Abstract
We compared the period of the rhythm of plasma melatonin, driven by the hypothalamic circadian pacemaker, to in vitro periodicity in cultured peripheral fibroblasts to assess the effects on these rhythms of a polymorphism of PER3 (rs57875989), which is associated with sleep timing. In vitro circadian period was determined using luminometry of cultured fibroblasts, in which the expression of firefly luciferase was driven by the promoter of the circadian gene Arntl (Bmal1). The period of the melatonin rhythm was assessed in a 9-d forced desynchrony protocol, minimizing confounding effects of sleep-wake and light-dark cycles on circadian rhythmicity. In vitro periods (32 participants, 24.61±0.33 h, mean±sd) were longer than in vivo periods (31 participants, 24.16±0.17 h; P<0.0001) but did not differ between PER3 genotypes (P>0.4). Analyses of replicate in vitro assessments demonstrated that circadian period was reproducible within individuals (intraclass correlation=0.62), but in vivo and in vitro period assessments did not correlate (P>0.9). In accordance with circadian entrainment theory, in vivo period correlated with the timing of melatonin (P<0.05) at baseline and with diurnal preference (P<0.05). Individual circadian rhythms can be reliably assessed in fibroblasts but may not correlate with physiological rhythms driven by the central circadian pacemaker.—Hasan, S., Santhi, N., Lazar, A.S., Slak, A., Lo, J., von Schantz, M., Archer, S. N., Johnston, J. D., Dijk, D.-J. Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin.
Keywords: sleep, PER3, Bmal1, lentivirus, clock genes
Circadian rhythms are endogenously generated rhythms that continue to oscillate with a near-24-h period in the absence of periodic cycles in the external environment (1). They are a fundamental characteristic of most living systems. In humans, their disruption is associated with negative health outcomes, such as observed in shift work and circadian rhythm sleep disorders (2–4). Mammalian circadian rhythms depend on a network of interacting genes and proteins, including transcriptional activators such as CLOCK, NPAS2, and ARNTL (BMAL1), which induce transcription of the clock genes Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2), as well as other clock-controlled output genes. PER and CRY proteins form multimeric complexes that translocate to the nucleus, where they inhibit the transcriptional activators and thus suppress their own expression (5, 6). The transcriptional-translational oscillator, which may interact with a cytosolic metabolic oscillator (7), is operative in many tissues, and circadian rhythms can be observed in the central nervous system and in nearly all peripheral cell types, although some differences may exist between central and peripheral oscillators (8–11).
The near-24-h period of the transcriptional-translational feedback oscillator appears to be determined primarily by post-transcriptional events, such as phosphorylation, which affects stability of the PER proteins in particular (12, 13). The mammalian circadian system is organized in a hierarchical manner such that a central circadian pacemaker located in the suprachiasmatic nucleus directly drives rhythms in pineal melatonin synthesis, for example, through a well-defined neural connection, and also orchestrates peripheral oscillators through neural, endocrine, and physiological signals (14–16). The appropriate timing (phase) of circadian behavioral and physiological rhythms relative to external cycles is accomplished through entrainment of the central circadian clock to the light-dark cycle (17). According to models of entrainment (18–20) and confirmed by many empirical observations (21–23), the intrinsic period of the central circadian clock is a key determinant of the phase relationship between the endogenous oscillator and the light-dark cycle and thereby a biological determinant of individual differences in behavior and physiology. In individuals with a long intrinsic period of the central circadian pacemaker, the nadir of the core body temperature rhythm, the onset of nocturnal melatonin, and the preferred timing of sleep are all located at a later clock time than in individuals with a shorter circadian period (22, 23). It is, therefore, necessary to assess circadian period in order to identify causes underlying sleep timing disorders.
In familial advanced sleep-phase syndrome (FASPS), a mutation affecting a casein kinase Iε (CK1ε) phosphorylation site of PER2 (24) as well as a mutation in the CK1δ gene (25) have been reported. PER3 is also phosphorylated by CK1ε (26, 27) and interacts with PER1 and PER2 in nuclear translocation (27–29), which is blocked by inhibition of CK1ε (30). Because changes to the phosphorylation status of PER2 led to a dramatic 4-h advance in sleep-wake timing and an associated 1-h advance in intrinsic period in FASPS (24, 31), we previously hypothesized that a variable number tandem repeat (VNTR) polymorphism in PER3 (rs57875989) would also be associated with circadian period phenotypes because the polymorphism codes for an 18-aa length difference that contains multiple putative CK1ε phosphorylation sites (32). Indeed, delayed sleep-phase syndrome (DSPS) and evening preference are associated with polymorphisms in PER3 and in particular with the VNTR polymorphism (32–36). However, the absence of PER3 has been shown to have negligible effects on circadian period in mice (37, 38), and the VNTR polymorphism is thought now to exert its effects primarily through sleep regulatory mechanisms and not through effects on circadian period (39–42). Nevertheless, direct assessments of the effects of this or other clock gene variants on the period of the human central circadian pacemaker are not available. In fact, very few assessments of intrinsic circadian period are available for any circadian rhythm sleep disorder.
Assessment of the intrinsic period of the human central circadian pacemaker requires that the period of a reliable physiological marker of the central pacemaker can be monitored over a prolonged period of time (several days at least) while minimizing the influence of confounding factors, such as the light-dark cycle, feedback from the sleep-wake cycle, and other behaviors (19). Protocols include the classic free-run protocol in sighted individuals living in the laboratory (43); assessment of period in short-term constant routine or nap protocols (44–46); assessment of period of temperature and melatonin rhythms in blind individuals living in society (47, 48); and forced desynchrony protocols in which the sleep-wake cycle and associated light-dark cycles are scheduled to a noncircadian period, thereby distributing confounding factors uniformly across the circadian cycle (19, 49, 50). Theoretically, the latter protocol should provide the most robust assessment of central circadian period (19, 51). These protocols are very costly and labor intensive, and more recently, in vitro assessment of circadian period in peripheral tissues has been introduced as a potential alternative (46, 52, 53). Whether and how in vitro assessments of intrinsic period relate to in vivo assessments is an open question, because the latter reflect directly circadian oscillations driven by the central circadian clock of the brain, and in vitro protocols may only reflect the properties of peripheral circadian clocks in a particular cell type or tissue (6, 54, 55). The availability of systems allowing the determination of in vitro period on a large scale and at a low cost makes it an important question whether individual differences in the in vivo period of the central circadian pacemaker are reflected in circadian rhythms assessed in the in vitro systems. Some published data suggest that this is the case (46), while others highlight the potential confounding factors that could lead to differences between circadian periods assessed by in vivo and in vitro systems (56, 57). However, to date, no direct comparison of in vitro assessments to in vivo intrinsic period as assessed in a forced desynchrony protocol is available. We therefore assessed in vitro circadian periods in cultured fibroblasts from participants characterized for the PER3 VNTR polymorphism and compared those to the periods of the plasma melatonin rhythms assessed in a forced desynchrony protocol in the same individuals.
MATERIALS AND METHODS
Ethical permission and recruitment of human volunteers
The study was given a favorable ethical opinion by the University of Surrey Ethics Committee and conformed to the Declaration of Helsinki. A group of 271 young healthy male and female participants without sleep complaints were recruited through advertisements and posters. They all provided written informed consent and underwent a screening procedure that included multiple health questionnaires assessing physical and mental health, and psychological profile including personality, intelligence, and emotional status, as well as chronotype and sleep assessment (described in ref. 36).
Participants were also genotyped for the PER3 VNTR as described previously (36, 58), Thirty-six healthy individuals (19 females and 17 males) aged 20.5–32.4 yr, who passed a full physical examination and investigation including full blood count and coagulation screen, biochemical profile, and urine analyses for drugs of abuse, were selected to participate in the study. The sample, which was stratified for the 3 genotypes of the PER3 VNTR, comprised 14 PER35/5 (homozygous for the longer, 5-repeat allele), 8 PER34/5 (heterozygous), and 14 PER34/4 (homozygous for the shorter, 4-repeat allele) subjects. The protocol included 2 wk of sleep diary and actigraphy monitoring (Actiwatch L; Philips Respironics, Best, The Netherlands) before the laboratory study period. The data from the first week were used to compute the participants' habitual bedtime and wake time, which they were then required to maintain until admission to the sleep laboratory and compliance was monitored. Of the 36 individuals, 1 was excluded (only for the in vivo part of the study) without replacement after the adaptation night for medical reasons. Thus, 35 continued in the in vivo study, and complete melatonin data for reliable assessment of in vivo circadian period were obtained in 31 subjects.
Assessment of circadian period in vivo
This version of the forced desynchrony protocol was modified from Dijk et al. (59) and Czeisler et al. (49) and similar to the implementation by Scheer et al. (60). It lasted 9 calendar days (Fig. 1A). The protocol began with a baseline 8-h sleep episode at habitual bedtime, followed by a 16-h wake period. Following the second 16-h wake episode, subjects began the forced desynchrony segment of seven 28-h days (sleep/wake: 9.33/18.67 h). During the forced desynchrony segment, each successive sleep episode started 4 h later than the previous one. Subjects spent their scheduled sleep episodes in individual bedrooms and their scheduled wake episodes in a light-controlled communal area, which was shared with other participants. To minimize relative coordination between the 28-h day and the endogenous circadian rhythm, the laboratory environment was maintained free of time cues, light levels were kept low (<5 lux) during the waking periods, and participants slept in darkness (19).
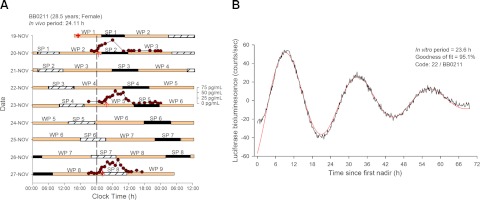
Figure 1.
A) Double raster plot of the forced desynchrony protocol of participant BB0211. A cutaneous biopsy (+) was collected during the baseline wake period (WP1) for isolating fibroblast cells used for the in vitro period assessment. After an 8-h baseline sleep period (SP1), and a 16-h baseline wake period (WP2), the participant was scheduled to a 28-h sleep-wake cycle consisting of sleep periods (black bars) of 9 h:20 min (SP2-8) and wake periods (tan bars) of 18 h:40 min (WP3-8). To better illustrate the timing of the sleep periods, consecutive sleep periods are plotted both next to (hatched and black boxes) and below each other. Melatonin profiles assessed from 3 blood sampling periods are plotted (dark red circles) together with the timing of the dim light melatonin onset (red arrows). DLMOs occurred at 00:29 (SP2); 03:25 (WP5) and 01:30 (SP8), and the derived in vivo period was 24.11. B) Detrended Bmal1-luciferase oscillation in fibroblasts from BB0211. The three full cycles included (from first nadir) in the analysis are shown in the graph, after detrending by subtracting the 24-h moving average of the data included from the raw data. The red curve indicates the largest sinusoidal component (Sin fit) in the detrended data, with its period and goodness of fit (% of variance).
To determine the in vivo circadian period, plasma samples, with a scheduled sampling frequency of 1 to 2 h, were collected during 28-h periods on 3 evenly spaced occasions, as shown in Fig. 1A. During the first and third occasions, plasma sampling was initiated several hours before the start of the sleep episode, which in this segment of the protocol coincided with the participants' habitual bedtime. The second sampling occasion coincided with the segment of the forced desynchrony, where the sleep episode started 12 h out of phase with the participants' habitual bedtime. Plasma samples were frozen at −20°C until use for melatonin quantification. Melatonin levels were quantified using radioimmunoassay (Stockgrand Ltd, Guildford, UK). The phase of the melatonin rhythm was defined as the time when melatonin concentration levels reached 25% of the amplitude of the melatonin rhythm [dim light melatonin onset (DLMO)]; a linear interpolation between the melatonin values just above and just below the 25% value was used to identify this time (61). The amplitude of the melatonin rhythm was defined as the maximum melatonin concentration in a 24-h period minus the baseline values (values observed during the day), with the melatonin maximum value being the median of the 3 highest values. The slope from a linear regression fitted to the 3 DLMOs was used to compute the in vivo circadian period for each participant (τ=24 h+slope; ref. 48). Because of incomplete data collection during the second sampling occasion, in one of the 31 participants only 2 DLMOs were available for computing the circadian period.
Choice of in vitro system
Although melatonin is produced in human skin (62), it would not be a convenient marker of the fibroblast circadian clock, mainly because its synthesis is not rhythmic (63). We therefore used the Bmal1-luc assay in which expression of firefly luciferase is driven by the promoter of the circadian gene Arntl (Bmal1). This approach is well established in the literature (52, 64) and therefore allows us to compare our data to the literature.
Lentivirus production and titration
Lentivirus was constructed by cotransfection of the envelope plasmid pMD2.G and the packaging plasmid psPAX2 (both originally constructed in D. Trono's laboratory, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland) into HEK293T cells (Thermo Fisher Scientific, Waltham, MA, USA). The lentiviral particles contained a reporter construct (pBluFpuro) in which expression of firefly luciferase is driven by the mouse circadian Bmal1 promoter (Bmal1::luc), as described previously (52, 64). All plasmids were generously provided by Professor S. A. Brown (University of Zürich, Zurich, Switzerland).
HEK293T cells were cultivated in 150-cm2 flasks with 30 ml of HEK medium [Dulbecco's modified Eagle medium (DMEM) including Glutamax (Life Technologies, Carlsbad, CA, USA), 10% fetal calf serum (FCS; Thermo Fisher Scientific), 1% penicillin-streptomycin (Life Technologies), and 1 mM sodium pyruvate (Sigma-Aldrich, St Louis, MO, USA)] at 37°C and 5% CO2. These cells were transfected when 70–90% confluent. For each 150-cm2 flask, 10.5 μg of plasmid (pMD2.G and psPAX2, 3 μg for each; pBluFpuro, 4.5 μg) at a concentration of 100 ng/μl was precomplexed with 30 μl of X-tremeGene 9 DNA Transfection Reagent (Roche, Basel, Switzerland) in 865 μl of Optimem (Life Technologies) at ambient temperature for 30 min. Medium was then aspirated from the HEK293T cells and replaced with 20 ml of fresh HEK medium supplemented with the transfection reagent:DNA complex. After 21 h at 37°C, transfection medium was aspirated and replaced with HEK medium (20 ml/flask). At 48 h post-transfection, the lentivirus-containing HEK medium was collected and replaced with fresh HEK medium. After a further 24 h (72 h post-transfection), the lentivirus-containing HEK medium was again collected. On collection, each batch was cleared of cell debris by centrifugation (3300 g, 10 min), and the resulting supernatant was sterilized by passing through 0.22-μm filters. Lentivirus particles were pelleted by centrifugation (3500 g for 22 h at 4°C), resuspended in 50 μl of serum-free DMEM, and kept in aliquots at −80°C until use. The stocks of lentivirus were titrated using QuickTiter Lentivirus Quantitation Kit (Cell Biolabs, San Diego, CA, USA) to enable us to use the same amount of lentivirus particles for the infection of each replicate of fibroblast cultures, thereby minimizing any possible variation on the protocol and consequently on the in vitro data.
Fibroblast isolation, infection, and synchronization
Fibroblasts were successfully isolated (as detailed below) from 32 participants including 4 subjects for whom in vivo period was not available. In the middle of the afternoon on the day before the scheduled baseline day of the forced desynchrony protocol (red cross in Fig. 1A), one cutaneous biopsy (2 mm diameter) per subject was collected from the upper buttock, and fibroblast cultures were generated as described elsewhere (46, 52). Briefly, the cells were cultivated in 35-mm dishes with 0.2 ml of fibroblast medium [DMEM including 2 mM glutamine (Thermo Fisher Scientific), 20% FCS and 1% amphotericin B (Sigma Aldrich)] at 37°C and 5% CO2. From 3 to 7 replicates of fibroblasts per individual were cultivated and used to assess circadian period. On reaching 40–50% confluence, each 35-mm dish of fibroblasts was infected with 6 × 1010 lentivirus particles dissolved in 2 ml DMEM including Glutamax, 20% FCS, 1% penicillin–streptomycin, and 8 μg/ml protamine sulfate (Sigma-Aldrich). Afer 6 d, fibroblast circadian rhythms were synchronized by treatment with 150 nM dexamethasone (Sigma-Aldrich) (65). After rinsing the cells twice with phosphate-buffered saline, luciferin medium [DMEM without phenol red, including 2 mM l-glutamine, 25 mM hydroxyethyl piperazineethanesulfonic acid (HEPES) buffer (Life Technologies), 20% FCS, and 1% penicillin-streptomycin] supplemented with 0.1mM of endotoxin-free beetle luciferin (Promega, Madison, WI, USA) was added to each dish. Bioluminescence was measured using a real-time (10-min resolution) and light-tight luminometry device (LumiCycle; Actimetrics, Wilmette, IL, USA) directly within the culture incubator for 6–7 d at 37°C (8, 52). During the process of expansion of fibroblast cultures (from fibroblast isolation until bioluminescence measurement), the passage number (≤1 passage difference between cell cultures) and confluence state of our cells were tightly controlled in order to minimize any potential effects on the in vitro data.
Assessment of circadian period in vitro
For each replicate of fibroblast measurements, the first circadian cycle was excluded from the analysis, as it may vary according to the condition of synchronization (52) and acute effects of dexamethasone. Thereafter, 3 full cycles were included between the first and fourth nadir from the measurement, as shown in an example (Fig. 1B). The 24-h moving average of the 3 cycles was subtracted from the raw data, in order to remove any baseline drift. Next, circadian period was computed with LumiCycle Analysis 2.44 software (46), which identifies the sinusoidal component (Sin fit) with the largest amplitude (from the amplitude spectrum) in the data. Then, the damping of the oscillations was accounted for using a negative exponential function. Our luminometry device allowed simultaneous assessment of 32 cultures. In general, we assessed period simultaneously in as many subjects as possible, and replicates within subjects were run on separate occasions. All analyses were conducted in a procedure blinded to genotype. An individual's period was calculated as the average of the replicates.
Statistical analysis
Data were analyzed using SAS v9.1 and v9.2 (SAS Institute, Cary, NC, USA). Procedure Corr was used to assess Spearman's rank-order correlations between variables. Correlations were computed using the nonparametric model, which converts each variable to ranks. Nonparametric correlations were chosen because nonparametric methods are more robust, as they rely on fewer assumptions (such as a monotonic relationship, which is less restrictive than the linear relationship in the parametric correlations). We used procedure Univariate for the Kolmogorov-Smirnov 1-sample test (most conservative for test of normality) to check that the frequency distributions of in vivo and in vitro periods are normally distributed, and also for the inspection of the probability plots of the residuals for ascertaining the normal distributions. Procedure NPAR1WAY [Kolmogorov-Smirnov 2-sample test (asymptotic)] was also used for the comparison of the in vivo with the in vitro distributions. To compare in vivo and in vitro periods and to assess the effect of genotype and subject on the circadian period, procedure Mixed for analysis of variance was applied. We used an unstructured covariance structure in these analyses. Procedure Mixed was also used to compute intraclass correlation coefficients (ICCs; reliability coefficients). ICCs were computed with their 95% confidence intervals (CIs), as described previously (66). The ICC indicates the ratio of the between-subject variance to the total variance (within-subject variance plus between-subject variance). The ICC can have any value between 0 and 1; values > 0.5 (moderate to high ICC) are indicative of stability of observations (replicates) within an individual. The ICC was used to assess individuality and stability of a particular individual's repeated measurements of in vitro period.
RESULTS
In vitro circadian period in human fibroblast is longer than in vivo period in healthy individuals
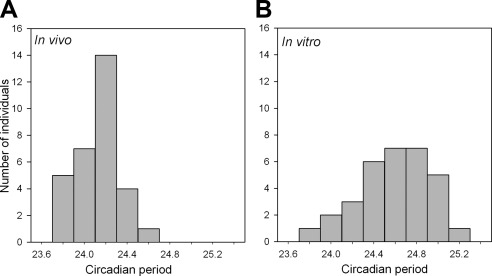
In total, data from 35 subjects were available (see Table 1). In the forced desynchrony protocol, the timing of the plasma melatonin rhythm drifted to a later hour in most participants (see example in Fig. 1A), i.e., the in vivo period was on average longer than 24 h. The frequency distribution of the in vivo periods did not deviate significantly (P>0.15) from a normal distribution. In vitro circadian rhythmicity could be reliably detected in 32 subjects. The frequency distribution of the in vitro periods also did not deviate significantly (P>0.15) from a normal distribution, but the range of periods was wider for the in vitro periods (23.7<τinvitro≤25.3 vs. 23.7<τinvivo≤24.7; Fig. 2). In vitro periods (n=32, 24.61±0.33 h, average±sd) were significantly longer than in vivo periods (n=31, 24.16±0.17 h; P<0.0001). The two distributions were statistically compared and found to be significantly different (KSa=2.848; P>KSa<0.0001).
Table 1.
Demographics
| Variable | Genotype |
Total (n) | ||
|---|---|---|---|---|
| PER34/4 | PER34/5 | PER35/5 | ||
| Gender | ||||
| Male (n) | 7 | 3 | 7 | 17 |
| Female (n) | 7 | 5 | 6 | 18 |
| Total (n) | 14 | 8 | 13 | 35 |
| Age (yr) | 25.8 ± 3.6 | 25.6 ± 3.2 | 25.5 ± 3.5 | 25.6 ± 3.4 |
| BMI (kg/m2) | 22.2 ± 1.9 | 22.6 ± 2.6 | 21.9 ± 2.8 | 22.2 ± 2.4 |
| MEQ | 50.1 ± 7.8 | 50.3 ± 8.5 | 49.5 ± 9.3 | 22.2 ± 2.4 |
Demographics of the 35 subjects for whom we have in vivo and/or in vitro periods. In 28 subjects, both in vivo and in vitro period assessments were available. In 4 subjects only in vitro periods and in three 3 subjects only in vivo period assessments were available. Values are means ± sd. BMI, body mass index; MEQ, morningness-eveningness questionnaire.
Figure 2.
Frequency distributions of in vivo (A; n=31) and in vitro (B; n=32) circadian periods (τ). Numbers of observations (individuals) were assessed for 8 period bins of 0.2 h each (e.g., 23.7<τ≤23.9, 23.9<τ≤24.1).
Replicability of in vitro circadian period measurements within individuals
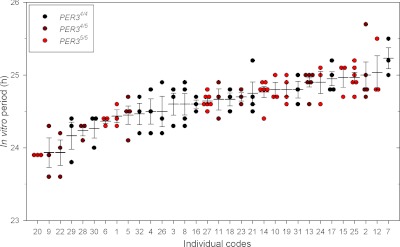
Individuality and stability of the in vitro period among the 116 measurements for the 32 subjects (3.6 replicates/individual on average) were assessed by plotting all individual observations (Fig. 3). The data show relatively low within-subject variation and also considerable between-subject variation. Analysis of variance to assess the effect of subject on the 116 in vitro measurements yielded a significant effect of subject (P<0.0001; procedure Mixed). The ICC was 0.62 (95% CI: 0.45–0.76). This confirms individuality and stability of period in these 32 sets of human fibroblasts.
Figure 3.
Individuality and stability of in vitro period assessments in 32 participants. In each participant, 3–7 replicates of fibroblasts were cultivated and used to assess in vitro period. Each assessment (dots) as well as the mean ± se (horizontal lines) is plotted for each participant. Data are ordered from short to long periods. PER34/4: n = 13 (black circles); PER34/5: n = 8 (dark red circles); PER35/5: n = 11 (red circles). Overall ICC (95% CI) = 0.62 (0.45, 0.76), confirming individuality in these 32 human fibroblasts. Coefficient of within-individual variance (95% CI) = 0.01 (0.01, 0.01).
Validity of in vivo circadian period as a measure of endogenous circadian rhythmicity
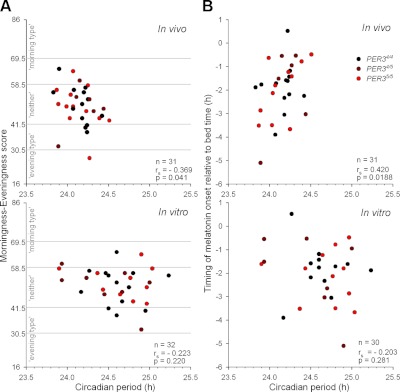
A valid measure of intrinsic period of the central circadian pacemaker should predict the timing of physiology and behavior during entrainment. We therefore assessed the association between both the in vivo and in vitro periods and two markers of timing of rhythmicity during entrainment. The timing of melatonin relative to the start of the dark onset (i.e., habitual bedtime), also known as the phase angle of entrainment, was evaluated. While no association was found for the in vitro period (P>0.2), a significant correlation was found between the in vivo period and the timing of melatonin onset (rs=0.420, P<0.05; Fig. 4B). This correlation was such that the shorter the observed in vivo period, the more advanced the timing of the melatonin rhythm relative to habitual bedtime.
Figure 4.
Association of in vivo (top panels) and in vitro periods (bottom panels) with morningness-eveningness (in vivo: n=31; in vitro: n=32; A) and the timing of melatonin onset relative to bedtime (B). Horizontal gray lines in A indicate cutoffs between diurnal types (16–30: definitely evening type; 31–41: moderately evening type; 42–58: neither type; 59–69: moderately evening type; 70–86: definitely morning type). B) Relationship between circadian periods (in vivo: n=31; in vitro: n=30) and timing of melatonin onset relative to habitual bedtime. Spearman correlations computed over all individuals are indicated at bottom right of each graph.
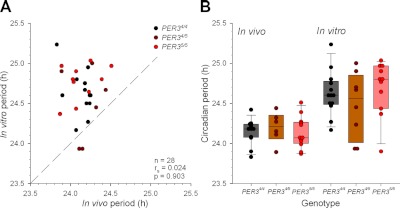
To establish whether the circadian periods were also related to diurnal preference, which to a large extent measures the preferred timing of sleep and waking activities, associations between in vivo and in vitro assessments and the morningness-eveningness questionnaire (MEQ) scores were computed. A significant negative correlation between the in vivo period and the MEQ score (rs=−0.369, P<0.05; Fig. 4A) was observed. The direction of this correlation shows that “morning types” have a shorter in vivo circadian period than “evening types.” For the in vitro periods, no significant correlations were observed, either with the timing of melatonin onset relative to habitual bedtime or the MEQ scores (P>0.2). Finally, in vitro and in vivo periods were directly compared (Fig. 5A). No significant correlation (P>0 .9) between in vivo and in vitro period assessments was observed.
Figure 5.
A) Association between in vivo and in vitro circadian periods. Dashed line represents the line of unity. B) In vivo (PER34/4: n=12; PER34/5: n=6; PER35/5: n=13) and in vitro (PER34/4: n=13; PER34/5: n=8; Per35/5: n=11) periods by genotype. Each dot represents the circadian period for each individual. Vertical box plot represents the median (midline) with the 25th (bottom rectangle) and 75th (top rectangle) percentiles, and errors bars show the 10th and 90th percentile values per genotype. Note that the 10th and 90th percentiles could not be computed in PER34/5 (n<9) because ≥9 individual data points are required.
Effects of PER3 VNTR genotype
There was no statistically significant effect of PER3 genotype on either the in vitro circadian periods (P>0.4), or in the in vivo periods (P>0.7; see distribution of periods among genotypes in Fig. 5B). Even when in vitro and in vivo periods were directly compared separately for each genotype, no significant correlation was found (see Table 2).
Table 2.
Summary statistics (Spearman's correlations)
| Variables compared and genotype |
In vivo |
In vitro |
||||
|---|---|---|---|---|---|---|
| n | rs | P | n | rs | P | |
| Circadian period vs. morningness-eveningness score | ||||||
| All | 31 | −0.369 | 0.041 | 32 | −0.223 | 0.220 |
| PER34/4 | 13 | −0.500 | 0.082 | 13 | −0.176 | 0.565 |
| PER34/5 | 6 | −0.029 | 0.957 | 8 | −0.491 | 0.217 |
| PER35/5 | 12 | −0.497 | 0.100 | 11 | −0.119 | 0.727 |
| Circadian period vs. timing of melatonin onset relative to habitual bed time | ||||||
| All | 31 | 0.420 | 0.019 | 30 | −0.203 | 0.281 |
| PER34/4 | 13 | 0.367 | 0.218 | 12 | −0.102 | 0.752 |
| PER34/5 | 6 | 0.371 | 0.469 | 7 | −0.433 | 0.333 |
| PER35/5 | 12 | 0.509 | 0.091 | 11 | −0.124 | 0.717 |
| In vivo period vs. in vitro period | ||||||
| All | 28 | 0.024 | 0.903 | |||
| PER34/4 | 12 | −0.282 | 0.375 | |||
| PER34/5 | 6 | 0.160 | 0.827 | |||
| PER35/5 | 10 | 0.404 | 0.247 | |||
Nonparametric correlations between in vivo and in vitro period and morningness-eveningness score, as well as timing of melatonin onset relative to habitual bedtime.
The ICCs computed per genotype showed that the PER35/5 [ICC (95% CI)=0.72 (0.46, 0.88), n=11 subjects, 4.0 replicates/subject on average] and PER34/5 [ICC=0.64 (0.33, 0.87), n=8, 3.8 replicates on average] had a higher replicability of repeated measurements per individual compared to the PER34/4 genotype [ICC=0.42 (0.16, 0.73), n=13, 3.2 replicates on average]. According to established benchmarks, the first two ICCs indicate high repeatability, whereas the third ICC indicates low repeatability (67).
DISCUSSION
The results of this study confirm several previous observations on the characteristics of human circadian rhythms and also provide new insights. The estimated in vivo period of 24.16 ± 0.17 h assessed with the forced desynchrony protocol, the gold standard method to determine the period of the central circadian pacemaker, is remarkably similar to a previously reported estimate of 24.15 ± 0.2 h (50). The melatonin rhythm, which is driven by the suprachiasmatic nucleus through a well described neural pathway to the pineal, is one of the most robust and widely used physiological markers of endogenous circadian timing (61). The validity of the in vivo assessment of melatonin as a measure of the period of the central circadian pacemaker was also confirmed by the significant correlation between period and the timing of melatonin during entrainment such that a shorter period was associated with an earlier timing, which is in accordance with previous reports (22, 23) and entrainment theory. In addition, this period assessment correlated with diurnal preference as reported by others (22) such that a shorter circadian period was associated with morningness. All of this suggests that this methodology to assess in vivo period is reliable, reproducible and valid, albeit labor intensive and expensive.
Assessments of circadian period in fibroblasts also confirmed several previous observations. Our estimate of the group average of fibroblast period of 24.61 ± 0.33 is similar to the values of 24.50 ± 0.75 and 24.63 ± 0.47 h published by others (refs. 46, 52, respectively). In the current study, in vitro periods were significantly longer than in vivo periods by 27 min on average. In a previous study (46) that used constant routine and nap protocols, the in vitro periods were longer by 40 min than the in vivo periods in sighted individuals (mean age: 39.80±19.27 yr) but not so in blind individuals (mean age: 55.38±7.54 yr).
Differences between in vivo and in vitro periods and their mutual association may depend on details of the protocol or participants. In our study in healthy individuals without circadian rhythm sleep disorders, in vivo periods did not correlate significantly with the in vitro periods. This contrasts with a recent study in which the in vitro periods were reported to be directly proportional to the in vivo periods (46). These discrepancies may be related to the fact that we used a forced desynchrony protocol, whereas in a previous study (46), constant routine (sighted subjects) or at-home measurements (blind subjects) were used. The sample size used to assess in vivo and in vitro period is substantially larger in the current study than in the individual studies in the previous study (46). However, the studies also differ in a number of other important respects (see Table 3). There is a considerable difference in age at biopsy collection between subjects in the current study (25.1±3.4 yr, n=28) and the previous study (Basel subjects: 51.11±22.79 yr, n=9; Novosibirsk subjects: 30.55±8.97 yr, n=11; Guildford blind subjects: 55.38±7.54 yr, n=8). In the current study, biopsy samples were obtained 1 d before the in vivo measurements, whereas in the previous study (46), biopsies were collected 2.44 ± 1.67 yr (Basel), 6.27 ± 2.00 yr (Novosibirsk), and 11.38 ± 1.85 yr (Guildford) after the in vivo assessment. In addition, the proportion of female subjects was 50.0% in our study, as compared to 33.3% (Basel), 81.8% (Novosibirsk) and 12.5% (Guildford) in Pagani et al.
Table 3.
Differences between the current study and a previous study (46)
| Laboratory | Female (%) | Sex (n) |
Subjects (N) | Average age at biopsy (yr) | Average time to biopsy | r | r2 | P | |
|---|---|---|---|---|---|---|---|---|---|
| F | M | ||||||||
| Basel | 33.33 | 3 | 6 | 9 | 51.11 | 2.44 yr | 0.679 | 0.460 | 0.0445 |
| Novosibirsk | 81.82 | 9 | 2 | 11 | 30.55 | 6.27 yr | 0.824 | 0.680 | 0.0018 |
| Guildford | 12.50 | 1 | 7 | 8 | 55.38 | 11.38 yr | 0.811 | 0.658 | 0.0268 |
| SSRC | 50.00 | 14 | 14 | 28 | 25.1 | 1 d | 0.024 | 0.00058 | 0.9029 |
SSRC, Surrey Sleep Research Centre.
The lack of correlation between in vivo and in vitro periods in our study and the moderate correlation observed in Pagani et al. (46) raises the question as to whether the in vitro period assessment is a reliable characteristic of an individual, given that it is different from the period of the central pacemaker. The high ICC in our study demonstrates that also for our in vitro periods, replicates from different subjects were both stable and specific to the individual. This individuality is observed even though it has become clear that in vitro period assessment is exquisitely sensitive to factors such as pH (56), and unidentified serum factors (57). This implies that meaningful peripheral fibroblast period assessment relies on in vivo signals. This is also in addition to any further confounding effects on the fibroblast clock that may be due to the overexpression of an artificial Bmal1 promoter construct. Thus, while in vitro period assessment may be reliable and reproducible within subjects, there are good reasons to explain why this would not reflect the in vivo period originating from the central circadian pacemaker.
Despite the fact that both in vitro and the in vivo periods are constant characteristics of the individual, the distributions of in vitro and in vivo period were very different (P<0.0001). This finding suggests that the parameters of peripheral rhythms are quite distinctive from central rhythms and can be interpreted as corroborating the differences between the central and peripheral oscillators with respect to molecular mechanisms (68) as well as the coupling of individual oscillators (9, 10, 69). These differences between central and peripheral oscillators are well emphasized in a recent study that measured period and phase in multiple tissues in wild-type and Per3−/− mice (11). Thus, the different period estimates may reflect some tissue-specific control of circadian clocks (6).
The PER3 VNTR genotype had no effect on circadian period assessed either in vivo or in vitro. According to the ICCs computed in each genotype for the in vitro period data, the higher replicability in PER35/5 may indicate that the circadian rhythms in this genotype are more robust, and may be related to the fact that PER35/5 homozygotes in one of our previous studies (40) had stronger correlations between melatonin and sleep-wake timing. Large-sample epidemiological studies have shown that the PER3 VNTR polymorphism associates with individual differences in sleep timing and thus represents a possible genetic regulator of human circadian rhythms (32, 35, 36, 41). Subsequent laboratory studies in smaller samples have suggested that these effects are mediated by changes in sleep homeostasis, and no effect of this polymorphism on the circadian phase or amplitude of cortisol, melatonin and RNA levels of several clock genes in leukocytes was found in young adults (39, 40), although a small effect of the PER3 VNTR on circadian phase in older individuals was recently reported (70). The absence of an effect of the polymorphism on either in vivo or in vitro period is in accordance with those data in young adults, although our current sample size will not have allowed us to detect small differences in period.
In summary, our data show that the period of circadian rhythms at the peripheral level can be reliably assessed in fibroblasts and is stable within an individual. Within this sample of healthy individuals without circadian rhythm sleep disorders, in vitro period does not correlate with the in vivo period driven by the central circadian pacemaker. However, in our present sample in vivo period is a determinant of differences in the timing of behavior. These data provide insights into the differences between in vivo and in vitro circadian rhythmicity in humans and have implications for the use of in vitro assessments in sleep and circadian medicine.
Acknowledgments
The authors thank Prof. S. A. Brown (Institute of Pharmacology and Toxicology, University of Zurich, Zurich, Switzerland) for generously providing the lentiviral plasmids, Dr. E. Maywood (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK) for providing training related to the real-time luminometry technique, Dr. W. Qasim and E. Chan (Institute of Child Health, University College London, London, UK) for training in lentivirus production, P. McGabe for statistical advice, and the staff of the Surrey Clinical Research Centre for their help.
This research was supported by the UK Biotechnology and Biological Sciences Research Council, grant BB/F022883/1.
Footnotes
- CI
- confidence interval
- CK1
- casein kinase I
- DLMO
- dim light melatonin onset
- DMEM
- Dulbecco's modified Eagle medium
- DSPS
- delayed sleep-phase syndrome
- FASPS
- familial advanced sleep-phase syndrome
- FCS
- fetal calf serum
- ICC
- intraclass correlation
- MEQ
- morningness-eveningness questionnaire
- VNTR
- variable number tandem repeat
REFERENCES
- 1. Hastings M. H., Maywood E. S., O'Neill J. S. (2008) Cellular circadian pacemaking and the role of cytosolic rhythms. Curr. Biol. 18, R805–R815 [DOI] [PubMed] [Google Scholar]
- 2. Boggild H., Knutsson A. (1999) Shift work, risk factors and cardiovascular disease. Scand. J. Work. Environ. Health 25, 85–99 [DOI] [PubMed] [Google Scholar]
- 3. Davis S., Mirick D. K. (2006) Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control 17, 539–545 [DOI] [PubMed] [Google Scholar]
- 4. Barion A. (2011) Circadian rhythm sleep disorders. Dis. Mon. 57, 423–437 [DOI] [PubMed] [Google Scholar]
- 5. Lee C., Etchegaray J. P., Cagampang F. R., Loudon A. S., Reppert S. M. (2001) Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107, 855–867 [DOI] [PubMed] [Google Scholar]
- 6. Takahashi J. S., Hong H. K., Ko C. H., McDearmon E. L. (2008) The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reddy A. B., O'Neill J. S. (2011) Metaclocks. EMBO Rep. 12, 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamazaki S., Numano R., Abe M., Hida A., Takahashi R., Ueda M., Block G. D., Sakaki Y., Menaker M., Tei H. (2000) Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 [DOI] [PubMed] [Google Scholar]
- 9. Kowalska E., Brown S. A. (2007) Peripheral clocks: keeping up with the master clock. Cold Spring Harb. Symp. Quant. Biol. 72, 301–305 [DOI] [PubMed] [Google Scholar]
- 10. Pendergast J. S., Friday R. C., Yamazaki S. (2010) Distinct functions of Period2 and Period3 in the mouse circadian system revealed by in vitro analysis. PLoS One 5, e8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pendergast J. S., Niswender K. D., Yamazaki S. (2012) Tissue-specific function of period3 in circadian rhythmicity. PLoS One 7, e30254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H. M., Chen R., Kim H., Etchegaray J. P., Weaver D. R., Lee C. (2011) The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc. Natl. Acad. Sci. U. S. A. 108, 16451–16456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng Q. J., Logunova L., Maywood E. S., Gallego M., Lebiecki J., Brown T. M., Sladek M., Semikhodskii A. S., Glossop N. R., Piggins H. D., Chesham J. E., Bechtold D. A., Yoo S. H., Takahashi J. S., Virshup D. M., Boot-Handford R. P., Hastings M. H., Loudon A. S. (2008) Setting clock speed in mammals: the CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron 58, 78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schibler U., Ripperger J., Brown S. A. (2003) Peripheral circadian oscillators in mammals: time and food. J. Biol. Rhythms 18, 250–260 [DOI] [PubMed] [Google Scholar]
- 15. Guo H., Brewer J. M., Lehman M. N., Bittman E. L. (2006) Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J. Neurosci. 26, 6406–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stratmann M., Schibler U. (2006) Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J. Biol. Rhythms 21, 494–506 [DOI] [PubMed] [Google Scholar]
- 17. Duffy J. F., Kronauer R. E., Czeisler C. A. (1996) Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J. Physiol. 495(Pt. 1), 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pittendrigh C. S., Daan S. (1976) A functional analysis of circadian pacemakers in nocturnal rodents IV, entrainment: pacemaker as clock. J. Comp. Physiol. 106, 291–331 [Google Scholar]
- 19. Klerman E. B., Dijk D. J., Kronauer R. E., Czeisler C. A. (1996) Simulations of light effects on the human circadian pacemaker: implications for assessment of intrinsic period. Am. J. Physiol. 270, R271–R282 [DOI] [PubMed] [Google Scholar]
- 20. Daan S. (2000) The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jurgen Aschoff, and the natural entrainment of circadian systems. J. Biol. Rhythms 15, 195–207 [DOI] [PubMed] [Google Scholar]
- 21. Sharma V. K., Chandrashekaran M. K., Singaravel M. (1998) Relationship between period and phase angle differences in Mus booduga under abrupt versus gradual light-dark transitions. Naturwissenschaften 85, 183–186 [DOI] [PubMed] [Google Scholar]
- 22. Duffy J. F., Rimmer D. W., Czeisler C. A. (2001) Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115, 895–899 [DOI] [PubMed] [Google Scholar]
- 23. Wright K. P., Jr., Gronfier C., Duffy J. F., Czeisler C. A. (2005) Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythms 20, 168–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toh K. L., Jones C. R., He Y., Eide E. J., Hinz W. A., Virshup D. M., Ptacek L. J., Fu Y. H. (2001) An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291, 1040–1043 [DOI] [PubMed] [Google Scholar]
- 25. Xu Y., Padiath Q. S., Shapiro R. E., Jones C. R., Wu S. C., Saigoh N., Saigoh K., Ptacek L. J., Fu Y. H. (2005) Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 434, 640–644 [DOI] [PubMed] [Google Scholar]
- 26. Akashi M., Tsuchiya Y., Yoshino T., Nishida E. (2002) Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol. Cell. Biol. 22, 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takano A., Isojima Y., Nagai K. (2004) Identification of mPer1 phosphorylation sites responsible for the nuclear entry. J. Biol. Chem. 279, 32578–32585 [DOI] [PubMed] [Google Scholar]
- 28. Yagita K., Yamaguchi S., Tamanini F., van Der Horst G. T., Hoeijmakers J. H., Yasui A., Loros J. J., Dunlap J. C., Okamura H. (2000) Dimerization and nuclear entry of mPER proteins in mammalian cells. Genes Dev. 14, 1353–1363 [PMC free article] [PubMed] [Google Scholar]
- 29. Loop S., Pieler T. (2005) Nuclear import of mPER3 in Xenopus oocytes and HeLa cells requires complex formation with mPER1. FEBS J. 272, 3714–3724 [DOI] [PubMed] [Google Scholar]
- 30. Walton K. M., Fisher K., Rubitski D., Marconi M., Meng Q. J., Sladek M., Adams J., Bass M., Chandrasekaran R., Butler T., Griffor M., Rajamohan F., Serpa M., Chen Y., Claffey M., Hastings M., Loudon A., Maywood E., Ohren J., Doran A., Wager T. T. (2009) Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J. Pharmacol. Exp. Ther. 330, 430–439 [DOI] [PubMed] [Google Scholar]
- 31. Jones C. R., Campbell S. S., Zone S. E., Cooper F., DeSano A., Murphy P. J., Jones B., Czajkowski L., Ptacek L. J. (1999) Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat. Med. 5, 1062–1065 [DOI] [PubMed] [Google Scholar]
- 32. Archer S. N., Robilliard D. L., Skene D. J., Smits M., Williams A., Arendt J., von Schantz M. (2003) A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 26, 413–415 [DOI] [PubMed] [Google Scholar]
- 33. Ebisawa T., Uchiyama M., Kajimura N., Mishima K., Kamei Y., Katoh M., Watanabe T., Sekimoto M., Shibui K., Kim K., Kudo Y., Ozeki Y., Sugishita M., Toyoshima R., Inoue Y., Yamada N., Nagase T., Ozaki N., Ohara O., Ishida N., Okawa M., Takahashi K., Yamauchi T. (2001) Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2, 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pereira D. S., Tufik S., Louzada F. M., Benedito-Silva A. A., Lopez A. R., Lemos N. A., Korczak A. L., D'Almeida V., Pedrazzoli M. (2005) Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep 28, 29–32 [PubMed] [Google Scholar]
- 35. Archer S. N., Carpen J. D., Gibson M., Lim G. H., Johnston J. D., Skene D. J., von Schantz M. (2010) Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep 33, 695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lazar A. S., Slak A., Lo J. C., Santhi N., von Schantz M., Archer S. N., Groeger J. A., Dijk D. J. (2012) Sleep, diurnal preference, health and psychological well-being: A prospective single-allelic-variation study. Chronobiol. Int. 29, 131–146 [DOI] [PubMed] [Google Scholar]
- 37. Shearman L. P., Jin X., Lee C., Reppert S. M., Weaver D. R. (2000) Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol. Cell. Biol. 20, 6269–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van der Veen D. R., Archer S. N. (2010) Light-dependent behavioral phenotypes in PER3-deficient mice. J. Biol. Rhythms 25, 3–8 [DOI] [PubMed] [Google Scholar]
- 39. Viola A. U., Archer S. N., James L. M., Groeger J. A., Lo J. C., Skene D. J., von Schantz M., Dijk D. J. (2007) PER3 polymorphism predicts sleep structure and waking performance. Curr. Biol. 17, 613–618 [DOI] [PubMed] [Google Scholar]
- 40. Archer S. N., Viola A. U., Kyriakopoulou V., von Schantz M., Dijk D. J. (2008) Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep 31, 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dijk D. J., Archer S. N. (2010) PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med. Rev. 14, 151–160 [DOI] [PubMed] [Google Scholar]
- 42. Hasan S., van der Veen D. R., Winsky-Sommerer R., Dijk D. J., Archer S. N. (2011) Altered sleep and behavioral activity phenotypes in PER3-deficient mice. Am. J. Physiol. 301, R1821–1830 [DOI] [PubMed] [Google Scholar]
- 43. Aschoff J. (1965) Circadian rhythms in man. Science 148, 1427–1432 [DOI] [PubMed] [Google Scholar]
- 44. Carskadon M. A., Dement W. C. (1980) Distribution of REM sleep on a 90 minute sleep-wake schedule. Sleep 2, 309–317 [PubMed] [Google Scholar]
- 45. Munch M., Knoblauch V., Blatter K., Schroder C., Schnitzler C., Krauchi K., Wirz-Justice A., Cajochen C. (2005) Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol. Aging 26, 1307–1319 [DOI] [PubMed] [Google Scholar]
- 46. Pagani L., Semenova E. A., Moriggi E., Revell V. L., Hack L. M., Lockley S. W., Arendt J., Skene D. J., Meier F., Izakovic J., Wirz-Justice A., Cajochen C., Sergeeva O. J., Cheresiz S. V., Danilenko K. V., Eckert A., Brown S. A. (2010) The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS One 5, e13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lewy A. J., Newsome D. A. (1983) Different types of melatonin circadian secretory rhythms in some blind subjects. J. Clin. Endocrinol. Metab. 56, 1103–1107 [DOI] [PubMed] [Google Scholar]
- 48. Lockley S. W., Skene D. J., Arendt J., Tabandeh H., Bird A. C., Defrance R. (1997) Relationship between melatonin rhythms and visual loss in the blind. J. Clin. Endocrinol. Metab. 82, 3763–3770 [DOI] [PubMed] [Google Scholar]
- 49. Czeisler C. A., Duffy J. F., Shanahan T. L., Brown E. N., Mitchell J. F., Rimmer D. W., Ronda J. M., Silva E. J., Allan J. S., Emens J. S., Dijk D. J., Kronauer R. E. (1999) Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181 [DOI] [PubMed] [Google Scholar]
- 50. Duffy J. F., Cain S. W., Chang A. M., Phillips A. J., Munch M. Y., Gronfier C., Wyatt J. K., Dijk D. J., Wright K. P., Jr., Czeisler C. A. (2011) Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl. 3), 15602–15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Czeisler C. A., Dijk D. J., Kronauer R. E., Brown E. N., Duffy J. F., Allan J. S., Shanahan T. L., Rimmer D. W., Ronda J. M., Mitchell J. F., Silva E. J., Emens J. S. (2000) Is there an intrinsic period of the circadian clock? Response. Science 288, 1174–1175 [PubMed] [Google Scholar]
- 52. Brown S. A., Fleury-Olela F., Nagoshi E., Hauser C., Juge C., Meier C. A., Chicheportiche R., Dayer J. M., Albrecht U., Schibler U. (2005) The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 3, e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Akashi M., Soma H., Yamamoto T., Tsugitomi A., Yamashita S., Yamamoto T., Nishida E., Yasuda A., Liao J. K., Node K. (2010) Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc. Natl. Acad. Sci. U. S. A. 107, 15643–15648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McDearmon E. L., Patel K. N., Ko C. H., Walisser J. A., Schook A. C., Chong J. L., Wilsbacher L. D., Song E. J., Hong H. K., Bradfield C. A., Takahashi J. S. (2006) Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science 314, 1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kornmann B., Schaad O., Bujard H., Takahashi J. S., Schibler U. (2007) System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 5, e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lee S. K., Achieng E., Maddox C., Chen S. C., Iuvone P. M., Fukuhara C. (2011) Extracellular low pH affects circadian rhythm expression in human primary fibroblasts. Biochem. Biophys. Res. Commun. 416, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pagani L., Schmitt K., Meier F., Izakovic J., Roemer K., Viola A., Cajochen C., Wirz-Justice A., Brown S. A., Eckert A. (2011) Serum factors in older individuals change cellular clock properties. Proc. Natl. Acad. Sci. U. S. A. 108, 7218–7223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vandewalle G., Archer S. N., Wuillaume C., Balteau E., Degueldre C., Luxen A., Maquet P., Dijk D. J. (2009) Functional magnetic resonance imaging-assessed brain responses during an executive task depend on interaction of sleep homeostasis, circadian phase, and PER3 genotype. J. Neurosci. 29, 7948–7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dijk D. J., Czeisler C. A. (1995) Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 15, 3526–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. U. S. A. 106, 4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klerman E. B., Gershengorn H. B., Duffy J. F., Kronauer R. E. (2002) Comparisons of the variability of three markers of the human circadian pacemaker. J. Biol. Rhythms 17, 181–193 [DOI] [PubMed] [Google Scholar]
- 62. Slominski A., Pisarchik A., Semak I., Sweatman T., Wortsman J., Szczesniewski A., Slugocki G., McNulty J., Kauser S., Tobin D. J., Jing C., Johansson O. (2002) Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 16, 896–898 [DOI] [PubMed] [Google Scholar]
- 63. Slominski A., Tobin D. J., Zmijewski M. A., Wortsman J., Paus R. (2008) Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 19, 17–24 [DOI] [PubMed] [Google Scholar]
- 64. Brown S. A., Kunz D., Dumas A., Westermark P. O., Vanselow K., Tilmann-Wahnschaffe A., Herzel H., Kramer A. (2008) Molecular insights into human daily behavior. Proc. Natl. Acad. Sci. U. S. A. 105, 1602–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Balsalobre A., Brown S. A., Marcacci L., Tronche F., Kellendonk C., Reichardt H. M., Schutz G., Schibler U. (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 [DOI] [PubMed] [Google Scholar]
- 66. Hankinson S. E., Manson J. E., Spiegelman D., Willett W. C., Longcope C., Speizer F. E. (1995) Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol. Biomarkers Prev. 4, 649–654 [PubMed] [Google Scholar]
- 67. Clendenen T. V., Arslan A. A., Lokshin A. E., Idahl A., Hallmans G., Koenig K. L., Marrangoni A. M., Nolen B. M., Ohlson N., Zeleniuch-Jacquotte A., Lundin E. (2010) Temporal reliability of cytokines and growth factors in EDTA plasma. BMC Res. Notes 3, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peirson S. N., Butler J. N., Duffield G. E., Takher S., Sharma P., Foster R. G. (2006) Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem. Biophys. Res. Commun. 351, 800–807 [DOI] [PubMed] [Google Scholar]
- 69. Welsh D. K., Logothetis D. E., Meister M., Reppert S. M. (1995) Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14, 697–706 [DOI] [PubMed] [Google Scholar]
- 70. Viola A. U., Chellappa S. L., Archer S. N., Pugin F., Gotz T., Dijk D. J., Cajochen C. (2011) Interindividual differences in circadian rhythmicity and sleep homeostasis in older people: effect of a PER3 polymorphism. [E-pub ahead of print] Neurobiol. Aging doi: 10.1016/j.neurobiolaging.2011.10.024 [DOI] [PubMed] [Google Scholar]