Abstract
β-Adrenergic receptor (AR) blockers provide substantial clinical benefits, including improving overall survival and left ventricular (LV) function following myocardial infarction (MI), though the mechanisms remain incompletely defined. The transverse-tubule (T-tubule) system of ventricular myocytes is an important determinant of cardiac excitation-contraction function. T-tubule remodeling occurs early during LV failure. We hypothesized that β-AR blockers prevent T-tubule remodeling and thereby provide therapeutic benefits. A murine model of MI was utilized to examine the effect of β-AR blockers on T-tubule remodeling following LV MI. We applied the in situ imaging of T-tubule structure from Langendorff-perfused intact hearts with laser scanning confocal microscopy. We found that MI caused remarkable T-tubule remodeling near the infarction border zone and moderate LV remodeling remote from the MI. Metoprolol and carvedilol administered 6 d after MI for 4 wk each increased the T-tubule integrity at the remote and border zones. At the molecular level, both β-AR blockers restored border and remote zone expression of junctophilin-2 (JP-2), which is involved in T-tubule organization and formation of the T-tubule/sarcoplasmic reticulum junctions. In contrast, β-AR blockers had no significant effects on caveolin-3 expression. In summary, our data show that β-AR antagonists can protect against T-tubule remodeling after MI, suggesting a novel therapeutic mechanism of action for this drug class. Preservation of JP-2 expression may contribute to the beneficial effects of metoprolol and carvedilol on T-tubule remodeling.—Chen, B., Li, Y., Jiang, S., Xie, Y.-P., Guo, A., Kutschke, W., Zimmerman, K., Weiss, R. M., Miller, F. J., Anderson, M. E., Song, L.-S. β-Adrenergic receptor antagonists ameliorate myocyte T-tubule remodeling following myocardial infarction.
Keywords: heart failure, β-AR blockers
β-Adrenergic receptor (AR) blockers decrease contractility in normal hearts (1) and cause an initial decline in ventricular function in patients (2). However, long-term therapy with β-AR blockers is clinically proven to improve the survival of patients with chronic heart failure, hemodynamic and left ventricular (LV) function, and quality of life as well as reduce hospitalization for worsening chronic heart failure, mortality, and morbidity (2–6). Of particular importance, long-term administration of β-AR blockers reduces risk of mortality by 30–65% (4). Despite the well-established clinical benefits of β-AR blockers, the molecular mechanism of action, particularly how β-AR blockers protect the heart and improve LV pump function, is still not completely understood.
The transverse tubules (T tubules) are regularly spaced invaginations of the surface membrane along the Z-line regions, which form a highly organized membrane network in mammalian ventricular myocytes (7). The myocyte T-tubule system is an important determinant of the cardiac excitation-contraction function (7–13). Alterations in T-tubules have been documented in failing ventricular myocytes from patients with heart failure (14, 15) and animal models of heart failure (14, 16–20). T-tubule remodeling is directly linked to sarcoplasmic reticulum (SR) Ca2+ release dysfunction in failing myocytes (16–18) and in artificially detubulated myocytes (21, 22). We recently reported that T-tubule remodeling is an important early event in the progression of heart failure, starting during the compensated hypertrophic stage, and the extent of T-tubule integrity correlates well with LV function (23). Down-regulation of junctophilin-2 (JP-2), a protein related to the formation of the T-tubule/SR junctional membrane complex, (24) causes T-tubule disorganization both in cultured myocytes and in mice expressing JP-2 shRNA construct (23, 25). The fact that β-AR blockers improve cardiac function and reduce mortality in chronic heart failure patients lead us to hypothesize that the therapeutic benefit from β-AR blockers includes effects at the ultrastructural level; i.e., on T-tubule remodeling.
In the present study, we used a murine myocardial infarction (MI) model to test the hypothesis that β-AR blockers ameliorate T-tubule remodeling following MI. We applied in situ confocal imaging of the T-tubule network from Langendorff-perfused intact hearts to examine different regions of the myocardium relative to the MI. Here we demonstrate that a selective blocker (metoprolol, targeting only β1-AR) and a nonselective β-AR blocker (carvedilol, antagonizing β1-, β2-, and α1-AR) similarly prevented T-tubule remodeling at both the remote and border zones following MI. Strikingly, we found that β-AR blocker treatment restored the levels of JP-2 in affected zones to normal. Taken together, our findings suggest that JP-2 expression and corresponding restoration of T-tubule integrity is one mechanism by which β-AR blockers improve LV function following MI.
MATERIALS AND METHODS
Mouse MI model
Animal experiments were performed according to the protocol approved by the University of Iowa Institutional Animal Care and Use Committee. We performed MI surgery in mice as published previously (26, 27). Briefly, male mice at 9–10 wk of age were anesthetized with sodium pentobarbital (75 mg/kg, i.p.). The animals were intubated and ventilated with room air using a TOPO Volume/Pressure Small Animal Ventilator (Kent Scientific, Torrington, CT, USA). A left thoracotomy was performed, the heart was exposed, and the pericardium was removed. A suture was passed underneath the left anterior descending (LAD) branch of the coronary artery ∼3 mm from the tip of the left atrium along the anterolateral border of the heart (approximately mid-LAD coronary artery), and a surgical knot was tied to occlude the coronary artery. Successful ligation of the artery was confirmed by blanching of the myocardium. At the end of the procedure, a chest tube (28-gauge, venal catheter) was placed between the fourth and fifth ribs, and then the chest wall was closed. The control group had a similar procedure, with the passage of a suture under the LAD artery but without occlusion. MI mice were divided into 3 groups and were administered saline, metoprolol (3 mg/kg/d, i.p.), or carvedilol (1 mg/kg/d, i.p.) daily beginning 6 d after MI procedure and continued for 4 wk. All mice were sacrificed at 5 wk after surgery. The infarct size of MI mice was measured based on echocardiography.
Echocardiography
Transthoracic echocardiograms were performed in the University of Iowa Cardiology Animal Phenotyping Core Laboratory using a Vevo 2100 Imager (VisualSonics, Toronto, ON, Canada), prior to sacrificing the mice. Conscious sedation was achieved with midazolam (0.2 to 0.3 mg s.c.). The anterior chest was shaved, and prewarmed ultrasonic gel was applied. Two-dimensional images were acquired in LV short- and long-axis planes with a 40-MHz linear array probe, yielding 200 frames/s. LV mass, volume, and ejection fraction (EF) were calculated with the biplane area-length method (28). Regions demonstrating akinesis or dyskinesis were visually identified, planimetered, and expressed as percentages of total LV end-diastolic silhouette.
In situ confocal imaging of myocyte T-tubule structure in intact hearts
These studies were performed as described previously (23). Briefly, intact mouse hearts were Langendorff perfused at room temperature with Ca2+-free Tyrode's solution (137 mM NaCl, 5.4 mM KCl, 10 mM HEPES, 10 mM glucose, 1 mM MgCl2, and 0.33 mM NaH2PO4, pH adjusted to 7.4 with NaOH, oxygenated with 95% O2 and 5% CO2) containing 2.5 μM MM 4-64 (AAT Bioquest Inc., Sunnyvale, CA, USA), a lipophilic fluorescence indicator of membrane structure, for 20 min. The hearts were then placed in the perfusion chamber attached on the stage of a confocal microscope (See Fig. 2 in ref. 23), and perfused with indicator free/Ca2+ free solution (with continuous oxygenation). The membrane structure of epicardial myocytes was analyzed in situ by confocal microscopy (LSM510, Carl Zeiss MicroImaging Inc., Wetzlar, Germany). The microscope was equipped with ×63 (NA=1.4) oil-immersion lens. The optical pinhole was set to 1 Airy disc (axial resolution <1 μm). T-tubule images were acquired from the infarction zone, border zone, remote zone, and right ventricle of each heart. The border zone of MI heart was defined as the immediate neighboring regions around the infarction zone, as detected visually and also under confocal microscope. The remote zone included the areas ∼2 mm from the infarction region and the LV posterior wall. T-tubule images were analyzed offline with custom routines composed with the IDL program (ITT VIS Inc., Boulder, CO, USA), as recently described (23).
Figure 2.
Differential T-tubule remodeling among different regions of myocardium following MI. A–F) T-tubule images from sham-treated left ventricle (sham LV; A), sham-treated right ventricle (sham RV; B), the infarction zone (C), border zone (D), remote zone (E), and right ventricle (RV) of infarcted heart (F). G) Average data of TTpower from different regions; n = 9, 9, 8, 8, 8, 8 mice/group, respectively. **P < 0.01 vs. sham, ††P < 0.01 vs. MI border zone, P < 0.01 among groups; 1-way ANOVA.
Western blot analysis
Frozen hearts (whole hearts or tissues from different regions) were homogenized and then sonicated in lysis buffer (50 mM Tris, pH 7.5; 150 mM NaCl; 10 mM NaF; 1 mM Na3VO4; 5 mM EGTA; 5 mM EDTA; 0.5% Triton X-100; 0.5% Na deoxycholate; 0.1% SDS), containing protease inhibitors (Sigma, P8340). Tissue lysates were then centrifuged at 12,000 g for 10 min to remove insoluble debris. Protein concentrations were determined by using the Pierce BCA assay (Pierce; Thermo Scientific, Rockford, IL, USA). Samples (8 μg) were separated by SDS-PAGE (4–12% Bis-Tris gel; Invitrogen, Carlsbad, CA, USA) and transferred to PVDF membranes. Primary antibodies that recognize JP-2 (1:2000, sc-51313; Santa Cruz Biotechnology, Santa Cruz, CA, USA), caveolin-3 (Cav-3; 1:10,000, 610420; BD Transduction Laboratories, Lexington, KY, USA), and GAPDH (1:10,000, 2118; Cell Signaling, Danvers, MA, USA) were used. HRP-linked anti-goat IgG (1:10,000), anti-mouse (1:10,000), and anti-rabbit IgG (1:10000) were used to visualize bound primary antibodies with the SuperSignal chemiluminescence substrate (Pierce; Thermo Scientific). The protein bands were quantified using ImageJ 1.43d software (U.S. National Institutes of Health, Bethesda, MD, USA).
Statistics
Data are expressed as means ± se. Student's t tests and 1-way ANOVA were applied when appropriate. Values of P < 0.05 were considered statistically significant.
RESULTS
β-AR blockers improve cardiac function and attenuate myocardial hypertrophy after MI
We first examined how two widely prescribed β-AR blockers, metoprolol and carvedilol, affect LV function following MI. Echocardiography was used to assess cardiac function. We measured infarction size, end diastolic volume, end systolic volume, and LV EF following MI. LV infarct size was not significantly different among vehicle-, metoprolol-, and carvedilol-treated groups (35.0±2.4; 35.4±3.7; and 33.7±2.5%, respectively). However, metoprolol and carvedilol similarly improved LV function (Fig. 1A–C), consistent with findings from rat MI models (29) and a genetic model of sympathetic hyperactivity-induced heart failure (30). In addition, both β-AR antagonists significantly attenuated MI-induced cardiac hypertrophy at the end of 4 wk treatment, as shown by changes in heart weight/body weight (HW/BW) ratio. To investigate the subcellular mechanism underlying the beneficial effects of β-AR blockers on gross cardiac function, we next examined myocyte T-tubule structure using in situ T-tubule imaging technique.
Figure 1.
Metoprolol and carvedilol improved cardiac function and myocardial hypertrophy in MI mice. A–C) End diastolic volume (EDV; A), end systolic volume (ESV; B) and LV EF (C) were assessed by echocardiography. D) Heart weight to body weight (HW/BW) ratio. n = 17, 15, 12, 13 mice/group, respectively. *P < 0.05, **P < 0.01 vs. sham vehicle; †P < 0.05, ††P < 0.01 vs. MI vehicle.
T-tubule remodeling occurs in both border and remote zones following MI
Previous studies have shown that T-tubule remodeling occurs in both myocytes isolated from remote zones of rat and pig hearts after MI (18, 31) and in the septal myocardium of mouse hearts following large LV infarction, which affects almost the entire LV free wall (17). However, no studies have examined whether differential remodeling in T-tubule structure between different regions/chambers exists following MI. Taking advantage of the ability to perform in situ confocal imaging of Langendorff-perfused intact hearts, we evaluated the effects of MI on the organization of the T-tubule structure at different regions relative to the infarcted area in the LV. As expected, myocytes of both the LV and right ventricular (RV) myocardium from sham-operated hearts displayed highly organized T-tubule structure (Fig. 2B). In the infarction zone, the T-tubule structure was entirely lost (Fig. 2C). Myocytes adjacent to the infarction region (border zone) displayed severe T-tubule remodeling (Fig. 2D). Myocytes from the LV posterior wall (remote zone) had less pronounced but substantial remodeling (Fig. 2E), whereas the RV wall was not affected (Fig. 2F). We next conducted a power spectrum analysis of the strength of the repetitiveness of structure, termed T-tubule power (TTpower). The higher the organized pattern of the T-tubule structure, the higher the TTpower. Our data demonstrate that the TTpower was decreased in both the surviving myocytes from the border and the “unaffected” myocytes from remote zones (Fig. 2G), signifying the loss of T-tubule organization in these regions. Also, the difference in TTpower was significant between the border and remote zones, suggesting that, following MI, the damage to the border zone is more pronounced but remote zone is also affected.
β-AR blockers protects against T-tubule remodeling after MI
The therapeutic benefit of β-AR blockers with regard to LV function led us to hypothesize that β-AR blockers prevent the extensive ultrastructural remodeling in LV myocytes following MI. As shown in Figs. 3 and 4, β-AR blockers protected against T-tubule remodeling in both the border and remote zones, and the effects of metoprolol and carvedilol were comparable. This improvement in T-tubule structure was evidenced by a significant increase in TTpower with administration of both β-AR blockers as compared to vehicle (Fig. 4A–C). Furthermore, the response was limited to the chamber of infarction (i.e., left ventricle), and β-AR blockers did not alter the TTpower in the right ventricle (Figs. 3 and 4D).
Figure 3.
Metoprolol and carvedilol protected against T-tubule remodeling following MI. Images are representative from different zones relative to the infarction under different treatments.
Figure 4.
Metoprolol and carvedilol protected against T-tubule remodeling following MI. Averaged TTpower from LV MI zone (A), LV border zone (B), LV remote zone (C), and RV (D) relative to the infarction region under different treatments; n = 9, 8, 8, 7 mice/group, respectively. *P < 0.05, **P < 0.01 vs. sham vehicle; †P < 0.05, ††P < 0.01 vs. MI vehicle; P < 0.01 among groups (B, C); 1-way ANOVA.
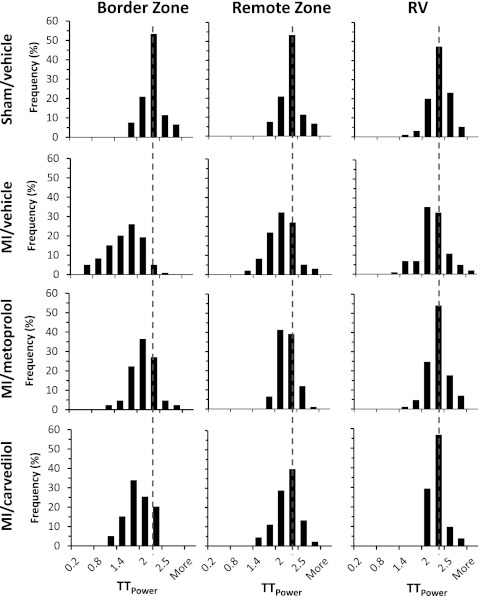
An examination of the histogram distribution of TTpower revealed that MI shifted the histogram of TTpower to the left, especially in the border zone, and a slight leftward shift was detected in the remote zone (Fig. 5). In contrast, both β-AR blockers shifted the histograms back to the right, indicating an improvement of T-tubule structure. Our findings to this point indicate that β-AR blocker therapy after MI provides measurable, beneficial effects on myocyte T-tubule remodeling.
Figure 5.
Histogram distribution of TTpower from different zones relative to the infarction region under different treatments. MI shifted the histogram of TTpower to the left, especially in the border zone, and caused a slight leftward shift in the remote zone. Both β-AR blockers shifted the histograms back to the right. Each group is from 80–100 images of 7–9 mice.
β-AR blockers restore JP-2 expression in infarcted hearts
We recently reported that, in an aortic banding pressure overload rat model, LV expression of JP-2 correlates with T-tubule remodeling, and depletion of JP-2 with viral transfection of JP-2 shRNA in cultured cardiomyocytes results in a loss of T-tubule integrity (23). Similar results were observed in transgenic mouse hearts expressing JP-2 shRNA (25). Therefore, we assessed whether the mechanism by which β-AR blockers protect against T-tubule remodeling following MI is through regulation of JP-2 expression. We first assessed JP-2 protein levels in whole heart lysates. Our data demonstrate that MI resulted in a 50% decrease in JP-2 level (Fig. 6A, B). Interestingly, both β-AR blockers restored JP-2 expression. Expression of caveolin-3 (Cav-3) has also been associated with T-tubule organization (32). However, Cav-3 was unchanged, although there is a trend of increase following MI (Fig. 6A, C), in accord with findings by others (33). Furthermore, both β-AR blockers did not alter Cav-3 expression as compared to vehicle.
Figure 6.
Metoprolol and carvedilol treatment restored JP-2, but not Cav-3 protein expression in infarction hearts. A) Representative Western blotting bands of JP-2, Cav-3, and loading control GAPDH. B) Average data on JP-2 protein level. C) Average data on Cav-3 protein level. n = 7, 6, 7, 6 hearts/group, respectively. **P < 0.001 vs. sham vehicle; ††P < 0.01 vs. MI vehicle.
We next examined JP-2 protein expression in different regions of infarcted hearts, i.e., border zone, remote zone and right ventricle (Fig. 7). We found JP-2 expression levels were decreased in the border and remote zones (48 and 62% of sham control levels, respectively), whereas JP-2 expression in the right ventricle was similar to sham. In the affected regions, both β-AR blockers restored JP-2 expression to normal levels. Notably, these changes in JP-2 expression levels correspond with T-tubule remodeling observed at different regions relative to the infarction (Fig. 4). Collectively, these data demonstrate that β-AR blockers protect against T-tubule remodeling following MI through normalization of JP-2 expression.
Figure 7.
Regional difference of infarcted hearts in JP-2 expression and effects of β-AR blockers. A) Representative Western blotting bands of JP-2 and loading control GAPDH from tissue lysates of different regions (border zone, remote zone, and RV). B) Average data on JP-2 protein level at different regions of infarcted hearts. n = 3–5 samples/group. **P < 0.001 vs. sham vehicle; †P < 0.05 vs. MI vehicle; no difference in RV samples among different groups.
DISCUSSION
The therapeutic benefits of β-AR blockers in patients with heart failure were established over a decade ago, yet the mechanisms remain to be fully defined. Herein we report that β-AR blockers metoprolol and carvedilol protect against the ultrastructural subcellular membrane changes that accompany LV failure. Specifically, T-tubule remodeling following MI was mitigated with administration of β-AR blockers. We also found that β-AR blockers restored expression of JP-2, which was dramatically reduced in MI. Our data reveal a novel mechanism by which β-AR blockers improve patient outcomes following heart failure.
LV function of patients with MI is improved with long-term treatment with β-AR blockers. A number of studies using MI animal models have reported, at the cellular level, that the improvement of LV function is associated with increasing Ca2+ handling function through preventing alterations in Ca2+ cycling proteins or restoration of normal ryanodine receptor activity (29, 34–36). However, carvedilol, the nonselective β-AR blocker, may act through a different mechanism than metoprolol to improve cardiac function after MI, as evidenced by data that metoprolol enhances Ca2+ transients, whereas carvedilol alters the myocardial redox state (30) and collagen deposition (37). It is well accepted that, at the structural (organ) level, β-AR blockers reduce or reverse ventricular remodeling in chronic heart failure following MI (38–41). Our data presented in this study extend these observations and demonstrate, at an ultrastructural level, that MI triggered a loss of T-tubule integrity in both the border and remote zones, and β-AR blockers protected against the deleterious T-tubule remodeling. Taken together, these findings suggest that the ultrastructural reverse remodeling induced by β-AR blockers is, at least partly, responsible for beneficial effects of β-AR blockers observed in heart failure.
Several pieces of data led us to conclude that β-AR blockers improve T-tubule integrity via JP-2 restoration. First, JP-2 is down-regulated in human hypertrophic cardiomyopathy, (42) mouse hypertrophic and dilated cardiomyopathy model, (43) and rat LV pressure overload model (23, 44). Here we found that JP-2 was markedly down-regulated in a murine model of ischemic cardiomyopathy. Next, JP-2 is implicated in the formation of T-tubule/SR junctions (24). Third, JP-2 expression correlates with T-tubule remodeling, and knockdown of JP-2 aggravates the integrity of the T-tubule structure. Finally, a recent report from the Wehrens group (25) using inducible JP-2-knockdown mice provide compelling evidence for a role for JP-2 in maintaining a normal T-tubule network and the architecture between T-tubule and SR membrane. Validating our hypothesis, β-AR blockers restored JP-2 expression in both the border and remote zones following MI, revealing that one cellular mechanism by which β-AR blockers improve T-tubule integrity is through normalization of JP-2 expression in these regions. However, it remains unclear how β-AR blockers mechanistically change JP-2 expression in the infarcted heart. We postulate that excessive sympathetic stress, e.g., high catecholamine levels post MI, causes intracellular Ca2+ overload and activates Ca2+-dependent gene transcription. A second possibility is that Ca2+-dependent proteases are activated and lead to JP-2 degradation. β-AR blockers may effectively attenuate JP-2 down-regulation through these pathways, though future studies are necessary to completely understand the mechanism.
Similar to JP-2, Cav-3 has also been implicated in T-tubule structural organization. In particular, Cav-3 is associated with JP-2 (43) and colocalizes with JP-2 at the T-tubules in postnatal cardiomyocytes (45). Genetic deletion of Cav-3 results in T-tubule disorganization (32). However, we did not observe a significant alteration in Cav-3 expression following MI, consistent with a previous report (33). Furthermore, our results demonstrate that changes in Cav-3 expression do not occur in response to β-AR blockers following MI. Therefore Cav-3 does not appear to be involved in β-AR blockers mediated t-tubule improvement in heart failure.
In summary, our data reveal that T-tubule remodeling at the subcellular ultrastructural level is one mechanism by which β-AR blockers provide therapeutic benefit. This study provides insights into new therapeutic strategies to improve T-tubule integrity and cardiac function in patients with heart failure.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants NIH R01 HL090905 (L.S.S.) and RR026293 (R.M.W.) and American Heart Association Scientific Development grant 0635056N (L.S.S.). S.J. was supported in part by an American Heart Association postdoctoral fellowship award (11POST7640011). The authors declare no conflicts of interest.
Footnotes
- AR
- adrenergic receptor
- Cav-3
- caveolin-3
- EF
- ejection fraction
- JP-2
- junctophilin-2
- LAD
- left anterior descending
- LV
- left ventricular
- MI
- myocardial infarction
- RV
- right ventricular
- SR
- sarcoplasmic reticulum
- T tubule
- transverse tubule
- TTpower
- transverse tubule power
REFERENCES
- 1. Epstein S., Robinson B. F., Kahler R. L., Braunwald E. (1965) Effects of beta-adrenergic blockade on the cardiac response to maximal and submaximal exercise in man. J. Clin. Invest. 44, 1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall S. A., Cigarroa C. G., Marcoux L., Risser R. C., Grayburn P. A., Eichhorn E. J. (1995) Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J. Am. Coll. Cardiol. 25, 1154–1161 [DOI] [PubMed] [Google Scholar]
- 3. Janosi A., Ghali J. K., Herlitz J., Czuriga I., Klibaner M., Wikstrand J., Hjalmarson A. (2003) Metoprolol CR/XL in postmyocardial infarction patients with chronic heart failure: experiences from MERIT-HF. Am. Heart. J. 146, 721–728 [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M., Colucci W. S., Swedberg K. (2003) Beta-blockers in chronic heart failure. Circulation 107, 1570–1575 [DOI] [PubMed] [Google Scholar]
- 5. Packer M., Coats A. J., Fowler M. B., Katus H. A., Krum H., Mohacsi P., Rouleau J. L., Tendera M., Castaigne A., Roecker E. B., Schultz M. K., DeMets D. L. (2001) Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 344, 1651–1658 [DOI] [PubMed] [Google Scholar]
- 6. Packer M., Fowler M. B., Roecker E. B., Coats A. J., Katus H. A., Krum H., Mohacsi P., Rouleau J. L., Tendera M., Staiger C., Holcslaw T. L., Amann-Zalan I., DeMets D. L. (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106, 2194–2199 [DOI] [PubMed] [Google Scholar]
- 7. Song L. S., Guatimosim S., Gomez-Viquez L., Sobie E. A., Ziman A., Hartmann H., Lederer W. J. (2005) Calcium biology of the transverse tubules in heart. Ann. N. Y. Acad. Sci. 1047, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang S. Q., Song L. S., Lakatta E. G., Cheng H. (2001) Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410, 592–596 [DOI] [PubMed] [Google Scholar]
- 9. Brette F., Orchard C. (2003) T-tubule function in mammalian cardiac myocytes. Circ. Res. 92, 1182–1192 [DOI] [PubMed] [Google Scholar]
- 10. Bito V., Heinzel F. R., Biesmans L., Antoons G., Sipido K. R. (2008) Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovasc. Res. 77, 315–324 [DOI] [PubMed] [Google Scholar]
- 11. Ibrahim M., Al Masri A., Navaratnarajah M., Siedlecka U., Soppa G. K., Moshkov A., Al-Saud S. A., Gorelik J., Yacoub M. H., Terracciano C. M. (2010) Prolonged mechanical unloading affects cardiomyocyte excitation-contraction coupling, transverse-tubule structure, and the cell surface. FASEB J. 24, 3321–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franzini-Armstrong C. (1999) The sarcoplasmic reticulum and the control of muscle contraction. FASEB J. 13(Suppl. 2), S266–S270 [DOI] [PubMed] [Google Scholar]
- 13. Song L. S., Wang S. Q., Xiao R. P., Spurgeon H., Lakatta E. G., Cheng H. (2001) beta-Adrenergic stimulation synchronizes intracellular Ca(2+) release during excitation-contraction coupling in cardiac myocytes. Circ. Res. 88, 794–801 [DOI] [PubMed] [Google Scholar]
- 14. Lyon A. R., MacLeod K. T., Zhang Y., Garcia E., Kanda G. K., Lab M. J., Korchev Y. E., Harding S. E., Gorelik J. (2009) Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc. Natl. Acad. Sci. U. S. A. 106, 6854–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crossman D. J., Ruygrok P. N., Soeller C., Cannell M. B. (2011) Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One 6, e17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song L. S., Sobie E. A., McCulle S., Lederer W. J., Balke C. W., Cheng H. (2006) Orphaned ryanodine receptors in the failing heart. Proc. Natl. Acad. Sci. U. S. A. 103, 4305–4310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Louch W. E., Mork H. K., Sexton J., Stromme T. A., Laake P., Sjaastad I., Sejersted O. M. (2006) T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J. Physiol. 574, 519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Heinzel F. R., Bito V., Biesmans L., Wu M., Detre E., von Wegner F., Claus P., Dymarkowski S., Maes F., Bogaert J., Rademakers F., D'Hooge J., Sipido K. (2008) Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ. Res. 102, 338–346 [DOI] [PubMed] [Google Scholar]
- 19. Xie Y. P., Chen B., Sanders P., Guo A., Li Y., Zimmerman K., Wang L. C., Weiss R. M., Grumbach I. M., Anderson M. E., Song L. S. (2012) Sildenafil prevents and reverses transverse-tubule remodeling and Ca2+ handling dysfunction in right ventricle failure induced by pulmonary artery hypertension. Hypertension 59, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu C. Y., Jia Z., Wang W., Ballou L. M., Jiang Y. P., Chen B., Mathias R. T., Cohen I. S., Song L. S., Entcheva E., Lin R. Z. (2011) PI3Ks maintain the structural integrity of T-tubules in cardiac myocytes. PLoS One 6, e24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brette F., Despa S., Bers D. M., Orchard C. H. (2005) Spatiotemporal characteristics of SR Ca(2+) uptake and release in detubulated rat ventricular myocytes. J. Mol. Cell. Cardiol. 39, 804–812 [DOI] [PubMed] [Google Scholar]
- 22. Brette F., Rodriguez P., Komukai K., Colyer J., Orchard C. H. (2004) beta-adrenergic stimulation restores the Ca transient of ventricular myocytes lacking t-tubules. J. Mol. Cell. Cardiol. 36, 265–275 [DOI] [PubMed] [Google Scholar]
- 23. Wei S., Guo A., Chen B., Kutschke W., Xie Y. P., Zimmerman K., Weiss R. M., Anderson M. E., Cheng H., Song L. S. (2010) T-tubule remodeling during transition from hypertrophy to heart failure. Circ. Res. 107, 520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takeshima H., Komazaki S., Nishi M., Iino M., Kangawa K. (2000) Junctophilins: a novel family of junctional membrane complex proteins. Mol. Cell 6, 11–22 [DOI] [PubMed] [Google Scholar]
- 25. Van Oort R. J., Garbino A., Wang W., Dixit S. S., Landstrom A. P., Gaur N., De Almeida A. C., Skapura D. G., Rudy Y., Burns A. R., Ackerman M. J., Wehrens X. H. (2011) Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation 123, 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang R., Khoo M. S., Wu Y., Yang Y., Grueter C. E., Ni G., Price E. E., Thiel W., Guatimosim S., Song L. S., Madu E. C., Shah A. N., Vishnivetskaya T. A., Atkinson J. B., Gurevich V. V., Salama G., Lederer W. J., Colbran R. J., Anderson M. E. (2005) Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 11, 409–417 [DOI] [PubMed] [Google Scholar]
- 27. Erickson J. R., Joiner M. L., Guan X., Kutschke W., Yang J., Oddis C. V., Bartlett R. K., Lowe J. S., O'Donnell S. E., Aykin-Burns N., Zimmerman M. C., Zimmerman K., Ham A. J., Weiss R. M., Spitz D. R., Shea M. A., Colbran R. J., Mohler P. J., Anderson M. E. (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133, 462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Francis J., Weiss R. M., Wei S. G., Johnson A. K., Felder R. B. (2001) Progression of heart failure after myocardial infarction in the rat. Am. J. Physiol. 281, R1734–R1745 [DOI] [PubMed] [Google Scholar]
- 29. Sun Y. L., Hu S. J., Wang L. H., Hu Y., Zhou J. Y. (2005) Effect of beta-blockers on cardiac function and calcium handling protein in postinfarction heart failure rats. Chest 128, 1812–1821 [DOI] [PubMed] [Google Scholar]
- 30. Bartholomeu J. B., Vanzelli A. S., Rolim N. P., Ferreira J. C., Bechara L. R., Tanaka L. Y., Rosa K. T., Alves M. M., Medeiros A., Mattos K. C., Coelho M. A., Irigoyen M. C., Krieger E. M., Krieger J. E., Negrao C. E., Ramires P. R., Guatimosim S., Brum P. C. (2008) Intracellular mechanisms of specific beta-adrenoceptor antagonists involved in improved cardiac function and survival in a genetic model of heart failure. J. Mol. Cell. Cardiol. 45, 240–249 [DOI] [PubMed] [Google Scholar]
- 31. Kemi O. J., Hoydal M. A., Macquaide N., Haram P. M., Koch L. G., Britton S. L., Ellingsen O., Smith G. L., Wisloff U. (2011) The effect of exercise training on transverse tubules in normal, remodeled, and reverse remodeled hearts. J. Cell. Physiol. 226, 2235–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minetti C., Bado M., Broda P., Sotgia F., Bruno C., Galbiati F., Volonte D., Lucania G., Pavan A., Bonilla E., Lisanti M. P., Cordone G. (2002) Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency. Am. J. Pathol. 160, 265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ratajczak P., Damy T., Heymes C., Oliviero P., Marotte F., Robidel E., Sercombe R., Boczkowski J., Rappaport L., Samuel J. L. (2003) Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc. Res. 57, 358–369 [DOI] [PubMed] [Google Scholar]
- 34. Maczewski M., Mackiewicz U. (2008) Effect of metoprolol and ivabradine on left ventricular remodelling and Ca2+ handling in the post-infarction rat heart. Cardiovasc. Res. 79, 42–51 [DOI] [PubMed] [Google Scholar]
- 35. Rehsia N. S., Dhalla N. S. (2010) Mechanisms of the beneficial effects of beta-adrenoceptor antagonists in congestive heart failure. Exp. Clin. Cardiol. 15, e86–95 [PMC free article] [PubMed] [Google Scholar]
- 36. Reiken S., Wehrens X. H., Vest J. A., Barbone A., Klotz S., Mancini D., Burkhoff D., Marks A. R. (2003) Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 107, 2459–2466 [DOI] [PubMed] [Google Scholar]
- 37. Wei S., Chow L. T., Sanderson J. E. (2000) Effect of carvedilol in comparison with metoprolol on myocardial collagen postinfarction. J. Am. Coll. Cardiol. 36, 276–281 [DOI] [PubMed] [Google Scholar]
- 38. Colucci W. S., Kolias T. J., Adams K. F., Armstrong W. F., Ghali J. K., Gottlieb S. S., Greenberg B., Klibaner M. I., Kukin M. L., Sugg J. E. (2007) Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the reversal of ventricular remodeling with Toprol-XL (REVERT) trial. Circulation 116, 49–56 [DOI] [PubMed] [Google Scholar]
- 39. Doughty R. N., Whalley G. A., Gamble G., MacMahon S., Sharpe N. (1997) Left ventricular remodeling with carvedilol in patients with congestive heart failure due to ischemic heart disease. Australia-New Zealand Heart Failure Research Collaborative Group. J. Am. Coll. Cardiol. 29, 1060–1066 [DOI] [PubMed] [Google Scholar]
- 40. Khattar R. S., Senior R., Soman P., van der Does R., Lahiri A. (2001) Regression of left ventricular remodeling in chronic heart failure: comparative and combined effects of captopril and carvedilol. Am. Heart. J. 142, 704–713 [DOI] [PubMed] [Google Scholar]
- 41. Doughty R. N., Whalley G. A., Walsh H. A., Gamble G. D., Lopez-Sendon J., Sharpe N. (2004) Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation 109, 201–206 [DOI] [PubMed] [Google Scholar]
- 42. Landstrom A. P., Kellen C. A., Dixit S. S., van Oort R. J., Garbino A., Weisleder N., Ma J., Wehrens X. H., Ackerman M. J. (2011) Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ. Heart Fail. 4, 214–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Minamisawa S., Oshikawa J., Takeshima H., Hoshijima M., Wang Y., Chien K. R., Ishikawa Y., Matsuoka R. (2004) Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochem. Biophys. Res. Commun. 325, 852–856 [DOI] [PubMed] [Google Scholar]
- 44. Xu M., Zhou P., Xu S. M., Liu Y., Feng X., Bai S. H., Bai Y., Hao X. M., Han Q., Zhang Y., Wang S. Q. (2007) Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol. 5, e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ziman A. P., Gomez-Viquez N. L., Bloch R. J., Lederer W. J. (2010) Excitation-contraction coupling changes during postnatal cardiac development. J. Mol. Cell. Cardiol. 48, 379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]