Abstract
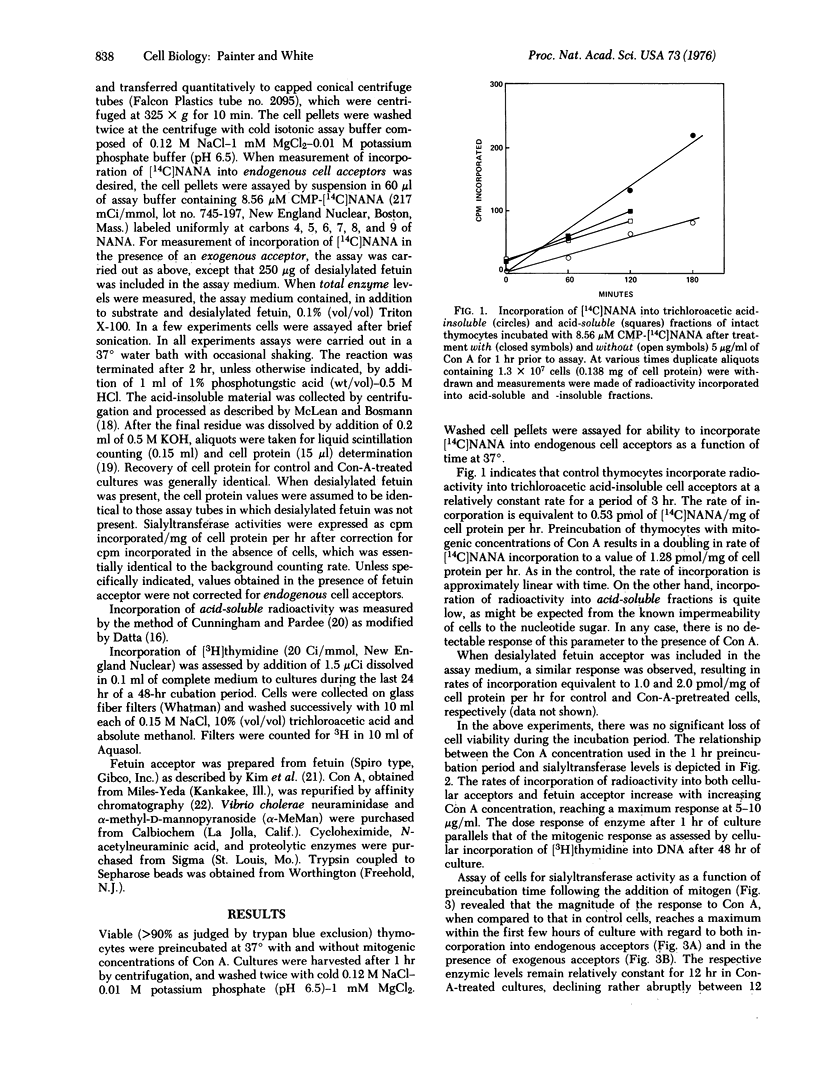
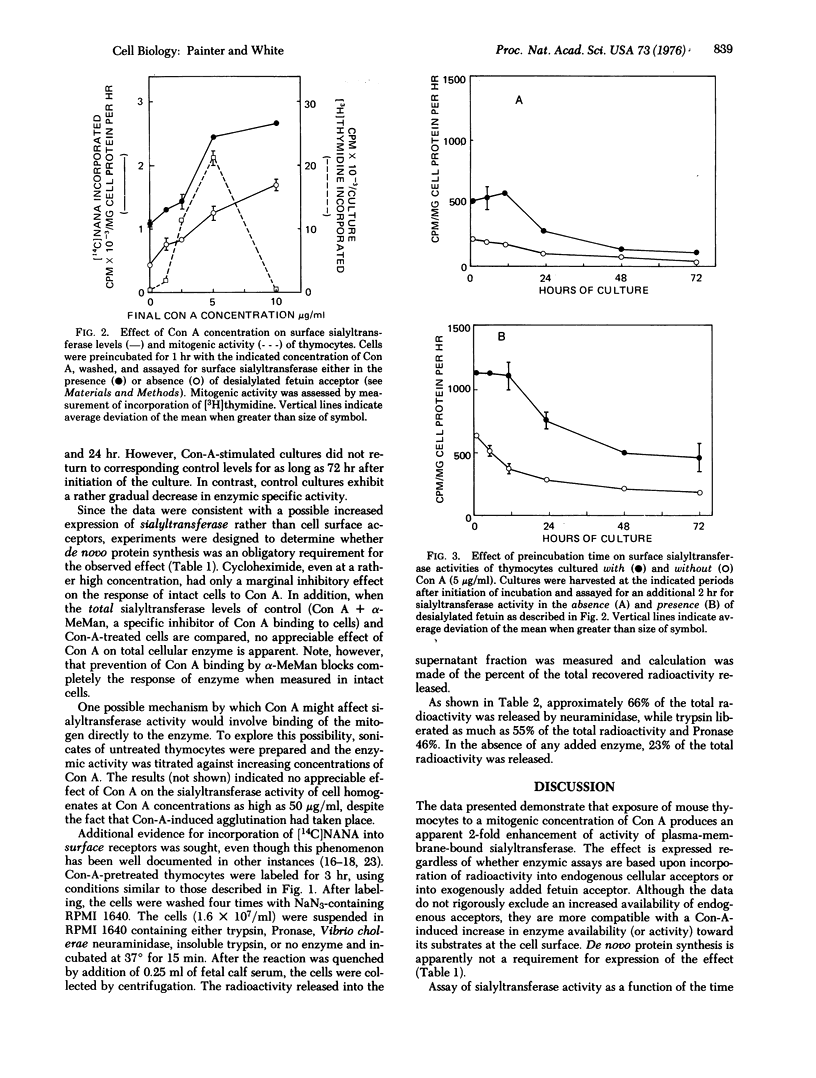
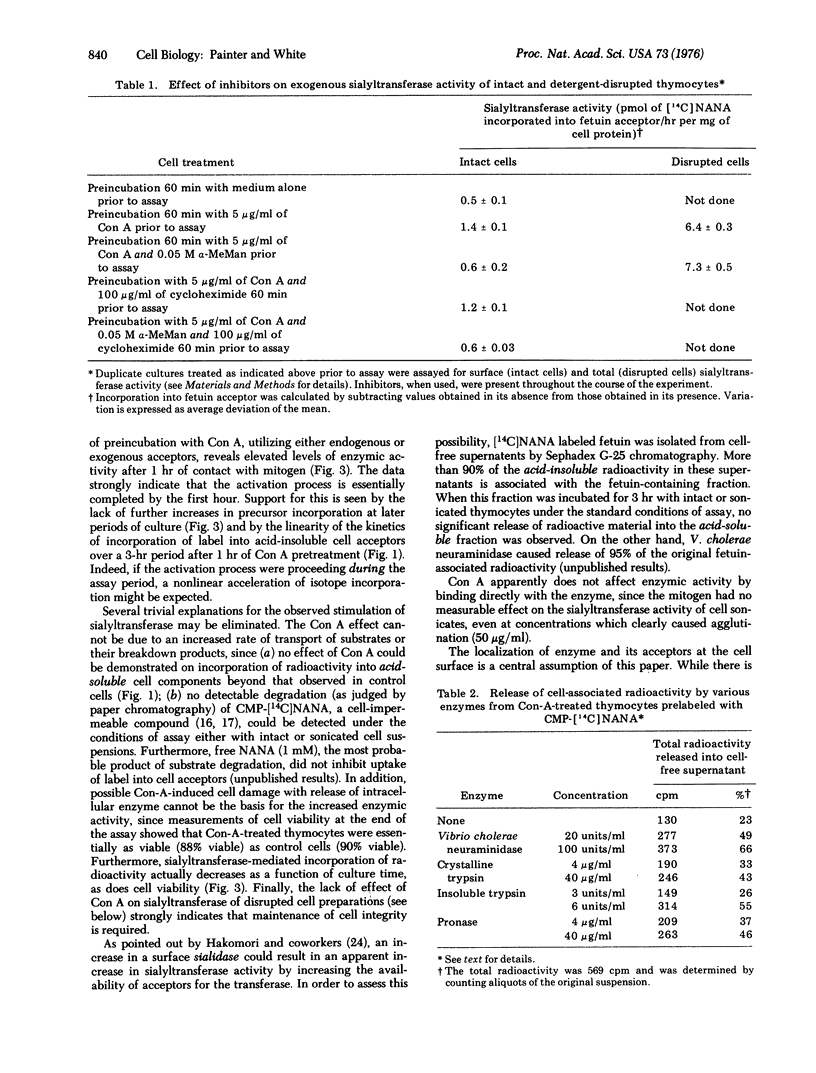
Incubation of mouse thymocytes with mitogenic concentrations of concanavalin A causes a 2-fold increase in cell-surface-associated (but not total cell) sialyltransferase activity (ectosialyltransferase, CMP-N-acetylneuraminate:D-galactosyl-glycoprotein N-acetylneuraminyltransferase, EC 2.4.99.1) as judged by incorporation of [14C]sialic acid into endogenous cell acceptors and into added desialylated fetuin acceptor. The concanavalin-A-induced enhancement of enzymic activity is essentially complete within 1 hr after addition of mitogen and remains at elevated levels for 12 hr, declining rapidly thereafter. Intact cells labeled previously with [14C]sialic acid and then incubated briefly with hydrolytic enzymes, including neuraminidase and insoluble trypsin, released 43-66% of total cell-associated radioactivity without appreciably changing cell viability. Alterations in sialyltransferase activity due to concanavalin A treatment could not be explained by a mitogen-mediated (a) uptake of radioactive precursors, (b) cell death, (c) increased product catabolism, or (d) activation of sialyltransferase by mitogen binding to the enzyme. Furthermore, the process does not require active protein synthesis. The results are consistent with a rapid concanavalin-A-induced exposure of potential enzymic activity that was previously inaccessible to substrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G., Leblond C. P. Formation of cell coat material for the whole surface of columnar cells in the rat small intestine, as visualized by radioautography with L-fucose-3H. J Cell Biol. 1970 Aug;46(2):409–416. doi: 10.1083/jcb.46.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. W., Heiniger H. J., Kandutsch A. A. Relationship between sterol synthesis and DNA synthesis in phytohemagglutinin-stimulated mouse lymphocytes. Proc Natl Acad Sci U S A. 1975 May;72(5):1950–1954. doi: 10.1073/pnas.72.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. D., Pardee A. B. Transport changes rapidly initiated by serum addition to "contact inhibited" 3T3 cells. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Effect of phytohaemagglutinin on the surface charge of human and murine lymphocytes. Nature. 1967 Nov 18;216(5116):694–695. doi: 10.1038/216694a0. [DOI] [PubMed] [Google Scholar]
- Datta P. Labeling of the external surface of hamster and mouse fibroblasts with (14C)sialic acid. Biochemistry. 1974 Sep 10;13(19):3987–3991. doi: 10.1021/bi00716a026. [DOI] [PubMed] [Google Scholar]
- Despont J. P., Abel C. A., Grey H. M. Sialic acids and sialyltransferases in murine lymphoid cells: indicators of T cell maturation. Cell Immunol. 1975 Jun;17(2):487–494. doi: 10.1016/s0008-8749(75)80052-9. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. The stepwise acceleration of phosphatidyl choline synthesis in phytohemagglutinin-treated lymphocytes. Biochim Biophys Acta. 1969 Mar 4;176(2):316–323. doi: 10.1016/0005-2760(69)90189-1. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. I. External labeling of cell surface galactose and galactosamine in glycolipid and glycoprotein of human erythrocytes. J Biol Chem. 1973 Jun 25;248(12):4311–4317. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. Surface carbohydrates of hamster fibroblasts. II. Interaction of hamster NIL cell surfaces with Ricinus communis lectin and concanavalin A as revealed by surface galactosyl label. J Biol Chem. 1975 Apr 10;250(7):2447–2451. [PubMed] [Google Scholar]
- Hayden G. A., Crowley G. M., Jamieson G. A. Studies on glycoproteins. V. Incorporation of glucosamine into membrane glycoproteins of phytohemagglutinin-stimulated lymphocytes. J Biol Chem. 1970 Nov 10;245(21):5827–5832. [PubMed] [Google Scholar]
- Hirano H., Parkhouse B., Nicolson G. L., Lennox E. S., Singer S. J. Distribution of saccharide residues on membrane fragments from a myeloma-cell homogenate: its implications for membrane biogenesis. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2945–2949. doi: 10.1073/pnas.69.10.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Brittinger G., Hirschhorn K., Weissmann G. Studies on lysosomes. XII. Redistribution of acid hydrolases in human lymphocytes stimulated by phytohemagglutinin. J Cell Biol. 1968 May;37(2):412–423. doi: 10.1083/jcb.37.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenti E., Avrameas S. The use of concanavalin A in the study of the dynamics of lymphocyte membrane glycans. FEBS Lett. 1973 Jun 1;32(2):238–242. doi: 10.1016/0014-5793(73)80841-5. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Krug U., Krug F., Cuatrecasas P. Emergence of insulin receptors on human lymphocytes during in vitro transformation. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2604–2608. doi: 10.1073/pnas.69.9.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackintosh P., Hardy D. A., Aviet T. Lymphocyte-typing changes after short-term culture. Lancet. 1971 May 15;1(7707):1019–1019. doi: 10.1016/s0140-6736(71)91415-2. [DOI] [PubMed] [Google Scholar]
- McLean R. J., Bosmann H. B. Cell-cell interactions: enhancement of glycosyl transferase ectoenzyme systems during Chlamydomonas gametic contact. Proc Natl Acad Sci U S A. 1975 Jan;72(1):310–313. doi: 10.1073/pnas.72.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt L. M., Grimes W. J. Cell surface glycolipid and glycoprotein glycosyltransferases of normal and transformed cells. J Biol Chem. 1974 Jul 10;249(13):4157–4165. [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. 2. Stimulation of "facilitated diffusion" of 3-O-methyl-glucose. Eur J Biochem. 1971 Apr 30;19(4):509–513. doi: 10.1111/j.1432-1033.1971.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Bernacki R. J. Ultrastructural evidence for ectoglycosyltransferase systems. Nature. 1975 Aug 21;256(5519):648–650. doi: 10.1038/256648a0. [DOI] [PubMed] [Google Scholar]
- Quastel M. R., Kaplan J. G. Early stimulation of potassium uptake in lymphocytes treated with PHA. Exp Cell Res. 1970 Nov;63(1):230–233. doi: 10.1016/0014-4827(70)90360-5. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wallach D. F., Ferber E. Concanavalin A augments the turnover of electrophoretically defined thymocyte plasma membrane proteins. Biochim Biophys Acta. 1974 Aug 9;356(3):288–299. doi: 10.1016/0005-2736(74)90269-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Natsuume S., Takahashi M. Letter: Lymphocyte membrane receptors in cultures treated with mitogen: enhancement of rosette forming activity. Jpn J Exp Med. 1975 Feb;45(1):49–51. [PubMed] [Google Scholar]
- Yogeeswaran G., Hakomori S. Cell contact-dependent ganglioside changes in mouse 3T3 gibroblasts and a suppressed sialidase activity on cell contact. Biochemistry. 1975 May 20;14(10):2151–2156. doi: 10.1021/bi00681a017. [DOI] [PubMed] [Google Scholar]
- Zagury D., Uhr J. W., Jamieson J. D., Palade G. E. Immunoglobulin synthesis and secretion. II. Radioautographic studies of sites of addition of carbohydrate moieties and intracellular transport. J Cell Biol. 1970 Jul;46(1):52–63. doi: 10.1083/jcb.46.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg K. J., Betel I. Regulation of amino acid uptake in lymphocytes stimulated by mitogens. I. Increase in AIB transport dependent on cell metabolism. Exp Cell Res. 1974 Mar 15;84(1):412–418. doi: 10.1016/0014-4827(74)90423-6. [DOI] [PubMed] [Google Scholar]



