Abstract
Background
The overall risk of hemolytic transfusion reactions from plasma (minor) incompatible platelet transfusions and the role of a critical anti-A or anti-B titer in predicting/preventing these reactions has not been clearly established.
Methods
We evaluated all apheresis platelet (AP) transfusions for three months. Using the gel titer method, we determined the anti-A and/or the anti-B IgG titer for all incompatible APs. Reported febrile transfusion reactions and hemolytic transfusion reactions (HTRs) were recorded; transfusions were not prospectively evaluated by the study team. A post-transfusion DAT and eluate were performed after a reported febrile or hemolytic reaction for patients who received plasma incompatible APs.
Results
647of 4,288 AP transfusions (15.1%) were plasma incompatible. Group O APs (N = 278) had significantly higher anti-A and anti-B titers than group A or B APs (p<0.0001). No group A or B APs had a titer >128 (0/342). For group O APs, 73 had titers ≥256 (26.3%), and 27 had titers ≥512 (9.7%). No HTRs were reported to any plasma incompatible AP transfusion during the study period. Two plasma incompatible AP transfusions were associated with fever/chills and positive DATs, of which one had a positive eluate. The incidence of a DAT and eluate positive febrile transfusion reaction in the plasma incompatible AP population is 0.15% (95% CI 0.0–0.86%).
Conclusion
A critical anti-A or B titer is not sufficient to predict the risk of hemolysis in patients receiving plasma incompatible APs, although underreporting of reactions to the blood bank may limit the generalizability of this study.
Keywords: platelet, apheresis, ABO, antibody titer, transfusion, incompatible, febrile transfusion reaction, hemolysis
INTRODUCTION
Hemolytic transfusion reactions are a known risk of ABO plasma (minor) incompatible apheresis platelet (AP) transfusions.1,2,3 Approximately 10–40% of patients today in the United States receive plasma incompatible platelet transfusions, but hemolytic reactions remain a rare event.1 The risk of a hemolytic transfusion reaction due to passive transfusion of anti-A and/or anti-B antibodies ranges widely from 1:2,000 to 1:46,176, depending on whether the evaluation is based on numbers of transfused products or transfusion events.1,4–6 These reactions are likely rare due to the capacity of the body to dilute incompatible ABO antibodies, as the A and B antigens are found on multiple epithelial tissues and are present on plasma proteins other than red cells.1,7,8 Moreover, while high titers of anti-A and anti-B should logically be more strongly associated with the development of symptoms than lower titers, case reports of adults who develop hemolysis due to plasma incompatible AP transfusions demonstrate that titers less than 128 can cause symptoms.1,8,9
Reducing the risk of hemolytic reactions due to plasma incompatible AP transfusions has been an area of increasing interest. The AABB standards state that “the transfusion service shall have a policy concerning transfusion of components containing significant amounts of incompatible ABO antibodies of unexpected red cell antibodies.”10 Many countries in Europe already have universal policies for preventing hemolytic reactions from plasma incompatible platelet transfusions.8 However, transfusion services in the United States do not have a defined uniform policy.9 A few labs have published data on the effect of using a critical anti-A and anti-B titer threshold to prevent and/or reduce the risk of these reactions.4,7,11 Based on these studies, it remains unclear whether this method is the best or most cost-effective approach. Moreover, to our knowledge, no study to date has systematically correlated clinical symptoms of hemolytic transfusion reactions, such as development of a fever, with the ABO antibody titer of the transfused product.
When plasma compatible platelets are not available in inventory and platelets are urgently needed, our institution currently reduces the risk of hemolytic transfusion reactions in adults by limiting the volume of transfused plasma incompatible AP products to 600cc in a 24 hour period. Under this protocol, a previous four year retrospective study at our institution demonstrated the incidence of overt hemolytic transfusion reactions to be 2 in 3816 plasma incompatible AP transfusions (0.05%); anti-A titers in the AP products that caused these reactions were 32 and 512.12 We hypothesized that evaluation of transfusion reactions consisting of any symptom suggestive of acute hemolysis, including isolated fever or chills, would increase the sensitivity of identifying a HTR due to plasma incompatible AP transfusions; thus, more accurately estimating the incidence of hemolysis from plasma incompatible AP transfusions, and more accurately defining the role of critical plasma incompatible titers. Consequently, we systematically evaluated the anti-A and anti-B titer for all plasma incompatible AP transfusions that occurred over a three month period, and correlated these titers with the development of clinical symptoms suggestive of a hemolytic transfusion reaction.
METHODS
Platelet Products
All platelet products evaluated were irradiated, leukoreduced apheresis platelets (APs). All individuals who received a plasma incompatible ABO AP transfusion from December 3, 2010 until February 27, 2011 were included in the study. The study was approved by the Johns Hopkins Medical Institutions Institutional Review Board.
The policy of the Johns Hopkins Hospital transfusion service is to provide plasma-compatible AP products for all children (age ≤ 18 years) and all oncology patients ≤ 40 kg. While every attempt is made to provide compatible products for all patients, those patients for whom plasma compatible AP products are not available are allowed up to 600 cc of incompatible plasma in a 24 hour period. If a patient requires greater than 600 cc of incompatible plasma, the attending blood bank physician is contacted, and approval of additional blood products and/or product manipulations are made.
Transfusion Reactions
At The Johns Hopkins Hospital, it is an institutional requirement to report all suspected transfusion reactions. Hemolytic transfusion reactions are defined as the presentation of clinical symptoms and signs of red cell hemolysis with supportive laboratory documentation of a hemolytic event due to incompatible antibodies (see http://www.aabb.org/programs/biovigilance/Documents/diagnose_full.html).13 Febrile transfusion reactions are clinically defined as a rise in 1°C to a level at or above 38.0°C and/or chills during or temporally associated with a platelet transfusion. Other potential etiologies of the fever are taken into consideration by the blood bank team. The final evaluation and diagnosis of a febrile transfusion or a hemolytic transfusion reaction was documented by transfusion medicine physicians. Chart audits were not performed to evaluate the consistency of transfusion reaction reporting, so it is not known to what extent there was underreporting of reactions.
For research purposes, a DAT and subsequent eluate (if the DAT was positive) was performed on post-transfusion patient blood samples after a reported febrile event for all patients who received plasma incompatible APs. The DAT and eluate were tested using standard procedures.14 Additionally, a visual hemolysis check was done on post-transfusion patient samples after a reported plasma incompatible-associated febrile or hemolytic reaction.
Chart Review
We retrospectively reviewed the available medical records on all patients who received a transfusion with a high IgG titer (defined as ≥ 512) and all patients who developed a fever associated with a plasma incompatible AP transfusion. We also identified two control groups. The first control group consisted of patients who were clinically similar to the high titer group and received a plasma incompatible transfusion with an IgG titer ≤ 32 (defined as low titer). The second control group consisted of patients who were clinically similar to the high titer group and received plasma compatible APs. We evaluated age, weight, primary diagnosis, and reported bleeding during the transfusion events. The change in hemoglobin from pre- and post-transfusion was evaluated and recorded if 1) no red cell product was administered before the second hemoglobin result, 2) both hemoglobins were obtained within 48 hours of the transfusion in question, and 3) the patient did not have clinical signs of bleeding.
ABO antibody titer methodology
While all AP samples were obtained prospectively, the subsequent ABO IgG antibody titration studies for all AP units were performed retrospectively and were performed 1–4 weeks after the transfusion. Consequently, titer results were not available to influence transfusion decisions or transfusion reaction evaluations. The samples collected from the AP units were centrifuged for five minutes and the supernatant was stored between 1–6°C. One of three skilled technologists serially titrated each sample utilizing a calibrated pipet with 0.9% saline until a negative reaction was obtained.
The titers were tested with A1 and/or B cells depending on the ABO type of the unit and the ABO type of the patient. The A1 and B cells (Immucor, Inc., Norcross, GA) were prepared using MTS™ Diluent 2, a hypotonic buffered saline solution to a 0.8% cell suspension. Each dilution was tested using ID-MTS™ Anti-IgG gel cards (Ortho Clinical Diagnostics, Inc., Raritan, NJ). It is important to note that MTS™ gel cards are designed to detect IgG, but IgM can contribute to positive readings to an undefined degree. The high throughput efficiency afforded by gel cards was desirable for the multiple titers required for the study. Fifty microliters of 0.8% A or B cells were added to the wells of the gel card followed by twenty-five microliters of the appropriate sample dilution. The cards were incubated at 37.0°C for fifteen minutes and then centrifuged for ten minutes. The reactions were graded from 0 to 4+. The titer was reported as the reciprocal of the highest dilution yielding a 1+ or greater reaction. Titers ≥ 512 were considered high for the purposes of this study. Control samples of known titer were stored at 4°C along with test samples and were run with each titer set. Results were consistently within a one tube dilution. The stability of the titer results were validated by testing the anti-A and -B titer of five different AP samples both on the day of platelet expiration, and after 2 weeks of storage 4°C. No titer result was different after storage.
Statistical analysis
Summary statistics and statistical analyses were performed using Vassarstats (http://faculty.vassar.edu/lowry/VassarStats.html), Microsoft Excel (Redmond, WA) and Stata v11.2 (College Station, TX). Confidence intervals for binary outcomes were calculated using exact methods. Sensitivity and positive predictive value calculations were also performed. True positives were defined as a plasma incompatible transfusion that resulted in a fever and a positive DAT. Hypothesis testing was performed for continuous variables with either a two-tailed Student’s t-test assuming unequal variance for two categories or an ANOVA for three categories; Fisher exact test was used for dichotomous frequency variables; and Kruskal-Wallis test for ordinal variables. Spearman correlation was used to compare the relationship between transfused volume and subsequent hemoglobin change, and a non-parametric test for trend (Cuzick method) was used to evaluate how hemoglobin changes trended among groups.
RESULTS
ABO titers
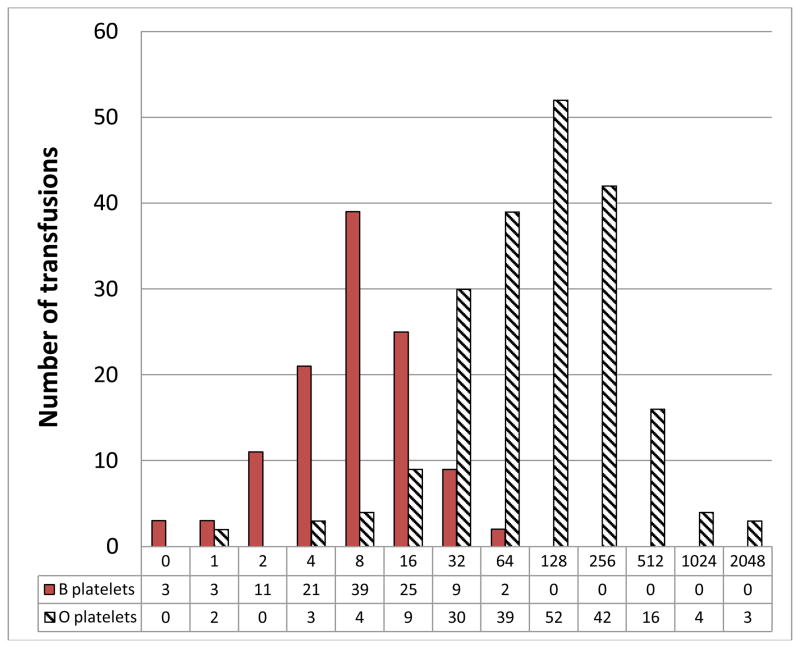
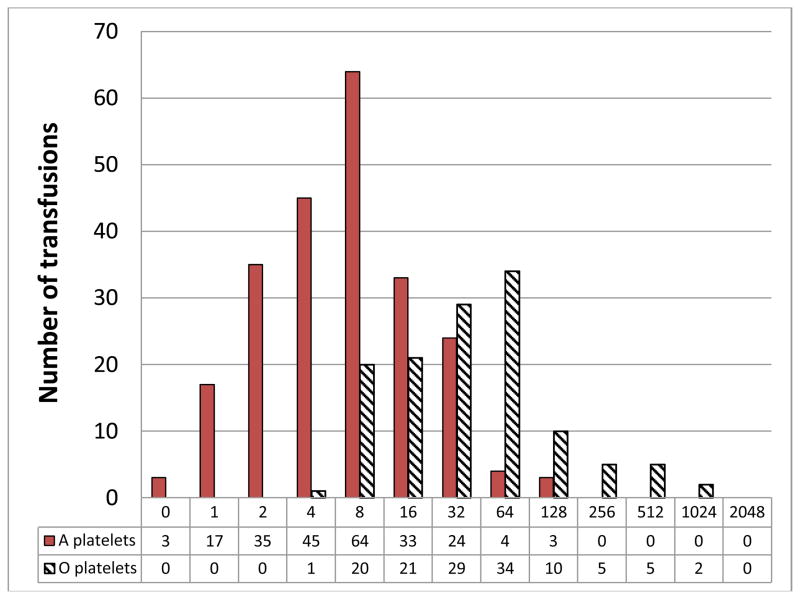
Over the 3 month study period, 4,288 AP units were transfused. Of these, 647 (15.1%) AP transfusions were to plasma incompatible patients. Samples from 620 of the 647 incompatible AP transfusions were available for titer evaluation. Figure 1 shows the IgG anti-A and anti-B titers found for the 620 AP products. No group A or B APs had an IgG titer >128 (0/342). In contrast, group O AP products (N = 288) had significantly higher IgG anti-A and anti-B titers than group A or B AP products (p<0.0001). Median group O anti-A titers were 128 (range: 1 – 2048) and median group O anti-B titers were 32 (range: 4 – 1024). For group O APs, 73 had IgG titers ≥ 256 (26.3%), and 27 (transfused to 17 different patients) had titers ≥ 512 (9.7%).
Figure 1.
Incompatible anti-A (top) and anti-B titer frequencies (bottom) from apheresis platelet units transfused to ABO incompatible patients*
*Titers for group O platelets transfused into AB recipients are split between the appropriate graphs. Numbers of platelets with each titer are shown below each titer level.
Transfusion reactions associated with ABO mismatched APs
During the study period, no hemolytic transfusion reactions were reported. Twenty-six transfusion reactions with fever were reported: 4 (15.4%) from plasma incompatible transfusions, 8 (30.8%) from major ABO incompatible transfusion, and 14 (53.8%) from ABO identical transfusions. The proportion of reactions with fever was similar between plasma incompatible AP transfusions (4/647, 0.62%) and all other AP transfusions (22/3641, 0.60%, p=0.67). As shown in Table 1, 3 of 4 (75%) were adjudicated as a febrile non-hemolytic transfusion reaction. None of the four patients received additional out-of-group APs during the 24 hours associated with the febrile reaction. There were two patients with febrile transfusion reactions (who each received two different plasma incompatible AP products) who had a positive post-transfusion DAT; one patient (products with anti-B titers of 16 and 2) had a negative eluate and one patient (products with anti-A titers of 2048 and 128) had a positive eluate suggestive of hemolysis. As acute hemolysis was not suspected clinically at the time of the transfusion, no supportive tests, such as LDH or haptoglobin, were obtained. Consequently, the DAT positive transfusion reactions were probable for acute hemolysis according to hemovigilance criteria.13 All four plasma incompatible AP transfusions that were associated with a fever were also associated with a drop in hemoglobin, regardless of antibody titer (median: −1.8 g/dL) between 4–20 hours after the transfusion. The incidence of developing a transfusion reaction associated with a positive DAT and eluate from a plasma incompatible AP transfusion was 1/647 (0.15%, 95% CI 0.0 – 0.86%); or 1 in 27 high titer (titer ≥ 512) transfusions (3.7%, 95% CI 0.09%–19.0%).
Table 1.
Transfusion reactions with reported fever after a plasma incompatibleAP transfusion over the 3 month study period.
| Gender | Age | Race | Weight (kg) | Primary Dx. | Patient ABO-Rh | Unit ABO-Rh | Transfused Vol. (cc) in a 24 hr. period | Ab. titer | Pre-tx. Temp (C) | Post-tx. Temp (C) | Chills (Y/N) | Change in Hgb (g/dL) | DAT | IgG/C3/ Both | Eluate | Final Dx. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | M | 21 | black | 58 | MDS | B+ | A+ | 194/193 | 16/2 | 36.6 | 38.6 | N | −2.2 | Pos | Both | Neg | FNHTR |
| Patient 2 | M | 52 | black | 84 | Granulocytic Sarcoma | B− | O+ | 385 | 1024 | 36.6 | 38.2 | Y | −0.3 | Neg | NA | NA | FNHTR |
| Patient 3 | F | 32 | white | 68.8 | ALL | B+ | O+ | 515 | 64 | 38.5 | 37.9 | Y | −1.9 | Neg | NA | NA | ATR |
| Patient 4 | F | 41 | black | 54.9 | AML | AB+ | O+ | 313/244 | 2048/ 128 | 37.4 | 38.4 | Y | −1.7 | Pos | Both | Anti-A | FNHTR |
FNHTR: Febrile non-hemolytic transfusion reaction, ATR: allergic transfusion reaction. Note that Patients 1 and 4 each received 2 plasma incompatible platelet products during the transfusion event in question.
Table 3.
Correlation of transfusion volume and pre-post transfusion hemoglobin change, according to incompatibility group
| Group | Spearman rho | P |
|---|---|---|
| Plasma compatible (n=28) | 0.25 | 0.21 |
| Titer ≤ 32 incompatible (n=23) | −0.17 | 0.44 |
| Titer ≥ 512 incompatible (n=13) | −0.43 | 0.14 |
Table 2 shows the clinical data from 17 patients who received an AP transfusion with an IgG titer ≥512 (19 transfusion events), clinically matched patients who received plasma incompatible AP transfusions with titers ≤32 (23 transfusion events), and matched controls who received ABO plasma compatible transfusions (28 transfusion events). None of the individuals in these three groups developed clinical symptoms of a hemolytic or febrile transfusion reaction during the analyzed transfusion events. The three groups were similar in gender distribution, patient age, patient weight, diagnosis, ABO group, and plasma volume transfused (p > 0.05). Patients in all 3 groups had a drop in hemoglobin. While, there was not a statistically significant difference in hemoglobin changes among the three groups (P=0.06), a test for trend showed larger hemoglobin decreases as incompatibility increased from plasma compatible to plasma incompatible low titer (≤32) and to plasma incompatible high titer (≥512) transfusions (P=0.02).
Table 2.
Demographic, transfusion, and lab data for 17 clinically asymptomatic plasma incompatible AP recipients of low and high antibody titer and 17 matched control recipients who received ABO compatible APs.
| No clinical reaction: Compatible plasma (n=28) | No clinical reaction: Incompatible titer ≤ 32 (n=23) | No clinical reaction: Incompatible titer ≥ 512 (n=19) | P | |
|---|---|---|---|---|
| Male (%) | 75 | 65.2 | 68.4 | 0.76 |
| Age, years | 59 (45–67) | 48 (29–65) | 57 (40–66) | 0.61 |
| Weight, kg | 77.1 (65.4–95.4) | 77.7 (65.0–94.0) | 74.5 (60.1–88.0) | 0.57 |
| Oncology Dx (%) | 100 | 100 | 89.5 | 0.07 |
| Patient ABO Group, A/ B/ AB (%) | 82.1/10.7/7.1 | 52.3/21.7/26.0 | 73.7/10.9/15.8 | 0.22 |
| Volume transfused, cc | 370 (200–587) | 281 (192–522) | 291 (220–365) | 0.26 |
| Incompatible anti A/B titer | NA | 32 (16–32) | 512 (512–1024) | < 0.0001 |
| Change in Hb, g/dL | −0.4 (0 to −0.7) | −0.7 (−0.4 to −0.8) | −0.8 (−0.5 to −1.0) | 0.06 |
Data are presented as medians (IQR) or as indicated.
Table 3 demonstrates the relationship between the volume of plasma transfused and the change in hemoglobin for the two groups who received incompatible plasma and a plasma compatible control group. There is a stronger negative correlation with volume transfused and decreasing hemoglobin among the plasma incompatible platelet transfusions, as compared to plasma compatible transfusions.
We lastly evaluated the sensitivity, specificity, and the positive predictive value of the ABO titer using different ABO titer thresholds to predict a DAT positive transfusion. The positive predictive value was low across all titer thresholds, and reached a value of 0.33 only when a titer threshold of 2048 was used. Sensitivity also was poor, and remained 0.5 until lower the titer threshold to ≥ 16. Sensitivity analysis is limited by the occurrence of only two DAT positive transfusion reactions. Specificity was 88% using a titer cutoff of ≥ 256 and 96% using a titer cutoff of ≥ 512.
DISCUSSION
The goal of this study is to estimate the frequency of clinical and subclinical hemolysis attributable to plasma incompatible AP transfusions and determine at what IgG titers and at what transfused volumes these reactions occur. Our study revealed a low incidence of any transfusion reaction to minor plasma incompatible AP transfusions. Additionally, there is evidence for subclinical hemolysis after plasma incompatible AP transfusion: we observed a greater drop in post transfusion hemoglobin with increasing ABO titers and volumes of plasma incompatible transfusion.
We find that a large proportion of our transfused plasma incompatible APs have a high IgG anti- A/B titer. Specifically, at our institution, nearly 10% of our transfused group O APs had a titer ≥512, and 26.3% had titers ≥ 256. This finding is consistent with prior reports. Specifically, Larsson et al. (2000)6 report a prevalence of 10–20%, and a more recent study by Josephson et al.11 report anti-A/A,B gel test titers of ≥ 256 in 39% of units.
Based on the large number of ABO “high” titer mismatched AP transfusions, one might expect to observe hemolytic transfusion reactions more frequently. However, hemolysis due to incidental infusion of ABO incompatible plasma is known to be uncommon. Larsson et al.6 found an incidence of 1 in 6600 when looking at all platelet transfusions, and Mair and Benson5 determined a hemolytic reaction frequency of 1 in 9000 when looking at only plasma incompatible platelet transfusions. To increase the sensitivity for observing plasma incompatible AP transfusion-related hemolysis, we evaluated any plasma incompatible AP transfusion reaction associated with any symptom consistent with hemolysis, including isolated fever and/or chills. We find evidence for a hemolytic reaction in 1 of 647 plasma incompatible AP transfusions, or 1 of 4288 AP transfusions.
To our knowledge, our study is the first to document a direct correlation between platelet product ABO plasma incompatibility and changes in hemoglobin. Mair and Benson5 evaluated 24 ABO compatible and 24 ABO incompatible platelet transfusions in a matched oncology cohort and found no significant change in hemoglobin between the 2 groups. Unlike Mair’s findings, we show incompatible plasma results in a slight increase in hemoglobin loss that in part depends on volume transfused and plasma incompatible titer.
Factors other than the anti-A and anti-B titer are also believed to contribute to the associated risk of hemolysis.1 Studies have shown that documented hemolytic transfusion reactions have occurred from plasma incompatible platelet transfusions with antibody titers considered to be low (≤ 64).1,12,15,16 It is well established that A and B antigens are known to be present on multiple tissues and plasma proteins in addition to red cells.1,7,8 Consequently, as potentially clinically significant anti-A or B antibodies are adsorbed by many tissues soon after transfusion, one would expect that only high titers of these antibodies could overcome this effect. Additionally, the total plasma volume of both the patient and the transfused unit may partially explain this apparent contradiction, and has been suggested to be of considerable importance1 for predicting hemolytic transfusion reactions to ABO mismatched platelet products.
Regulatory agencies including the AABB and CAP require that blood banks have a policy to prevent hemolytic reactions from plasma incompatible plasma-containing products. There is currently no standard policy in the US, and this is represented by the diversity of policies demonstrated in a recent survey by Fung et al.9 In this survey, only 83% (2623/3156 labs) of all labs have a policy. However, many labs have a non-specific policy, such as notifying an ordering physician or medical director if a plasma incompatible platelet transfusion occurs. Practice in Scotland and England is to screen for high titer platelet products and only transfuse products to plasma incompatible patients if the product has a titer lower than 50 or 100, respectively.4,17 About 2% of labs in the United States have also attempted and published similar policies.9 Cooling et al., for instance, proposed a critical titer of 128–2007, and most recently, Quillen et al., has proposed a critical titer of 250.4 These methods may not be feasible in all blood banks. Other strategies to prevent hemolytic reactions from plasma incompatible platelets have been proposed. Strategies to remove or reduce exposure to the offending plasma have been used by some transfusion services. More restrictive policies, such as using only ABO plasma compatible platelets have also been reported.9
Documentation that titer methods prevent hemolytic transfusion reactions is not clear. Quillen et al. note that since starting universal screening, they have not had a single hemolytic transfusion reaction to a platelet product, and they note that they had an incidence of 1 in 2460 prior to implementing universal screening.4 Josephson et al. hypothesize that their method could prevent 2 hemolytic reactions per year at their institution.11 Lastly, the hemovigilance system in England has reported that platelets account for 20% (9/44) of acute hemolytic reactions overall, and 33% (3/9) of acute hemolytic reactions in children.18,19
It has been recently shown that platelets stored in platelet additive solution (PAS) are effective and reduce adverse events associated with platelet transfusions.20–22 As PAS effectively reduces the plasma from a platelet product, it may also reduce the incidence of hemolysis due to incompatible plasma. It is not yet knownwhether PAS platelets will be a cost-effective strategy for the reduction of hemolytic reactions.
Our findings have limitations. Although our study included active surveillance with seven platelet coordinators that round daily on oncology platelet recipients, we did not have active surveillance for transfusion reactions for all platelet recipients. We did not screen all plasma incompatible AP transfusions for evidence of hemolysis; rather, we relied on reporting from the clinical units before conducting DATs. Due to the retrospective nature of this study, we were also unable to confirm that the two patients who did have a positive DAT after the incompatible transfusion, did not have a positive DAT prior to the transfusion of interest. Our results would likely have a higher sensitivity for detecting hemolytic reactions if we employed prospective, standardized evaluations for hemolysis, but performing these evaluations on every plasma incompatible transfusion is prohibitive. In evaluating fever, we did exclude TRALI and septic transfusion reactions, but we could not exclude other causes of fever, such as an underlying medical condition. Most patients were receiving platelet support due to aplasia from chemotherapy. Consequently, marrow suppression likely explains the fall in hemoglobin for most patients, regardless of the platelet transfusion. Lastly, we evaluated over 600 consecutive plasma incompatible AP transfusions, but our patient population of interest was still relatively small as far as observing for an overt hemolytic reaction, which is a rare event. Nevertheless, there were adequate numbers of febrile and other reactions to observe for more subtle signs of hemolysis. The MTS™ gel card method is designed for IgG detection but can detect IgM antibodies to an undefined extent, so our titer results are not exactly comparable to other methods. The relative contributions of IgM and IgG antibodies in plasma incompatible transfusion to in vivo hemolysis (both intra- and extra-vascular) is not known. Generalization of the clinical associations with the titer results presented is limited to other studies using this method.
In summary, our study demonstrates that hemolysis to plasma incompatible APs may be mild and unreported. While plasma compatibility is an important factor for predicting hemolysis, the IgG antibody titer, in itself, is of limited predictive value. Transfusion volume and recipient factors also likely affect the risk of hemolysis. Future studies evaluating plasma incompatible platelet transfusion should evaluate factors in addition to ABO plasma incompatible titer.
Acknowledgments
We gratefully acknowledge the nurses and laboratory medical technologists who performed the transfusion reaction workups. AART is supported by the Doris Duke Charitable Foundation (grant 2011036), NIH 1K23AI093152-01A1, and Johns Hopkins University Clinician Scientist Development Award.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Josephson CD, Castillejo M, Grima K, Hillyer CD. ABO-mismatched platelet transfusions: Strategies to mitigate patient exposure to naturally occurring hemolytic antibodies. Transfusion and Apheresis Science. 2010;42:83–88. doi: 10.1016/j.transci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg WB, McGinniss MH. Hemolytic transfusion reaction due to anti-A. Transfusion. 1975;15:1–9. doi: 10.1046/j.1537-2995.1975.15175103502.x. [DOI] [PubMed] [Google Scholar]
- 3.McLeod BC, Sassetti RJ, Weens JH, Vaithianathan T. Haemolytic transfusion reaction due to ABO incompatible plasma in a platelet concentrate. Scand J Haematol. 1982;28:193–196. doi: 10.1111/j.1600-0609.1982.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 4.Quillen K, Sheldon SL, Daniel-Johnson JA, Lee-Stoka H, Flegel WA. A practical strategy to reduce the risk of passive hemolysis by screening plateletpheresis donors for high-titer ABO antibodies. Transfusion. 2011;51:92–96. doi: 10.1111/j.1537-2995.2010.02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mair B, Benson K. Evaluation of changes in hemoglobin levels associated with ABO-incompatible plasma in apheresis platelets. Transfusion. 1998;38:51–55. doi: 10.1046/j.1537-2995.1998.38198141498.x. [DOI] [PubMed] [Google Scholar]
- 6.Larsson LG, Welsh VJ, Ladd DJ. Acute intravascular hemolysis secondary to out-of-group platelet transfusion. Transfusion. 2000;40:902–906. doi: 10.1046/j.1537-2995.2000.40080902.x. [DOI] [PubMed] [Google Scholar]
- 7.Cooling LL, Downs TA, Butch SH, Davenport RD. Anti-A and Anti-B titers in pooled group O platelets are comparable to apheresis platelets. Transfusion. 2008;48(10):2106–13. doi: 10.1111/j.1537-2995.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- 8.Cooling L. ABO and platelet transfusion therapy. Immunohematology. 2007;23(1):20–33. [PubMed] [Google Scholar]
- 9.Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma. Arch Pathol Lab Med. 2007;131:909–916. doi: 10.5858/2007-131-909-TOPCAP. [DOI] [PubMed] [Google Scholar]
- 10.Standards for Blood Banks and Transfusion Services. 26. Bethesda: AABB; 2009. [PubMed] [Google Scholar]
- 11.Josephson CD, Mullis NC, VanDenmark C, Hillyer CD. Significant numbers of apheresis-derived group O platelets have a “high-titer” anti-A/A,B: Implications for transfusion policy. Transfusion. 2004;44:805–808. doi: 10.1111/j.1537-2995.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 12.Fauzie D, Shirey RS, Thoman S, Bensen-Kennedy D, King KE. The risk of hemolytic transfusion reactions due to passively-acquired ABO antibodies: a retrospective study of non-group O adult recipients of group O plateletpheresis transfusions. Transfusion. 2004;44(Suppl):36A (abstract). [Google Scholar]
- 13.Biovigilance Criteria. http://www.aabb.org/programs/biovigilance/Documents/diagnose_full.html.
- 14.Technical Manual. 16. Bethesda: AABB; 2008. [Google Scholar]
- 15.Conway LT, Scott EP. Acute hemolytic transfusion reaction due to ABO incompatible plasma in a plateletpheresis concentrate. Transfusion. 1985;24:413–414. doi: 10.1046/j.1537-2995.1984.24585017836.x. [DOI] [PubMed] [Google Scholar]
- 16.Sapatnekar S, Sharma G, Downes KA, Wiersma S, McGrath C, Yomtovian R. Acute hemolytic transfusion reaction in a pediatric patient following transfusion of apheresis platelets. J Clin Apher. 2005;20:225–229. doi: 10.1002/jca.20072. [DOI] [PubMed] [Google Scholar]
- 17.Sadani DT, Urbaniak SJ, Bruce M, Tighes JE. Repeat ABO-incompatible platlet transfusions leading to hemolytic transfusion reaction. Transfus Med. 2006;16:375–379. doi: 10.1111/j.1365-3148.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, Chapman CE, Davison K, Gerrard R, Gray A, Knowles S, Love EM, Milkins C, McClelland DB, Norfolk DR, Soldan K, Taylor C, Revill J, Williamson LM, Cohen H SHOT Steering Group. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–282. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Stainsby D, Jones H, Wells AW, Gibson B, Cohen H SHOT Steering Group. Adverse outcomes of blood transfusion in children: Analysis of UK reports to the serious hazards of transfusion scheme 1996–2005. Br J Haematol. 2008;141:73–79. doi: 10.1111/j.1365-2141.2008.07022.x. [DOI] [PubMed] [Google Scholar]
- 20.de Wildt-Eggen J, Nauta S, Schrijver JG, van Marwijk Kooy M, Bins M, van Prooijen HC. Reactions and platelet increments after transfusion of platelet concentrates in plasma or an additive solution: a prospective, randomized study. Transfusion. 2000;40:398–403. doi: 10.1046/j.1537-2995.2000.40040398.x. [DOI] [PubMed] [Google Scholar]
- 21.Azuma H, Hirayama J, Akino M, Miura R, Kiyama Y, Imai K, Kasai M, Koizumi K, Kakinoki Y, Makiguchi Y, Kubo K, Atsuta Y, Fujihara M, Homma C, Yamamoto S, Kato T, Ikeda H. Reduction in adverse reactions to platelets by the removal of plasma supernatant and resuspension in a new additive solution (M-sol) Transfusion. 2009;49:214–8. doi: 10.1111/j.1537-2995.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 22.Kerkhoffs JL, Eikenboom JC, Schipperus MS, van Wordragen-Vlaswinkel RJ, Brand R, Harvey MS, de Vries RR, Barge R, van Rhenen DJ, Brand A. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108:3210–5. doi: 10.1182/blood-2006-04-020131. [DOI] [PubMed] [Google Scholar]