAMP-mediated PKA-dependent Ca2+ cycling with surface membrane channels. J Mol Cell Cardiol. 2009;47:456–474. doi: 10.1016/j.yjmcc.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kass RS, Sanguinetti MC. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984;84:705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipp P, Mechmann S, Pott L. Effects of calcium release from sarcoplasmic reticulum on membrane currents in guinea pig atrial cardioballs. Pflügers Archiv. 1987;410:121–131. doi: 10.1007/BF00581904. [DOI] [PubMed] [Google Scholar]
- 33.Shigekawa M, Iwamoto T. Cardiac Na+-Ca2+exchange : Molecular and pharmacological aspects. Circ Res. 2001;88:864–876. doi: 10.1161/hh0901.090298. [DOI] [PubMed] [Google Scholar]
- 34.Stimers JR. Cardiac Na+/K+ pump. In: Sperelakis N, Kurachi Y, Terzic A, Cohen MV, editors. Heart Physiology and Pharmacology. San Diego: Academic Press; 2001. pp. 407–416. [Google Scholar]
- 35.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 36.Shinagawa Y, Satoh H, Noma A. The sustained inward current and inward rectifier K+ current in pacemaker cells dissociated from rat sinoatrial node. J Physiol. 2000;523:593–605. doi: 10.1111/j.1469-7793.2000.t01-2-00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


