Abstract
Focal adhesion (FA) formation and disassembly play an essential role in adherence and migration of endothelial cells. These processes are highly regulated and involve various signaling molecules that are not yet completely identified. Lnk [Src homology 2-B3 (SH2B3)] belongs to a family of SH2-containing proteins with important adaptor functions. In this study, we showed that Lnk distribution follows that of vinculin, localizing Lnk in FAs. Inhibition of Lnk by RNA interference resulted in decreased spreading, whereas sustained expression dramatically increases the number of focal and cell-matrix adhesions. We demonstrated that Lnk expression impairs FA turnover and cell migration and regulates β1-integrin-mediated signaling via Akt and GSK3β phosphorylation. Moreover, the α-parvin protein was identified as one of the molecular targets of Lnk responsible for impaired FA dynamics and cell migration. Finally, we established the ILK protein as a new molecular partner for Lnk and proposed a model in which Lnk regulates α-parvin expression through its interaction with ILK. Collectively, our results underline the adaptor Lnk as a novel and effective key regulator of integrin-mediated signaling controlling endothelial cell adhesion and migration.—Devallière, J., Chatelais, M., Fitau, J., Gérard, N., Hulin, P., Velazquez, L., Turner, C. E. Charreau, B. LNK (SH2B3) is a key regulator of integrin signaling in endothelial cells and targets α-parvin to control cell adhesion and migration.
Keywords: adaptor protein, integrin-linked kinase, cell motility
The migration of endothelial cells (ECs) is an essential step in vascular remodeling and regeneration events. The loss of endothelial function and integrity initiates a cascade of events that may lead to vascular disorders, including atherosclerosis, vein graft atherosclerosis, restenosis after percutaneous revascularization, and transplant arteriosclerosis (TA; ref. 1). EC migration is a mechanically integrated molecular process that involves dynamic and coordinated changes in cell adhesion and cytoskeleton organization (2, 3). It requires a series of steps, starting with cellular extension and formation of membrane protrusion, termed lamellipodia, that push the leading front, and finishing by cell contraction, allowing forward progression. Attachment of protrusions to the extracellular matrix (ECM) is mediated by integrins that function as receptors for cell-adhesion molecules.
Integrins are a large family of heterodimeric proteins. Their ligation with ECM promotes clustering and subsequent integrin-mediated intracellular signal transduction. Unlike growth factor receptors, integrins have short cytoplasmic tails with no intrinsic enzymatic activity. To integrate signals and activate intracellular signaling pathways, integrins cocluster with serine, threonine, and tyrosine kinases, phosphatases, and adaptor proteins in large multiprotein complexes referred to as cell-matrix adhesions. These complexes connect integrins with the actin cystoskeleton and enable extracellular signals transduction that regulates adhesion and motility (4), especially by controlling cytoskeletal remodeling. Several types of cell-matrix adhesions are described in adherent cells and are classified according to their structure and size. Among them, focal adhesions (FAs) are comprised of integrins; protein kinases, such as focal adhesion kinase (FAK) and Src; adaptor proteins, such as Shc; actin-binding cytoskeletal proteins, including talin, parvins, α-actinin, paxillin, tensin, and vinculin; and signaling intermediates, such as Rho family GTPases (5). Migrating cells need highly regulated signaling pathways, which are not yet fully understood, to coordinately disassemble cell adhesions at the trailing edge and reform multiple new contacts at the cell's front.
Lnk [Src homology 2-B3 (SH2B3)] belongs with the closely related proteins SH2B1 (also termed SH2-B) and SH2B2 (also termed APS) to a subfamily of SH2-containing proteins with adaptor functions (6–8). Substantial data established that these adaptor proteins are regulators of growth factor and cytokine receptor-mediated pathways (9, 10). The Lnk protein contains a NH2-terminal proline-rich region, a pleckstrin homology (PH) domain, a Src homology 2 (SH2) domain, and potential tyrosine phosphorylation sites. Lnk−/− mice display an abnormal accumulation of erythroid cells, megakaryocytes, and B lymphocytes in the different hematopoietic compartments, indicating a defect in lymphoid and myeloid homeostasis (11, 12). Lnk negatively regulates stem cell factor (SCF) and c-Kit receptor signaling in B-cell precursors (11, 13, 14), hematopoietic cells (15, 16), endothelial progenitor cells (17, 18), and mast cells (19, 20). Lnk deficiency causes increased signaling through cytokine receptors that are critical for growth of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs), such as c-Kit and thrombopoietin (TPO) receptor, c-mpl (11, 20, 21, 22).
Novel functions of Lnk have recently emerged, notably its effect on the integrin signaling of various cells. Lnk appears to regulate HSC/HPC interaction and trapping on vascular cell adhesion molecule 1 (VCAM-1), controlling progenitor motility and interactions with the stem cell niche (22). A role for Lnk in integrin-mediated signaling pathways was also reported in thrombopoiesis, in which Lnk regulates crosstalk between integrin and cytokine pathways during megakaryocytic maturation (9). Finally, Lnk modulates outside-in signaling of the integrin αIIbβ3 pathway in platelets (23). Loss of Lnk reduces the capacity of platelets to spread on fibrinogen and impairs Fyn kinase activation and recruitment, leading to reduced tyrosine phosphorylation of the β3-integrin subunit and destabilization of thrombus development in vivo (23). Whether Lnk may affect integrin-mediated cytoskeleton organization or cell adhesion, and migration in ECs is an important issue that we address in this study.
We previously reported that the Lnk adaptor is expressed in vascular ECs, where it is rapidly phosphorylated and subsequently up-regulated by the proinflammatory cytokine tumor necrosis factor (TNF; refs. 24, 25). We also demonstrated that Lnk down-regulates expression of cellular adhesion molecules (E-selectin and VCAM-1) in activated vascular ECs via negative control on the TNF signaling pathway involving both the phosphatidylinositol 3-kinase (PI3K) and the ERK1/2 MAPK (25). The present study further examines the signaling events triggered by Lnk in the endothelium. Our findings demonstrate that the Lnk adaptor is an effective regulator of the integrin-mediated signaling pathway that affects EC adhesion and migration processes. Our results also identify the integrin-linked kinase (ILK) and α-parvin proteins as a new molecular partner and target, respectively, of the Lnk adaptor, thus providing an additional mechanism for Lnk-mediated regulatory functions in ECs.
MATERIALS AND METHODS
Reagents and antibodies
Mouse monoclonal anti-β1-integrin (CD29, clone TS2/16) was purchased from Pierce (Rockford, IL, USA). Goat polyclonal anti-Lnk antibody used for Western blot analysis was obtained from Serotec (Cergy St Christophe, France; 1:500 dilution). Rabbit polyclonal anti-Lnk antibody used for microscopy study was produced by rabbit immunization with a peptide designed in the Lnk C-term region (Covalab, Villeurbanne, France). Antibodies were immunopurified, and anti-Lnk reactivity and specificity were analyzed by Western blotting. Rabbit polyclonal or monoclonal antibodies directed against total and phosphorylated forms of Akt (at Ser473), glycogen synthase kinase 3β (GSK3β; at Ser9), paxillin (Tyr118), FAK (Tyr397), and total forms of α-parvin and ILK1 were purchased from Cell Signaling Technology (CST; Ozyme, St Quentin Yveline, France; 1:1000 dilution). Mouse monoclonal vinculin and PINCH-1 antibodies were obtained from Sigma-Aldrich (Lyon, France). Anti-phosphotyrosine antibody (clone 4G10) was purchased from Upstate Biotechnology (Euromedex, Mundolsheim, France). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody from Chemicon (Temecula, CA, USA) and mouse anti-tubulin antibody from Oncogene (Merk EuroLab, Val de Fontenay, France) were used for protein level normalization (1:1000 dilution). Horseradish peroxidase conjugated anti-mouse (1:2000 dilution; CST), anti-rabbit (1:1000 dilution; CST) and anti-goat (1:500 dilution; Serotec) IgG antibodies were used as secondary antibodies.
EC isolation, culture, and activation
Human ECs were isolated from umbilical veins [human umbilical vein ECs (HUVECs)] or renal arteries [human arterial ECs (HAECs)] and grown in early passages (passages 2–6), as described previously (26). ECs were cultured in EC basal medium (ECBM; Promocell, Heidelberg, Germany) supplemented with 10% FCS, 0.4% EC growth supplement/heparin, hydrocortisone (1 μg/ml), human basic fibroblast growth factor (1 ng/ml), human epidermal growth factor (0.1 ng/ml), 100 U/ml penicillin (Life Technologies, Cergy Pontoise, France) and 0.1 mg/ml streptomycin (Life Technologies) at 37°C in a 5% CO2 humidified air incubator. Mouse ECs were isolated from hearts of wild-type (WT) or Lnk−/− 129SV mice, as previously described (11), and grown in MCDB 105 medium supplemented with 20% FSC, 2 mM l-glutamine, 1 mM sodium pyruvate (Life Technologies), 1% nonessential amino acids (Life Technologies), 100 U/ml penicillin, and 0.1 mg/ml streptomycin at 37°C in a 5% CO2 humidified air incubator. Before activation, confluent EC monolayers were maintained for 48 h in ECBM for human cells or MCDB 105 medium for mouse cells supplemented with only 2% FCS before incubation with 10 μg/ml of anti-CD29 (from 15 to 45 min).
Generation of recombinant adenoviral vector encoding human Lnk
The recombinant adenovirus AdLnk was produced in human embryonic kidney 293 cells by the vector core laboratory of the University Hospital of Nantes (INSERM UMR649 Gene Therapy Laboratory, Nantes, France), as previously described (25, 27). The recombinant adenovirus AdTrack-GFP was used as a control (AdGFP). HAECs were cultured in 6-well plates at 70% confluence and infected with a multiplicity of infection from 10 to 100 infectious particles/cell for AdLnk and AdGFP. Adenoviral infections were carried out in ECBM supplemented with 1% FCS at 37°C, 5% CO2 under agitation. Expression of GFP and Lnk were measured 24 h after infection.
siRNAs and transfections
The 21-nt synthetic siRNA duplexes were prepared by Dharmacon (Thermo Scientific, Lafayette, CO, USA). We used 2 siRNAs that mediate the silencing of Lnk expression, J-019961-10 (Lnk1) and J-019961-11 (Lnk2). The day before transfection, ECs were seeded at a density of 1.5 × 105 cells/well in 6-well plates. HAECs were transfected with siRNAs that specially target Lnk or with a 21-nt irrelevant RNA duplex as a control, using Lipofectamine RNAiMax (Invitrogen, Cergy Pontoise, France) at a final concentration of 20 nM. The cells were analyzed 48 h after transfection by RT-PCR. HUVECs were used for plasmid transfection experiments. Transfections were performed using Lipofectamine reagent in combination with Plus (Invitrogen) and Xpress-tagged WT and quintuple (Quint) mutant α-parvin constructs, as previously described (28, 29).
Immunofluorescence and image analysis
For immunofluorescence, ECs were grown on glass coverslips. After treatment, cultures were washed with PBS, fixed for 10 min in PBS containing 1% paraformaldehyde, and permeabilized with PBS containing 4% BSA (Sigma-Aldrich) and 0.1% saponin (Sigma-Aldrich) for 20 min. Cells were washed again with PBS and blocked with 10% donkey or goat serum diluted in PBS with 4% BSA and 0.1% saponin for 1 h. Cells were then incubated with anti-vinculin (1:400 dilution) and/or anti-Lnk (1:100 dilution) antibodies overnight at 4°C. Cells were rewashed and incubated for 1 h with goat anti-mouse Alexa Fluor 488 and/or goat anti-rabbit Alexa Fluor 568 (1:2000 dilution; Invitrogen) depending on experiments. 4′,6-Diamidino-2-phenylindole (DAPI; 1:30,000 dilution; Invitrogen) was used for nuclear staining and phalloidin-TRITC (1:1000 dilution; Sigma-Aldrich) for F-actin labeling. Cells were washed in PBS containing 1% BSA, and mounted with ProLong antifade reagent (Molecular Probes, Eugene, OR, USA). Specimens were examined by immunofluorescence microscopy using a Nikon Diaphot microscope (Nikon, Tokyo, Japan). Deconvolution of microscopic images was performed with Huygens Essential software (Scientific Volume Imaging, Thousand Oaks, CA, USA).
Colocalization assays
An ImageJ plugin (U.S. National Institutes of Health, Bethesda, MD, USA) was used for automated quantification and visualization of colocalized fluorescent signals. The algorithm used was originally described by Jaskolski et al. (30). This automated method computes correlation of intensities between pairs of individual pixels in two different channels and calculates a correlation index (Icorr). Results are presented as a colocalization color map, where hot colors represent positive correlation (colocalization) and cold colors represent negative correlation (exclusion).
Quantification of FAs
Image acquisition, processing, and analysis were performed using MetaMorph imaging software (Molecular Devices, Sunnyvale, CA, USA). The number of FAs in each cell was evaluated from photographs of vinculin staining. Scaled images acquired from noninfected (NI) or transduced cells were thresholded in order to define items corresponding to FAs. A filter (0.5 μm) was applied to discard background noise. Then, filtered FAs were counted, and the number of FAs was divided by the cell surface area to obtain the density of FAs.
Nocodazole assays
Cells were treated with 10 μM nocodazole for 2 h to completely depolymerize microtubules. The drug was washed away, and the chase was performed in complete medium for the indicated times. Cells were fixed and permeablized before processing for immunofluorecence. For microtubule visualization, cells were fixed in methanol at −20°C for 10 min, rehydrated in TBS, and stained with a mouse monoclonal antibody against tubulin (1:200 dilution).
Immunoprecipitation (IP) and Western blot analysis
Cells were lysed in RIPA buffer containing protease and phosphatase inhibitors (Sigma-Aldrich). Protein concentration was determined using BCA protein assay reagent (Pierce). IP assays were performed using Dynabeads protein A (Invitrogen). Cell lysates were incubated overnight at 4°C with protein A and 10 μg of anti-Lnk or anti-α-parvin antibodies according to the manufacturer's protocol. Cell lysates and IP were resolved by SDS-PAGE (10%), and proteins were transferred to nitrocellulose membranes (ECL Hybond; Amersham, Little Chalfont, UK) using a Trans-Blot SD semidry electrophoretic transfer cell (Bio-Rad, Marnes-la-Coquette, France). Then, membranes were subjected to immunoblot analysis using the primary antibodies described above and appropriate peroxidase-conjugated secondary antibodies. Antibody-bound proteins were detected using an enhanced chemiluminescence (ECL) kit (Amersham) and luminescent image analyzer LAS-4000 (Fujifilm, Tokyo, Japan). Image analysis and blot quantification were performed with Multi Gauge software (Fujifilm). Results shown are representative of ≥3 independent experiments.
Adhesion assays
Cells were seeded (2×104 cells/well) in 96-well plates precoated with 1% gelatin, and medium was removed 30, 40, 50, or 60 min after seeding in order to remove nonadherent cells. Medium was replaced by 100 μl 3-[4,5-dimethylthiazol-2-yl] 2,5-diphenyltetrazodium (MTT) per well (1 mg/ml) diluted in complete medium, and cells were incubated at 37°C for 4 h. Then MTT solution was replaced by DMSO (100 μl/well) and optical density (OD) was recorded at 550 nm. All assays were performed in triplicate and metabolic activity of infected and NI cells was shown to be positively correlated to cell number.
Migration assays
Wound-healing assays
Cells were plated in 24-well plates and grown until confluence in complete medium. The monolayers were then wounded by scratching with a 10-μl pipette tip (VWR, Fontenay sous Bois, France), and detached cells were removed by washing with medium. The cells were incubated in complete ECBM medium and observed using a microscope (DMI6000B; Leica Microsystems SAS, Rueil Malmaison, France) equipped with an ×40 objective lens (HCX FL plan) for the indicated time. Images of three different segments of the cell-free area were recorded with a CCD camera (Coolsnap HQ2; Photometrics Roper Scientific SAS, Evry, France), and migration distance of individual cells from the front in the three different segments of the wound was measured using MetaMorph imaging software.
Boyden chamber assays
The transwell inserts (Costar, Corning, NY, USA) were precoated with a solution of 1% gelatin in PBS, and 20% FCS was added to ECBM medium in the bottom chamber. After 48 h of silencing (siRNACtl and siRNALnk) and 24 h of infection (AdGFP and AdLnk), HAECs were suspended in a 100-μl aliquot of ECBM containing 1% FCS and were added to the upper chamber. After 18 h of incubation, numbers of cells that had migrated through the membrane were estimated using MTT colorimetric assay.
CYTOOchip experiments
To investigate the role of Lnk in cell migration, we used a novel micropatterning assay termed CYTOOchip motility, purchased from CYTOO, SA (Grenoble, France). In this model, 1-dimensional (1D) cell migration occurs along oriented fibronectin fibers. We established that ECs plated on single 10-μm lines mimic the 3D phenotype (Supplemental Fig. S1) and migrate continuously, as previously demonstrated (31). This assay allowed us to quantify cell velocity. Briefly, after treatment, HAECs were collected by trypsinization and diluted to a concentration of 5 × 104 cells/ml. Then, 100 μl of cell suspension was seeded onto lines of 10 μm width. After 25 min, nonadherent cells were removed by 3 washes of PBS, and adherent cells in 4 wells of a CYTOO chamber were imaged at 37°C, 5% CO2, using a microscope (DMI6000B; Leica Microsystems) equipped with an ×20 objective lens for the indicated time period. All parameters (velocity and distance) were quantified using MetaMorph imaging software.
Proximity ligation assay
Cells were fixed with 1% paraformaldehyde, and samples were incubated with rabbit antibodies against ILK and goat antibodies against Lnk. Secondary antibodies conjugated with oligonucleotides (PLA probes Minus and Plus; Olink Bioscience, Uppsala, Sweden) were then added and incubated. The Duolink proximity ligation assay (PLA-Duolink; Olink Bioscience) was then performed as instructed by the manufacturer. Each distinct red spot represents a single ILK-Lnk complex (radius <40 nm).
RT-PCR
RNA was isolated using TriZol reagent (Invitrogen) and treated with Turbo DNase (Ambion; Applied Biosystems, Foster City, CA, USA) before RT. Transcript levels were quantified by quantitative RT-PCR with the following primers and probes from Applied Biosystems: Lnk (Hs01081958_g1) and HPRT-1 (Hs99999909_m1). Quantitative PCRs were performed using the ABI PRISM 7900 sequence detection application program (PE Biosystems; Applied Biosystems). For quantification, triplicates were normalized by the concomitant quantification of hypoxanthine-guanine phosphoribosyl transferase (HPRT). Relative expression was calculated according to the 2−ΔΔCt method, as described previously (32).
Statistical analysis
Results are expressed as means ± se for replicate experiments. Statistical analysis was performed using GraphPad Prism software (GraphPad, San Diego, CA, USA) by the parametric analysis of variance test, as appropriate. A value of P < 0.05 was considered statistically significant.
RESULTS
Cellular distribution of Lnk in ECs
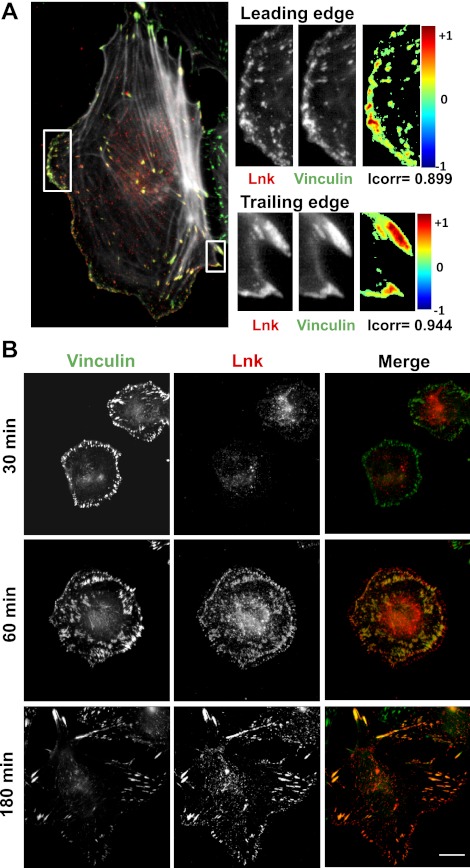
Although Lnk protein was shown to be expressed and to function in hematopoietic cells and ECs, limited data are available concerning its spatial location in cells (33, 34). To investigate the cellular localization of endogenous Lnk in human primary EC culture, we produced specific anti-Lnk rabbit polyclonal antibodies suitable for immunofluorescence microscopy. HAECs were plated on glass coverslips, and confluent monolayers were wounded to induce polarization and directional cell movement. At 2 h after cell migration induction, cells were fixed, permeabilized, and then incubated with anti-Lnk and anti-vinculin antibodies, followed by AlexaFluor 568 and 488, respectively. Deconvolution of microscopic images was performed with Huygens Essential software, and colocalization of the fluorescent signals was demonstrated as a colormap using ImageJ software. The majority of Lnk protein appeared to localize at the cell membrane and to the juxtanuclear region (Fig. 1A). In addition, Lnk and the FA protein vinculin appeared to be localized to overlapping areas of the cell. An overlay of Lnk and vinculin revealed a coincident localization, as determined by yellow fluorescence and a high Icorr value.
Figure 1.
Lnk colocalizes with adhesion sites in EC. A) HAECs were plated on glass coverslips, and confluent monolayers were wounded. After 2 h, the cells were fixed and immunostained for Lnk (red) and vinculin (green). Deconvolution of microscopic images was performed with Huygens Essential software. Colocalization colormap and correlation index (Icorr) were obtained as described in Materials and Methods, using ImageJ software. B) ECs were allowed to adhere to coated glass coverslips for various time periods (30, 60, and 180 min). The cells were fixed and immunostained for Lnk (red) and vinculin (green) and visualized as superimposition of images (merge). Scale bar = 10 μm.
To observe Lnk localization during the process of cell adhesion, the same analysis was performed at different time periods (30, 60, and 180 min) following EC adhesion on gelatin-coated glass coverslips (Fig. 1B). After 30 min, a distinctive ring staining of vinculin was observed in cells, while Lnk was diffuse throughout the cytosol. At 60 min after adhesion, Lnk presented a similar staining pattern as vinculin, forming small clusters all around the cell. In adherent ECs (180 min), Lnk and vinculin were still similarly localized, showing typical plaques at the periphery of the cells. Thus, Lnk is not present immediately at FA but appears to be recruited downstream of the vinculin protein during cell adhesion and spreading.
Altogether, these data suggest an interaction between the adaptor protein Lnk and FA complexes that occurs in adhering, quiescent, and migrating ECs and which is temporally regulated during the early stages of the adhesive process. This particular distribution of Lnk protein evokes its involvement in FA regulation.
Lnk modulates cell spreading and cell-matrix adhesion
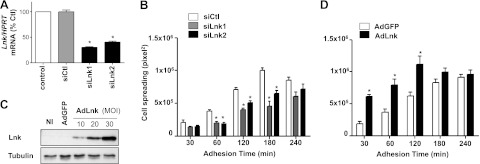
Spreading is an essential step in cell motility that requires a transition from round cells in suspension to polarized cells on ECM, involving FA formation as well as cytoskeleton reorganization. We took advantage of this cellular process to evaluate the contribution of Lnk to FA dynamics. We used RNA interference to silence the expression of Lnk or alternatively, recombinant adenoviral vector to overexpress Lnk protein. ECs were transfected or not (control) with nontargeting scrambled siRNA or specific Lnk-targeted siRNAs (siLnk1 and siLnk2), leading to the efficient down-regulation of Lnk transcripts (69.7% for siLnk1 and 60.1% for siLnk2 at mRNA level; Fig. 2A). We generated a recombinant adenoviral vector encoding the human Lnk cDNA and GFP as a reporter gene (AdLnk) to efficiently transduce ECs (25). This vector contains a single promoter, which, with the internal ribosome entry site, permits the translation of Lnk and GFP from a single transcript. Controls were NI cells and cells transduced with a recombinant adenovirus encoding GFP alone (AdGFP) to account for any effects that may be due to adenoviral infection. Endogenous expression of Lnk (NI, AdGFP) appeared to be weak and difficult to visualize, whereas Lnk overexpression was clearly confirmed by Western blot analysis (Fig. 2C). Lnk-deficient cells or cells overexpressing Lnk were plated on gelatin-coated coverslips, and the cell perimeter (cell spreading) was quantified after different time periods (30 to 240 min). As illustrated in Fig. 2B, the cell spreading was significantly diminished in the absence of Lnk after 180 min, with a 54.3 and 34.7% maximal decrease of the substrate contact area for the siLnk1 and siLnk2, respectively. On the contrary, Lnk overexpression promoted cell spreading with an increase of ∼60% of the cell area in contact with the collagen matrix 30 to 120 min after cell attachment (Fig. 2D). Furthermore, cells overexpressing Lnk were totally spread on ECM after 120 min, whereas control cells needed ≥180 min and cells lacking Lnk 240 min. Thus, Lnk expression enhanced ECs ability to fully spread on collagen, leading to a reduced time for complete spreading. Cells that are totally spread (240 min) on the collagen matrix did not display differences in cell surface area in the absence of or following overexpression of Lnk. Collectively, these results reveal that Lnk is required for efficient and rapid spreading on ECM and participates actively during early cell spreading.
Figure 2.
Lnk is required for efficient cell spreading. A) HAECs were transfected with the control siRNA (siCtl) or the Lnk-specific siRNAs (siLnk1, siLnk2), and silencing of Lnk was determined by qRT-PCR, Lnk mRNA level was normalized to HPRT-1. Histogram is representative of 3 independent experiments done in triplicate (percentage of basal level, mean ± se). B) After siRNA treatment, cells were plated on collagen-coated glass coverslip and allow to spread for 30, 60, 120, 180, and 240 min. Cells were fixed and immunostained for vinculin (green) to determine cell surface area (perimeter, pixel2) using MetaMorph software (means±se; n=40). Histogram is representative of 3 independent experiments. C) HAECs were either NI or infected with control AdGFP (MOI 30) or AdLnk (MOI 10, 20, 30). Cell lysates were analyzed by Western blot with an anti-Lnk antibody. Blots were reprobed with an anti-GAPDH antibody to ensure equal loading. D) After Ad infection, cells were treated as in B. *P < 0.05 vs. control.
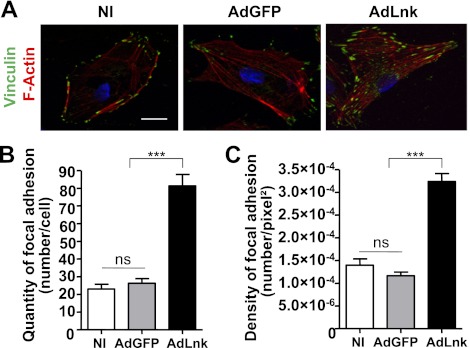
We noticed that spreading cells expressing Lnk attached more tightly to the ECM and showed a delayed detachment after trypsin/ethylene diamine tetracetic acid (EDTA) treatment during subculturing compared to control cells (data not shown). Combining this observation with the observed distribution of Lnk protein and the faster cell spreading of cells expressing Lnk, we hypothesize that Lnk may regulate FA rearrangement in ECs. Therefore, characterization of cell FAs in HAECs expressing Lnk was performed by double-staining with phalloidin-TRITC and anti-vinculin antibody (Fig. 3A). The number of FAs was quantified and revealed a 3.1-fold increase in Lnk-expressing cells (Fig. 3B). Furthermore, quantification of the density of vinculin-rich adhesions in spreading cells showed also a 2.8-fold increase (Fig. 3C). These findings indicate that Lnk affects the number and density of adhesive structures. It is also important to note that these additional FAs were associated with a change in cell morphology and cytoskeleton organization. F-actin staining revealed an increase in actin fibers in cells transduced with AdLnk compared with NI cells or control AdGFP. Cells transduced with AdLnk exhibited a highly organized microfilament network, with long actin fibers running through the whole cell body (Fig. 3A), demonstrating that Lnk expression induces structural changes in the cytoskeleton organization.
Figure 3.
Lnk increases the number of cell FAs and triggers actin cytoskeleton reorganization. ECs were plated on glass coverslips and infected with either control AdGFP or AdLnk for 24 h. A) For immunofluorescence, ECs were fixed with 1% paraformaldehyde and stained for F-actin (phalloidin-TRITC, red, to determine mean surface area), vinculin (for FAs, green), and nuclei (DAPI, blue). Scale bar = 10 μm. B) Number of FAs per cell was quantified using MetaMorph software (mean ± se; n=20). C) Density of FAs was obtained by dividing number of FAs by the cell surface area (mean ± se; n=20). Experiments were done ≥3 times. ns, not significant. ***P < 0.0001 vs. control.
Lnk impairs FA disassembly
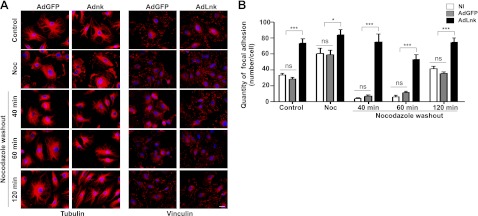
The increase in adhesion sites suggests that Lnk is a regulator of FA dynamics. Further, we asked whether Lnk enhances the formation of FAs or affects their turnover. To evaluate this, we used nocodazole treatment that synchronized the disassembly of adhesion sites. Initially, nocodazole incubation induces microtubule depolymerization that promotes FA assembly, then, after nocodazole washout, the microtubules regrow, causing the rapid and reversible disassembly of FAs in the entire cell population. As expected, we found that microtubule disruption on nocodazole treatment increased the number of FAs in control cells (NI or transduced with AdGFP), as shown by quantification of vinculin staining (Fig. 4). In both cell populations, microtubule regrowth and FA disassembly occurred simultaneously within 40 min after nocodazole washout. Finally, FAs reappeared 120 min after nocodazole washout. As previously shown, HAECs overexpressing Lnk displayed a significant increase in the number of FAs, even in the absence of nocodazole. Moreover, nocodazole washout failed to induce the disassembly of FAs, even though the microtubules were able to reform in these cells (Fig. 4). These data confirm the role of Lnk in FA turnover and demonstrate that Lnk expression blocks FA disassembly.
Figure 4.
Lnk blocks FA disassembly. A) HAECs were infected with control AdGFP (MOI 30) or AdLnk (MOI 30), and left untreated or incubated with nocodazole (10 μM, 2 h at 37°C). Cells were then fixed before or at the indicated times after nocodazole washout. Microtubules (right) and FA (left) were visualized by immunostaining with antibodies against tubulin or vinculin, respectively. Scale bar = 10 μm. B) Quantification of FA numbers during nocodazole treatment and washout (mean±se; n=3). Experiments were done ≥3 times. ns, not significant. *P < 0.05, ***P < 0.0001 vs. control.
Lnk decreases EC migration
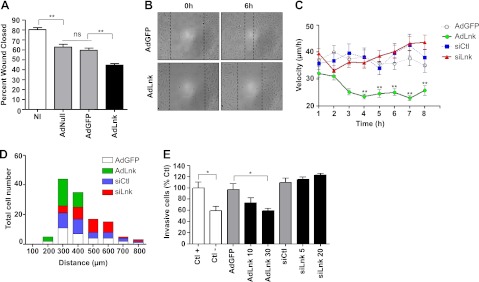
Cell migration is a multistep process, requiring the dynamic reorganization of the actin cytoskeleton and the rapid turnover of FAs. The significant increase of adhesive structures and modification in cell morphology and cytoskeleton architecture prompted us to examine migration of ECs expressing Lnk. To evaluate the role of Lnk in cell motility, we wounded confluent monolayers of ECs transduced with AdLnk or control AdGFP and then measured the migration of the cells into the cell-free area. Our results showed that Lnk overexpression significantly reduced EC migration by ∼30% as compared to controls (79.5±1.4% for NI and 64.1±2.3% for AdGFP vs. 48.4±1.9% for AdLnk of wound closed, P < 0.01; Fig. 5A). Representative phase-contrast micrographs illustrating repair of endothelial injuries are shown in Fig. 5B.
Figure 5.
Lnk decreases EC motility in migration assays. A) ECs were NI or transduced with either control adenoviruses (AdNull, AdGFP) or AdLnk and subjected to a scratch assay. Wound closure was followed for 18 h by time-lapse imaging. Percentage of wound closed 6 h after wounding was measured and expressed as a percentage of wound closed compared to control (t=0 h, mean±se; n=4). B) Representative pictures of healing for AdGFP- vs. AdLnk-transduced EC monolayers at 0 and 6 h after wounding. C, D) ECs were transduced with AdGFP, AdLnk, control siRNA (siCtl), or Lnk-specific siRNA and seeded onto 10-μm-wide lines of CYTOO chips. Cell migration was monitored for 8 h. Cell velocity (C) and total covered distances (D) were quantified using MetaMorph imaging software (mean±se; n=60). E) ECs were not transduced (Ctl+ and Ctl−) or transduced with AdGFP (MOI 30), AdLnk (MOI 10 and 30), control siRNA (20 nM), or Lnk-specific siRNA (5 and 20 nM). After 48 h of silencing and 24 h of infection, HAECs were plated to the upper chamber, and 20% FCS was added in the bottom chamber as chemoattractant molecule, except in Ctl− conditions. After 18 h incubation, numbers of cells that migrated through the membrane were estimated using MTT colorimetric assay (mean±se; n=3). ns, not significant. *P < 0.05, **P < 0.01 vs. control.
To further characterize the function of Lnk in EC migration, we performed CYTOOchip experiments that allow analysis of cell motility. This 1D migration assay confined cells to the field of view to avoid the use of cell-tracking hardware. Cells plated on wide tracks (10 or 20 μm width) exhibited typical formation of lamellipodia at the leading edge with a well-defined protrusion area, whereas cells confined to thin tracks (2.5 or 5 μm width) displayed a more elongated morphology and a filopodia-like structure due to geometrical constraints (Supplemental Fig. S1). As cells on wide tracks migrated in a similar manner to cells grown in the 2D model, we chose to perform our experiments on a 10-μm-wide line to complete 2D wound healing assays. Cell migration was monitored for 8 h, and migration parameters were analyzed for ≥60 cells for each group. HAECs overexpressing Lnk display a significant and lasting decrease in cell velocity of ∼60% compared to control cells (22.93±0.87 μm/h for AdLnk cells vs. 37.70±2.803 μm/h for AdGFP cells after 7 h monitoring, P < 0.01; Fig. 5C). No significant differences were observed between the cells transduced with control or Lnk siRNA, suggesting that absence of Lnk does not affect cell motility in this assay. Accordingly, Lnk−/− murine ECs showed no significant changes in cell motility as compared to WT cells (Supplemental Fig. S2). However, the distribution of the distance covered by cells showed a discrepancy between control and transduced cells. As expected, AdLnk cells (Fig. 5D, green) clearly migrated over shorter distances than control cells (Fig. 5D, white) with a maximum covered distance of 400 μm for AdLnk vs. 800 μm for AdGFP cells (Fig. 5D). In contrast, down-regulation of Lnk tends to increase migration distances. Most of the cells transduced with Lnk siRNA (Fig. 5D, red) covered distances between 500 and 600 μm, compared to control siRNA cells (Fig. 5D, blue), which more frequently covered 300 to 400 μm (Fig. 5D). Similarly, the absence of Lnk led to a trend toward increased migration in a chemotactic migration assay, whereas Lnk overexpression dose-dependently decreased cell migration in response to SVF (40±4.2% decrease for AdLnk MOI 30 as compared to AdGFP, P < 0.05; Fig. 5E). Collectively, these data demonstrate that Lnk expression inhibits cell motility and migration induced by wounding or a chemotactic agent.
Lnk is a key signaling adaptor of the integrin pathway
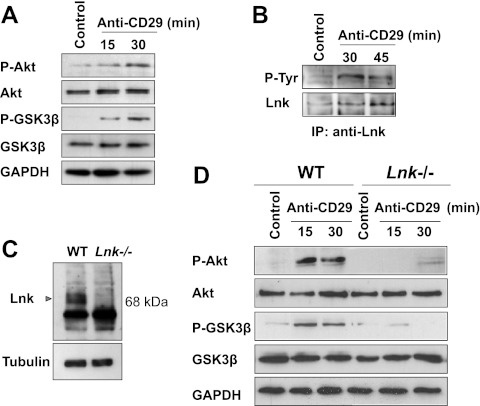
To investigate potential mechanisms by which Lnk modifies cell spreading and motility, we explored the role of Lnk in the integrin pathway. We first analyzed the effect of β1-integrin (CD29) cross-linking on Lnk protein phosphorylation on human ECs using an anti-β1-integrin mAb (clone TS2/16), previously described as an activating β1-integrin antibody (35). Phosphorylation of Akt at Ser473 and GSK3β at Ser9 was used as a control to assess CD29-mediated activation of the integrin pathway (Fig. 6A). Next, lysates were used to perform IP assays and indicated that Lnk is phosphorylated at tyrosine residues subsequent to stimulation with anti-CD29 mAb (Fig. 6B). These findings suggest that in ECs, the adaptor molecule Lnk may be involved in signaling events occurring during integrin pathway activation. To confirm these data, we isolated primary vascular ECs from Lnk−/− mice (11). Lack of Lnk expression in EC cultures from the knockout mice was confirmed by Western blot analysis (Fig. 6C). Next, primary cultures of ECs derived from Lnk−/− and WT mice were treated with an anti-β1-integrin mAb, and cell signaling was investigated on the cellular lysates. First, the phosphorylation of Akt and GSK3β kinases induced by β1-integrin ligation was examined on murine ECs by Western blotting. As expected, WT ECs showed a rapid and significant phosphorylation of both Akt and GSK3β (Fig. 6D). In contrast, anti-CD29 mAb failed to induce phosphorylation of Akt or GSK3β in Lnk−/− ECs, supporting the finding that Lnk plays a major role in the activation of the integrin pathway. Taken together, these results suggest that Lnk is a key regulator of the integrin pathway and is necessary for the activation of β1-integrin pathway.
Figure 6.
Activation of the integrin pathway in ECs induces phosphorylation of Lnk and requires Lnk expression. Human confluent EC monolayers were incubated with anti-CD29 (β1-integrin) or an irrelevant (control) antibody for the indicated periods. A) Activation of the integrin pathway was assessed by immunoblotting with phosphospecific Akt (P-Akt) and total Akt, phosphospecific GSK3β (P-GSK3β) and total GSK3β. Immunoblots shown are representative of 3 independent experiments. Blots were reprobed with an anti-GAPDH antibody to ensure equal loading. B) Representative analysis showing Lnk tyrosine phosphorylation and expression after incubation with anti-CD29 (30 and 45 min) or an irrelevant (control) antibody. IPs were performed using anti-Lnk antibodies, and the subsequent immunoblotting was achieved using anti-phosphotyrosine monoclonal antibody (top panel) and anti-Lnk antibodies (bottom panel). C) Mouse ECs were isolated from WT or Lnk-knockout (Lnk−/−) mice. Representative Western blot analysis compares Lnk expression in WT and Lnk−/− ECs. Equal loading was confirmed using an anti-tubulin antibody. D) Confluent mouse EC monolayers were incubated with anti-CD29 (β1-integrin) or an irrelevant (control) antibody for the indicated periods. Integrin pathway activation was determined by immunoblotting with P-Akt and total Akt, P-GSK3β and total GSK3β. Blots were reprobed using an anti-GAPDH antibody to ensure equal loading. Data shown are representative of 3 independent experiments.
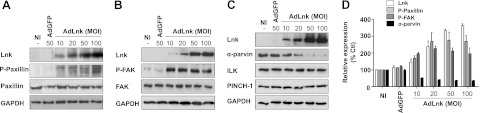
We next sought to determine whether Lnk was able to regulate expression and phosphorylation of FA proteins. HAECs were transduced with AdLnk (10 to 100 MOI) or AdGFP (50 MOI) for 24 h, and the phosphorylation status of two critical proteins of the integrin pathway, paxillin on Tyr118 and FAK on Tyr397, was examined. Paxillin is a multidomain scaffold protein localized to FAs that mediates protein-protein interactions to facilitate signaling (36, 37). Tyrosine phosphorylation of paxillin protein provides additional docking sites for other structural and signaling components (38). The tyrosine kinase FAK is a major regulator of focal contact structure, involved in both FA disassembly and cell protrusion (39, 40). On integrin engagement, FAK autophosphorylates at the Tyr397 residue, resulting in the recruitment of several kinases and induction of downstream signaling pathways. Using phosphospecific antibodies, we found that Lnk expression was associated with a significant phosphorylation of paxillin (2.7±0.3-fold increase as compared to basal level, P < 0.05) and FAK (2.4±0.5-fold increase as compared to basal level, P < 0.05) in comparison with control or AdGFP, even when the Lnk protein overexpression was small (MOI 10; Fig. 7A, B). Moreover, phosphorylation at Tyr118 of paxillin promoted by Lnk expression in a dose-dependent manner was observed (Fig. 7D). These data show that Lnk regulates the phosphorylation status of FAK and paxillin proteins, key mediators of the integrin signaling.
Figure 7.
Lnk elicits the phosphorylation of the FA proteins FAK and paxillin and down-regulates α-parvin expression in ECs. ECs were either NI or infected with control AdGFP (MOI 50) or AdLnk (at MOI ranging from 10 to 100). Cells were lysed 24 h after transduction, and cytosolic proteins were harvested as described in Materials and Methods. A–C) Paxillin and P-Paxillin (Tyr118; A), FAK and P-FAK (Tyr397; B), and α-parvin, ILK, and PINCH-1 expressions (C) were determined by Western blot. D) P-Paxillin, P-FAK, and α-parvin levels were quantified after normalization to GAPDH; results are expressed as a percentage of basal expression (n=3).
We also examined the effect of Lnk on the ILK-PINCH-1-α-parvin/actopaxin complex, which has emerged as a key transducer of β1- and β3-integrin signaling (23, 41). We found that increased levels of the Lnk protein did not affect ILK or PINCH-1 expression, whereas the α-parvin protein level was dramatically decreased in a dose-dependent manner (2.9±0.1-fold decrease as compared to basal level, P < 0.05; Fig. 7C, D). This significant regulation of the actin binding protein α-parvin by Lnk expression led us to further investigate whether reduced levels of α-parvin might be responsible for the accumulation of FAs and delayed migration observed in Lnk-expressing cells.
Lnk blocks FA disassembly and decreases EC migration through a regulatory effect on the α-parvin protein
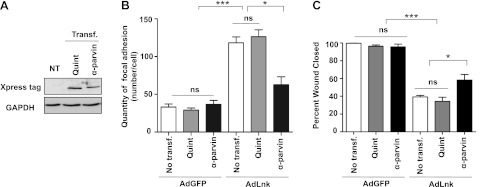
To test our hypothesis, we used plasmids encoding for an α-parvin phospho-mutant (Quint) or α-parvin WT cDNA. The Quint plasmid induces expression of a quintuple nonphosphorylatable mutant of α-parvin (29). Phosphorylation of α-parvin is essential for its activity; thus, the Quint plasmid was used as a control in our experiments. Transfection efficiency (Quint or WT α-parvin) was evaluated by Western blot analysis using Xpress antibody (Fig. 8A). HUVECs were then transfected with Quint mutant or α-parvin cDNA before Lnk expression, and the number of FAs was quantified using vinculin staining. As described previously, cells expressing Lnk displayed ∼3 times more adhesion sites than control cells even in presence of the Quint mutant protein (Fig. 8B). However, WT α-parvin expression decreased by ∼50% the number of vinculin-rich adhesions in Lnk cells, leading to an average of 62.6 ± 8.6 FAs/cell as compared to 118.17 ± 7.8 for AdLnk cells (Fig. 8B). Reexpression of α-parvin protein partially prevented the accumulation of FAs in cells expressing Lnk. A similar experiment was performed in the wound healing model. As illustrated in Fig. 8C, transfection of Quint and α-parvin plasmids does not affect migration of control cells (AdGFP). Moreover, Lnk expression results in a reduced HUVEC migration, as observed in HAECs. In contrast, α-parvin expression partially restores motility of Lnk cells compared to Quint-expressing cells (58.3±6.3% of AdLnk transfected α-parvin plasmid vs. 34.3±4.5% for AdLnk-transfected Quint plasmid of wound closed, P < 0.05). Thus, recombinant α-parvin expression rescued partially the reduced migration capacity of Lnk cells. These results indicate that the increase in the number of adhesions and the impaired migratory phenotype of Lnk cells are partly due to a reduced expression of α-parvin protein.
Figure 8.
Lnk expression increases FA numbers and impairs migration of ECs through inhibition of α-parvin expression. A) HUVECs were either nontransfected (NT) or transfected with plasmids encoding wild type (α-parvin) or nonphosphorylable (Quint) α-parvin cDNA and immunoblotted using anti-Xpress tag. B) HUVECs were transfected with α-parvin or Quint plasmids and were subsequently infected with either control AdGFP or AdLnk. Numbers of FAs per cell were quantified using MetaMorph software after immunostaining with antibody against vinculin (mean±se; n=20). C) Cells were subjected to scratch assay, and wound closure was followed for 36 h by time-lapse videomicroscopy. Percentage of wound closed 33 h after wounding was measured using MetaMorph Imaging software. Results are expressed as percentage of wound closed compared to control (t=0 h; mean±se; n = 3). Experiments were done ≥3 times in triplicate. ns, not significant. *P < 0.05, ***P < 0.0001 vs. control.
Lnk forms complex with ILK and inhibits ILK-α-parvin interaction
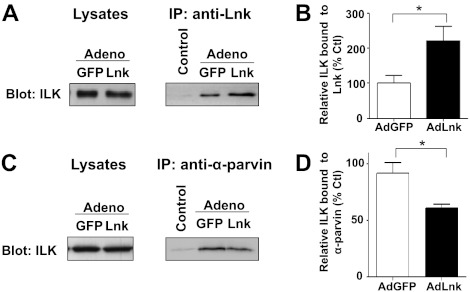
We next sought to identify molecular binding partners by which Lnk could mediate the down-regulation of α-parvin. Lysates of ECs infected with control AdGFP or AdLnk were immunoprecipitated with anti-Lnk antibody and then analyzed by Western blotting using anti-ILK. Lysates immunoprecipitated with irrelevant antibody were used as controls. As illustrated in Fig. 9A, Lnk coprecipitates efficiently with ILK, thereby indicating ILK-Lnk complex formation. Moreover, immunoprecipitated proteins from cells infected with AdLnk showed an increase in the amount of ILK protein associated with Lnk (Fig. 9B), despite similar ILK expression in all samples. These results indicate that increased expression of Lnk in ECs promotes ILK-Lnk complex formation. As a control, lysates were analyzed by Western blotting before IP (Fig. 9A, lysates) and indicated a comparable expression of ILK in all samples. ILK and proteins of the parvin family form a complex that localizes to integrin adhesions. To test whether Lnk could decrease α-parvin protein levels by inhibiting the formation of the ILK-α-parvin interaction, we performed IP using anti-α-parvin antibody. Infections were achieved using a low amount of viral particles (MOI 5). As a control, Western blot analysis of lysates, derived from the Lnk overexpressing or control cells were performed and confirmed a similar expression of ILK in samples (Fig. 9C, lysates). As illustrated in Fig. 9C, D, immunoprecipitates derived from the Lnk-overexpressing cells showed a slight, but consistent, decrease of the ILK-α-parvin association, indicating that increased expression of Lnk could inhibit ILK-α-parvin interaction. Thus, expression of Lnk promotes the formation of ILK-Lnk complex and concomitantly inhibits the formation of the ILK-α-parvin complex. Taken together, these data suggest that sustained expression of Lnk favors a binding of ILK to Lnk that could negatively modulate ILK-α-parvin association.
Figure 9.
Lnk forms complexes with ILK and inhibits ILK-α-parvin interaction. HAECs were infected with either control AdGFP or AdLnk at MOI 5. A) Cell lysates were analyzed by Western blot with an anti-ILK antibody as a loading control (left panel). Cell lysates were then immunoprecipitated with an anti-Lnk antibody and analyzed by Western blot with anti-ILK antibody (right panel). Control lane represents lysate immunoprecipitated with an irrelevant antibody. B) Relative expression of ILK was quantified by densitometry in IP anti-Lnk (percentage of AdGFP control, mean± se; n=3). C) Lysates of ECs (MOI 5) were analyzed by Western blotting with anti-ILK antibody (left panel). IP was performed as above using anti-α-parvin antibody, and precipitates were subjected to Western blot analysis for ILK (right panel). D) Relative expression of ILK was quantified by densitometry in IP anti-α-parvin (percentage of AdGFP control, mean±se; n=3). *P < 0.05 vs. control.
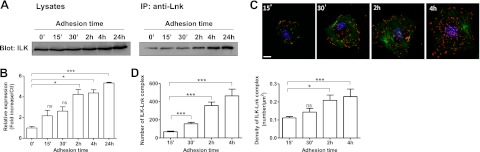
To investigate the physiological stimulus that could regulate the binding of Lnk to ILK, we have studied the effect of cell adhesion on ILK-Lnk complex formation. Lysates of ECs plated 0 min, 15 min, 30 min, 2 h, 4 h, and 24 h on gelatin-coated dishes were immunoprecipitated with anti-Lnk antibody and then analyzed by Western blotting using anti-ILK. Cells trypsinized and directly lysed are used as control (0 min) and referred as nonadherent cells. As illustrated in Fig. 10A, B, the poor quantity of ILK-Lnk complex observed in nonadherent cells increases gradually over adhesion time and reaches a 5.33 ± 0.1-fold increase after 24 h as compared to control. To confirm this physiological regulation of Lnk binding, we have used Duolink-PLA analysis, which detects proteins located within a radius of 40 nm (Fig. 10C). The number and the density of distinct red spots, which represent ILK-Lnk complex, were quantified and revealed a progressive rise over time, with a 4.66- and 2.1-fold increase, respectively, after 4 h on gelatin (Fig. 10D). Altogether, these results indicate that the formation of ILK-Lnk is activated during the adhesion process and confirm Lnk involvement in FA stabilization during adhesion.
Figure 10.
ILK-Lnk complex formation is regulated during cell adhesion. A) Lysates of HAECs plated 0 min (nonadherent cells), 15 min, 30 min, 2 h, 4 h, and 24 h on gelatin-coated dishes were analyzed by Western blot with an anti-ILK antibody as a loading control (left panel). Cell lysates were then immunoprecipitated with anti-Lnk antibody, and precipitates were analyzed by Western blot with anti-ILK antibody (right panel). B) Relative expression of ILK was quantified by densitometry in immunoprecipitated anti-Lnk and normalized with ILK expression of loading control (percentage of nonadherent cells vs. control, mean±se; n=3). C) Proximity ligation assay was performed on fixed cells plated 15 min, 30 min, 2 h, or 4 h, using rabbit antibodies against ILK and goat antibodies against Lnk. Cells were stained for F-actin (phalloidin-FITC, green, to determine mean surface area), ILK-Lnk complexes (red), and nuclei (DAPI, blue). Scale bar = 10 μm. D) Number (left panel) and density (right panel) of ILK-Lnk complexes (red spots in C) were quantified using MetaMorph software (mean ± se; n>20). *P < 0.05, ***P < 0.0001 vs. control.
DISCUSSION
Cell adhesion, migration, and proliferation are major processes controlling vascular remodeling and regeneration. In this study, we have demonstrated that Lnk is an adaptor protein involved in the integrin pathway and an effective regulator of adhesion and migration for ECs in vitro. An attempt to recapitulate our findings is provided in Fig. 11.
Figure 11.
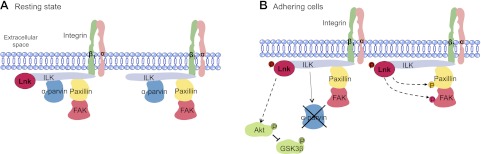
Model for Lnk signaling in ECs. A) On the basis of the present study, we propose that when cells are quiescent, a pool of nonphosphorylated Lnk constitutively binds to ILK. B) Adhesion of cells to ECM through the integrin β1 subunit initiates Lnk phosphorylation and up-regulation and its recruitment to FA via its interaction with ILK. There, Lnk decreases the protein level of α-parvin by inhibiting the formation of ILK-α-parvin complex and facilitates phosphorylation of Akt (Ser473) and GSK3β (Ser9), as well as FA proteins paxillin (Tyr118) and FAK (Tyr397). This signaling cascade, partly due to α-parvin down-regulation, blocks FA disassembly, increases cell attachment to the ECM, and reduces cell motility.
First, we identify Lnk as a novel contributor to the FA complex by studying its localization in EC. Microscopy analysis revealed a close proximity between Lnk and the actin-binding protein vinculin, suggesting the interaction of Lnk with FA complexes. This particular distribution was confirmed by IP experiments in which endogenous Lnk coimmunoprecipitated with ILK protein. ILK was originally described as a serine/threonine kinase, which directly associates with the cytoplasmic tail of β1 and β3 subunits (42). ILK can interact with several adaptor protein partners, including the FA protein paxillin, which directly binds vinculin (36, 43). Thus, the formation of a complex containing ILK and Lnk could explain the close localization of Lnk and vinculin observed in ECs and strongly implies a function for Lnk in FA function.
Our results further argue that Lnk blocks FA disassembly and reduces cell motility by decreasing the protein level of α-parvin in cells. This conclusion is supported by our experiments using WT and mutated forms of α-parvin that demonstrate that transfection of WT α-parvin but not Quint α-parvin significantly diminishes FA numbers and restores partial cell motility in Lnk-overexpressing cells. Quint α-parvin expression did not allow recovery of cell migration, suggesting that phosphorylation of α-parvin is necessary for this function. These data agree with previous observations showing that silencing of α-parvin impairs cell migration (29). Transcript analysis during the migration process revealed a significant decrease in Lnk mRNA concomitant with an enhanced α-parvin mRNA levels (data not shown). This observation demonstrates the physiological regulation of both proteins in ECs. These data support a role for Lnk as a negative regulator of cell migration that may correspond to the end of a promigratory signal and a return to cell quiescence. The observation that Lnk-deficient cells cover longer distances during 1D migration along oriented fibronectin fibers is in agreement with this hypothesis. However, the down-regulation of Lnk is not sufficient to increase cell motility, demonstrating the complexity of cell migration regulation and suggesting compensatory actions by other FA proteins.
Preceding reports indicated that siRNA-mediated silencing of ILK expression resulted not only in depletion of ILK but also in the modest and substantial reductions in α-parvin and PINCH-1 protein levels, respectively. These data suggested that PINCH-1, ILK, and, to a lesser extent, α-parvin depend on each other for maintenance of their protein levels in cells (44, 45). In this study, we were not able to observe any change in ILK and PINCH1 expression mediated by Lnk. Consequently, we hypothesize that depletion of α-parvin by Lnk is independent of the other two proteins. A possible explanation may be that Lnk stabilizes the ILK-PINCH-1 complex through a binding-dependent mechanism, avoiding their degradation.
Over the past several years, the ability of ILK to function as an adaptor protein rather than a kinase has been debated because of its atypical kinase domain (43, 46, 47, 48). Fukuda et al. (49) have reported crystal structure of the kinase domain of ILK fused to CH domain of the α-parvin and concluded that this complex was catalytically inactive, supporting a function as a pseudokinase. However, a recent study has demonstrated that ILK activity is markedly inhibited by α-parvin, suggesting that the presence of α-parvin maintains the kinase domain in an inactive conformation (50, 51). Here, we provide evidence that Lnk could negatively regulate the cellular level of α-parvin through its interaction with ILK protein. We further propose a model in which up-regulation of Lnk enhances ILK-Lnk complex formation at the expense of ILK-α-parvin association, blocking α-parvin access to FAs. Moreover, the ligation of integrin by cross-linking β1-integrin on Lnk−/− ECs has shown that the absence of Lnk totally abrogates the activation of Akt and GSK3β, which are the most widely used readouts for ILK activity (52, 53). This result suggests that activation of ILK and integrin signaling requires Lnk function. Consistent with this notion, Lnk-expressing cells assume an elongated morphology, which is similar to transfected cells constitutively expressing ILK (54). Moreover, expression of membrane-targeted ILK in fibroblasts induces a marked increase in the number of adhesions per cell (55), as observed in cells expressing Lnk. Presently, we can only speculate about the action of Lnk on ILK activity. The demonstrated regulation of ILK function by α-parvin and above-mentioned data are in agreement with the hypothesis that the kinase activity of ILK may be regulated by Lnk interaction through inhibition of ILK-α-parvin binding. However, the Lnk-mediated effect on ILK activity remains to be further explored. An important issue will be to determine whether Lnk binding is essential for full activation of ILK and contribute to the active conformation of the kinase or if its action is only mediated through its regulation of the α-parvin-ILK dissociation.
We suggest that Lnk may be a regulator of the integrin/ILK pathway, which is activated during various events, including attachment of cells to extracellular matrix. In our model, we have observed that Lnk is rapidly phosphorylated in response to β1-integrin activation. Moreover, we have shown that cell adhesion, a process mediated by integrin engagement, activates Lnk protein, resulting in the increase of Lnk-ILK complex formation. The relative levels (or the ratio) of the ILK-Lnk complex and the ILK-α-parvin complex are controlled by the expression level of Lnk protein, as an elevation of the expression level of Lnk increases the level of the ILK-Lnk complex and concomitantly decreases the level of the ILK-α-parvin complex. Thus, the modulation of α-parvin expression level by Lnk could provide a mechanism by which cells control adhesion/spreading and migration processes.
In our experiments, we have not observed any change in the PINCH protein expression. Nevertheless, the potential role of PINCH, which has been shown to actively participate in ILK signaling, should be further explored. An alternative explanation of the effect of Lnk on Akt and GSK3β phosphorylation could be the defective interaction of ILK with PINCH, which could mediate the downstream effects. PINCH has been found to inhibit protein phosphatase 1α (PP1α) activity, resulting in increased Akt phosphorylation (56, 57). It will be interesting to investigate whether PINCH contributes in the Lnk-mediated signaling by blocking phosphatase activity.
Lnk overexpression is concomitant with elevated FAK phosphorylation. The increased number of FA contacts observed in Lnk-expressing cells is consistent with the hyperphosphorylation of FAK and paxillin proteins. Nevertheless, the increased phosphorylation pattern of FAK and paxillin suggests a promigratory signal, while cell migration in Lnk cells is decreased. Our findings revealed the role of α-parvin inhibition in decreased cell motility and FA disassembly. However, recombinant α-parvin expression only partially rescued the reduced migration capacity of Lnk cells. These results suggest that additive mechanisms may be involved in the phenotype of Lnk cells. Phosphorylation of FAK at various tyrosine residues regulates FA turnover by mechanisms that are not well understood. In fact, it has been shown that fibroblasts from FAK−/− mice contain an abundance of FAs with maturation defects and reduced migration (58). Moreover, studies of cells lacking the tyrosine phosphatase SHP2 (SH2-domain-containing protein tyrosine phosphatase 2), a regulator of FAK phosphorylation, support the role of FAK in FA disassembly. SHP2−/− cells have increased FAK activity, but they also showed an accumulation of immature FAs and similar migration defects to FAK−/− cells (40). These reports support the importance of the balance of FAK phosphorylation in the control of FAs dynamics and maturation. According to these findings, we cannot exclude the possibility that Lnk could also have an effect on migration and FA number by altering the phosphorylation status of FAK.
Our results reveal that Lnk is required for efficient EC adhesion and spreading on collagen. This finding is underlined by observations that Lnk-deficient EC generated by siRNA treatment exhibit delayed spreading, whereas Lnk overexpression increases by ∼60% the cell area in contact with the collagen during the early stage of cell spreading. In addition, Lnk is concentrated to adhesion sites throughout spreading, supporting its important role in regulating cell adhesions. Takizawa et al. (23) have shown that Lnk is required for platelets to fully spread on fibrinogen and promotes integrin αIIbβ3-mediated actin cytoskeletal reorganization. Thus, the involvement of Lnk in cell spreading and integrin signaling seems not to be limited to ECs and could be a ubiquitous function of this adaptor protein, although its implication in different cell types remains to be demonstrated.
Previous reports provided evidence for an interaction between Lnk and actin, notably through a direct interaction with the actin binding protein filamin A (ABP-280), which facilitates actin assembly (33, 59). Filamin A has been shown to contribute to the mechanical stability of cells and to interact with several proteins that regulate cell adhesion, including β1-integrin (60). Nevertheless, whether Lnk may regulate filamin A activity remains to be determined. Another member of the Lnk family, SH2B2 (adaptor protein with PH and SH2 domains or APS) was shown to colocalize with F-actin in B cells (61) and was found to be a molecular partner for Enigma in insulin-induced membrane ruffles (62). During the review process, Lanning et al. (63) identified SH2B1β as a new FA protein in fibroblasts. Consistent with our findings, this study showed that in addition to regulating the actin cytoskeleton during cell migration and differentiation, SH2B1β phosphorylation increases the number of FAs, suggesting a mechanism by which SH2B1β may control cell motility. Together, our findings and those of others indicate that regulation of the actin cytoskeleton and FA number is a common function of members of SH2B adaptor family (SH2B1, SH2B2, and Lnk). Lnk −/− ECs were used in migration and adhesion assays and surprisingly do not present significant differences compared to WT cells. Therefore, the lack of Lnk in ECs may be compensated by other members of the SH2B family, and the role that SH2B1 and SH2B2 play in vascular cells should be further explored.
In summary, we have provided evidence for a new regulatory mechanism by which the Lnk adaptor controls EC adhesion and migration. Lnk takes part in integrin signaling through its direct interaction with ILK and is required for efficient phosphorylation of Akt and GSK3β during integrin activation. Lnk up-regulation is associated with a functional decrease in α-parvin expression. This correlates with an accumulation of FAs and delayed migration. We have previously established Lnk as a negative regulator of TNF signaling, indicating a potential role in controlling inflammation. Overall, our findings suggest that Lnk is a pivotal adaptor in vascular ECs that regulates at least two major signaling pathways (TNF and β-integrin) in the endothelium. Finally, the regulatory functions of Lnk in multiple interconnected intracellular signaling pathways highlights its therapeutic potential as a molecular target for the prevention of vascular diseases.
Supplementary Material
Acknowledgments
The authors thank the vector core of the University Hospital of Nantes, supported by the Association Française contre les Myopathies (AFM), for providing the adenoviral vectors.
This work was supported by Xenome, a European Commission-funded Integrated Project; by Life Sciences, Genomics, and Biotechnology for Health grant LSHB-CT-2006-037377; and by fellowships from La Fondation Progreffe and La Fondation Centaure. J.D. was supported by a grant from La Région Pays de la Loire. C.E.T. was supported by the U.S. National Institutes of Health (RO1-HL-070244). L.V. was supported by an INSERM-Avenir grant.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- DAPI
- 4′,6-diamidino-2-phenylindole
- EC
- endothelial cell
- ECBM
- endothelial cell basal medium
- ECM
- extracellular matrix
- FA
- focal adhesion
- FAK
- focal adhesion kinase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GSK3β
- glycogen synthase kinase 3β
- HAEC
- human arterial endothelial cell
- HPRT
- hypoxanthine-guanine phosphoribosyl transferase
- HUVEC
- human umbilical vein endothelial cell
- ILK
- integrin-linked kinase
- IP
- immunoprecipitation
- MTT
- 3-[4,5-dimethylthiazol-2-yl] 2,5-diphenyltetrazodium
- NI
- noninfected
- PH
- pleckstrin homology
- PI3K
- phosphatidylinositol 3-kinase
- Quint
- quintuple
- SH2
- Src homology 2
- SH2B3
- Src homology 2-B3
- TNF
- tumor necrosis factor
- VCAM-1
- vascular cell adhesion molecule 1
- WT
- wild type
REFERENCES
- 1. Mitchell R. N., Libby P. (2007) Vascular remodeling in transplant vasculopathy. Circ. Res. 100, 967–978 [DOI] [PubMed] [Google Scholar]
- 2. Lauffenburger D. A., Horwitz A. F. (1996) Cell migration: a physically integrated molecular process. Cell 84, 359–369 [DOI] [PubMed] [Google Scholar]
- 3. Sheetz M. P., Felsenfeld D., Galbraith C. G., Choquet D. (1999) Cell migration as a five-step cycle. Biochem. Soc. Symp. 65, 233–243 [PubMed] [Google Scholar]
- 4. Avraamides C. J., Garmy-Susini B., Varner J. A. (2008) Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 8, 604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaidel-Bar R., Itzkovitz S., Ma'ayan A., Iyengar R., Geiger B. (2007) Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang X., Li Y., Tanaka K., Moore K. G., Hayashi J. I. (1995) Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. U. S. A. 92, 11618–11622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takaki S., Watts J. D., Forbush K. A., Nguyen N. T., Hayashi J., Alberola-Ila J., Aebersold R., Perlmutter R. M. (1997) Characterization of Lnk. An adaptor protein expressed in lymphocytes. J. Biol. Chem. 272, 14562–14570 [DOI] [PubMed] [Google Scholar]
- 8. Yokouchi M., Suzuki R., Masuhara M., Komiya S., Inoue A., Yoshimura A. (1997) Cloning and characterization of APS, an adaptor molecule containing PH and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene 15, 7–15 [DOI] [PubMed] [Google Scholar]
- 9. Takizawa H., Eto K., Yoshikawa A., Nakauchi H., Takatsu K., Takaki S. (2008) Growth and maturation of megakaryocytes is regulated by Lnk/Sh2b3 adaptor protein through crosstalk between cytokine- and integrin-mediated signals. Exp. Hematol. 36, 897–906 [DOI] [PubMed] [Google Scholar]
- 10. Simon C., Dondi E., Chaix A., de Sepulveda P., Kubiseski T. J., Varin-Blank N., Velazquez L. (2008) Lnk adaptor protein down-regulates specific Kit-induced signaling pathways in primary mast cells. Blood 112, 4039–4047 [DOI] [PubMed] [Google Scholar]
- 11. Velazquez L., Cheng A. M., Fleming H. E., Furlonger C., Vesely S., Bernstein A., Paige C. J., Pawson T. (2002) Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J. Exp. Med. 195, 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Devallière J., Charreau B. (2011) The adaptor Lnk (SH2B3): An emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochem. Pharmacol. 82, 1391–1402 [DOI] [PubMed] [Google Scholar]
- 13. Takaki S., Sauer K., Iritani B. M., Chien S., Ebihara Y., Tsuji K., Takatsu K., Perlmutter R. M. (2000) Control of B cell production by the adaptor protein lnk. Definition of a conserved family of signal-modulating proteins. Immunity 13, 599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takaki S., Tezuka Y., Sauer K., Kubo C., Kwon S.-M., Armstead E., Nakao K., Katsuki M., Perlmutter R. M., Takatsu K. (2003) Impaired lymphopoiesis and altered B cell subpopulations in mice overexpressing Lnk adaptor protein. J. Immunol. 170, 703–710 [DOI] [PubMed] [Google Scholar]
- 15. Seita J., Ema H., Ooehara J., Yamazaki S., Tadokoro Y., Yamasaki A., Eto K., Takaki S., Takatsu K., Nakauchi H. (2007) Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc. Natl. Acad. Sci. U. S. A. 104, 2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bersenev A., Wu C., Balcerek J., Tong W. (2008) Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J. Clin. Invest. 118, 2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kwon S.-M., Suzuki T., Kawamoto A., Ii M., Eguchi M., Akimaru H., Wada M., Matsumoto T., Masuda H., Nakagawa Y., Nishimura H., Kawai K., Takaki S., Asahara T. (2009) Pivotal role of lnk adaptor protein in endothelial progenitor cell biology for vascular regeneration. Circ. Res. 104, 969–977 [DOI] [PubMed] [Google Scholar]
- 18. Kamei N., Kwon S.-M., Alev C., Ishikawa M., Yokoyama A., Nakanishi K., Yamada K., Horii M., Nishimura H., Takaki S., Kawamoto A., Ii M., Akimaru H., Tanaka N., Nishikawa S.-I., Ochi M., Asahara T. (2010) Lnk deletion reinforces the function of bone marrow progenitors in promoting neovascularization and astrogliosis following spinal cord injury. Stem Cells 28, 365–375 [DOI] [PubMed] [Google Scholar]
- 19. Kubo-Akashi C., Iseki M., Kwon S.-M., Takizawa H., Takatsu K., Takaki S. (2004) Roles of a conserved family of adaptor proteins, Lnk, SH2-B, and APS, for mast cell development, growth, and functions: APS-deficiency causes augmented degranulation and reduced actin assembly. Biochem. Biophys. Res. Commun. 315, 356–362 [DOI] [PubMed] [Google Scholar]
- 20. Tong W., Lodish H. F. (2004) Lnk inhibits Tpo-mpl signaling and Tpo-mediated megakaryocytopoiesis. J. Exp. Med. 200, 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tong W., Zhang J., Lodish H. F. (2005) Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood 105, 4604–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takizawa H., Kubo-Akashi C., Nobuhisa I., Kwon S.-M., Iseki M., Taga T., Takatsu K., Takaki S. (2006) Enhanced engraftment of hematopoietic stem/progenitor cells by the transient inhibition of an adaptor protein, Lnk. Blood 107, 2968–2975 [DOI] [PubMed] [Google Scholar]
- 23. Takizawa H., Nishimura S., Takayama N., Oda A., Nishikii H., Morita Y., Kakinuma S., Yamazaki S., Okamura S., Tamura N., Goto S., Sawaguchi A., Manabe I., Takatsu K., Nakauchi H., Takaki S., Eto K. (2010) Lnk regulates integrin alphaIIbbeta3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J. Clin. Invest. 120, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boulday G., Coulon F., Fraser C. C., Soulillou J.-P., Charreau B. (2002) Transcriptional up-regulation of the signaling regulatory protein LNK in activated endothelial cells. Transplantation 74, 1352–1354 [DOI] [PubMed] [Google Scholar]
- 25. Fitau J., Boulday G., Coulon F., Quillard T., Charreau B. (2006) The adaptor molecule Lnk negatively regulates tumor necrosis factor-alpha-dependent VCAM-1 expression in endothelial cells through inhibition of the ERK1 and -2 pathways. J. Biol. Chem. 281, 20148–20159 [DOI] [PubMed] [Google Scholar]
- 26. Coupel S., Leboeuf F., Boulday G., Soulillou J.-P., Charreau B. (2004) RhoA activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J. Am. Soc. Nephrol. 15, 2429–2439 [DOI] [PubMed] [Google Scholar]
- 27. Chatelais M., Devallière J., Galli C., Charreau B. (2011) Gene transfer of the adaptor Lnk (SH2B3) prevents porcine endothelial cell activation and apoptosis: implication for xenograft's cytoprotection. Xenotransplantation 18, 108–120 [DOI] [PubMed] [Google Scholar]
- 28. Nikolopoulos S. N., Turner C. E. (2000) Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J. Cell Biol. 151, 1435–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Clarke D. M., Brown M. C., LaLonde D. P., Turner C. E. (2004) Phosphorylation of actopaxin regulates cell spreading and migration. J. Cell Biol. 166, 901–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaskolski F., Mulle C., Manzoni O. J. (2005) An automated method to quantify and visualize colocalized fluorescent signals. J. Neurosci. Methods 146, 42–49 [DOI] [PubMed] [Google Scholar]
- 31. Doyle A. D., Wang F. W., Matsumoto K., Yamada K. M. (2009) One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 184, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 33. He X., Li Y., Schembri-King J., Jakes S., Hayashi J. (2000) Identification of actin binding protein, ABP-280, as a binding partner of human Lnk adaptor protein. Mol. Immunol. 37, 603–612 [DOI] [PubMed] [Google Scholar]
- 34. Li Y., He X., Schembri-King J., Jakes S., Hayashi J. (2000) Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T cell activation. J. Immunol. 164, 5199–5206 [DOI] [PubMed] [Google Scholar]
- 35. Arroyo A. G., Sánchez-Mateos P., Campanero M. R., Martín-Padura I., Dejana E., Sánchez-Madrid F. (1992) Regulation of the VLA integrin-ligand interactions through the beta 1 subunit, J. Cell Biol. 117, 659–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Turner C. E. (1998) Paxillin. Int. J. Biochem. Cell Biol. 30, 955–959 [DOI] [PubMed] [Google Scholar]
- 37. Turner C. E. (2000) Paxillin and focal adhesion signalling. Nat. Cell Biol. 2, E231–E236 [DOI] [PubMed] [Google Scholar]
- 38. Brown M. C., Turner C. E. (2004) Paxillin: adapting to change. Physiol. Rev. 84, 1315–1339 [DOI] [PubMed] [Google Scholar]
- 39. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 40. Von Wichert G., Haimovich B., Feng G.-S., Sheetz M. P. (2003) Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 22, 5023–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Legate K. R., Montañez E., Kudlacek O., Fässler R. (2006) ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 7, 20–31 [DOI] [PubMed] [Google Scholar]
- 42. Hannigan G. E., Leung-Hagesteijn C., Fitz-Gibbon L., Coppolino M. G., Radeva G., Filmus J., Bell J. C., Dedhar S. (1996) Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379, 91–96 [DOI] [PubMed] [Google Scholar]
- 43. McDonald P. C., Fielding A. B., Dedhar S. (2008) Integrin-linked kinase—essential roles in physiology and cancer biology. J. Cell Sci. 121, 3121–3132 [DOI] [PubMed] [Google Scholar]
- 44. Fukuda T., Chen K., Shi X., Wu C. (2003) PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J. Biol. Chem. 278, 51324–51333 [DOI] [PubMed] [Google Scholar]
- 45. Stanchi F., Grashoff C., Nguemeni Yonga C. F., Grall D., Fässler R., Van Obberghen-Schilling E. (2009) Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation, J. Cell Sci. 122, 1800–1811 [DOI] [PubMed] [Google Scholar]
- 46. Hannigan G., Troussard A. A., Dedhar S. (2005) Integrin-linked kinase: a cancer therapeutic target unique among its ILK, Nat. Rev. Cancer 5, 51–63 [DOI] [PubMed] [Google Scholar]
- 47. Boudeau J., Miranda-Saavedra D., Barton G. J., Alessi D. R. (2006) Emerging roles of pseudokinases. Trends Cell Biol. 16, 443–452 [DOI] [PubMed] [Google Scholar]
- 48. Wickström S. A., Lange A., Montanez E., Fässler R. (2010) The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 29, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fukuda K., Gupta S., Chen K., Wu C., Qin J. (2009) The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol. Cell 36, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maydan M., McDonald P. C., Sanghera J., Yan J., Rallis C., Pinchin S., Hannigan G. E., Foster L. J., Ish-Horowicz D., Walsh M. P., Dedhar S. (2010) Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3beta) phosphorylation. PLoS ONE 5, e12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hannigan G. E., McDonald P. C., Walsh M. P., Dedhar S. (2011) Integrin-linked kinase: Not so “pseudo” after all. Oncogene 30, 4375-4385 [DOI] [PubMed] [Google Scholar]
- 52. Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. U. S. A. 95, 11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Defilles C., Lissitzky J.-C., Montero M.-P., André F., Prévot C., Delamarre E., Marrakchi N., Luis J., Rigot V. (2009) alphavbeta5/beta6 integrin suppression leads to a stimulation of alpha2beta1 dependent cell migration resistant to PI3K/Akt inhibition. Exp. Cell Res. 315, 1840–1849 [DOI] [PubMed] [Google Scholar]
- 54. Khyrul W. A. K. M., LaLonde D. P., Brown M. C., Levinson H., Turner C. E. (2004) The integrin-linked kinase regulates cell morphology and motility in a rho-associated kinase-dependent manner. J. Biol. Chem. 279, 54131–54139 [DOI] [PubMed] [Google Scholar]
- 55. Boulter E., Grall D., Cagnol S., Van Obberghen-Schilling E. (2006) Regulation of cell-matrix adhesion dynamics and Rac-1 by integrin linked kinase. FASEB J. 20, 1489–1491 [DOI] [PubMed] [Google Scholar]
- 56. Eke I., Koch U., Hehlgans S., Sandfort V., Stanchi F., Zips D., Baumann M., Shevchenko A., Pilarsky C., Haase M., Baretton G. B., Calleja V., Larijani B., Fässler R., Cordes N. (2010) PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J. Clin. Invest. 120, 2516–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Meder B., Huttner I. G., Sedaghat-Hamedani F., Just S., Dahme T., Frese K. S., Vogel B., Köhler D., Kloos W., Rudloff J., Marquart S., Katus H. A., Rottbauer W. (2011) PINCH proteins regulate cardiac contractility by modulating integrin-linked kinase-protein kinase B signaling. Mol. Cell. Biol. 31, 3424–3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ilić D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 [DOI] [PubMed] [Google Scholar]
- 59. Kovacsovics T. J., Hartwig J. H. (1996) Thrombin-induced GPIb-IX centralization on the platelet surface requires actin assembly and myosin II activation. Blood 87, 618–629 [PubMed] [Google Scholar]
- 60. Kim H., McCulloch C. A. (2011) Filamin A mediates interactions between cytoskeletal proteins that control cell adhesion. FEBS Lett. 585, 18–22 [DOI] [PubMed] [Google Scholar]
- 61. Iseki M., Kubo C., Kwon S.-M., Yamaguchi A., Kataoka Y., Yoshida N., Takatsu K., Takaki S. (2004) Increased numbers of B-1 cells and enhanced responses against TI-2 antigen in mice lacking APS, an adaptor molecule containing PH and SH2 domains. Mol. Cell. Biol. 24, 2243–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barrès R., Gonzalez T., Le Marchand-Brustel Y., Tanti J.-F. (2005) The interaction between the adaptor protein APS and Enigma is involved in actin organisation. Exp. Cell Res. 308, 334–344 [DOI] [PubMed] [Google Scholar]
- 63. Lanning N. J., Su H.-W., Argetsinger L. S., Carter-Su C. (2011) Identification of SH2B1β as a focal adhesion protein that regulates focal adhesion size and number. J. Cell Sci. 124, 3095–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.