Abstract
We measured the effects of a diet in which d-β-hydroxybutyrate-(R)-1,3 butanediol monoester [ketone ester (KE)] replaced equicaloric amounts of carbohydrate on 8-wk-old male C57BL/6J mice. Diets contained equal amounts of fat, protein, and micronutrients. The KE group was fed ad libitum, whereas the control (Ctrl) mice were pair-fed to the KE group. Blood d-β-hydroxybutyrate levels in the KE group were 3-5 times those reported with high-fat ketogenic diets. Voluntary food intake was reduced dose dependently with the KE diet. Feeding the KE diet for up to 1 mo increased the number of mitochondria and doubled the electron transport chain proteins, uncoupling protein 1, and mitochondrial biogenesis-regulating proteins in the interscapular brown adipose tissue (IBAT). [18F]-Fluorodeoxyglucose uptake in IBAT of the KE group was twice that in IBAT of the Ctrl group. Plasma leptin levels of the KE group were more than 2-fold those of the Ctrl group and were associated with increased sympathetic nervous system activity to IBAT. The KE group exhibited 14% greater resting energy expenditure, but the total energy expenditure measured over a 24-h period or body weights was not different. The quantitative insulin-sensitivity check index was 73% higher in the KE group. These results identify KE as a potential antiobesity supplement.—Srivastava, S., Kashiwaya, Y., King, M. T. Baxa, U., Tam, J., Niu, G., Chen, X., Clarke, K., Veech, R. L. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet.
Keywords: brown fat, electron transport chain, sympathetic activity, insulin sensitivity, energy expenditure
Ketogenic (KG) diets have been successfully used for the treatment of drug-resistant epilepsy (1). The beneficial effects of a KG diet have also been demonstrated in a variety of neurological disorders, such as Parkinson's disease (2), traumatic brain injury (3), and Alzheimer's disease (4). Although the exact mechanisms involved are not clearly understood, increased mitochondrial biogenesis in hippocampi (5), increased production of the inhibitory neurotransmitter GABA (6), and reduced levels of the excitatory neurotransmitter glutamate (7) could be involved. Exposure to ketone bodies has been shown to improve cardiac efficiency by improving the free energy of ATP hydrolysis (8). Some studies have also shown benefits of KG diets in obese patients with diabetes (9), although the mechanisms remain poorly understood. Elevation of blood ketone levels is thought to play an important role in the protective effects of KG diets (10). However, the ketone body levels achieved with KG diets are modest. Further, to achieve effective ketosis with KG diets, almost complete avoidance of carbohydrates is required to keep blood insulin levels low to maintain adipose tissue lipolysis. Such high-fat, no-carbohydrate diets are unpalatable, leading to poor patient compliance (11). In addition, these diets have been associated with elevation of blood cholesterol and blood lipids in humans (12), and fatty liver and hepatic insulin resistance in mice (13). To circumvent the deleterious side effects of KG diets, we have synthesized an ester of the ketone body [d-3-hydroxybutyrate (βHB)] and (R)-1,3 butanediol, which, when added to a diet, increases plasma ketone levels without the need for a high-fat diet or starvation and without elevating plasma free fatty acids (FFAs; ref. 7). Thus, this ester makes it possible to dissociate the effects of ketone bodies from elevation of FFAs, which has hitherto been impossible. In addition, the levels of ketone bodies are not affected by the presence of insulin. It is also possible to regulate the ketone levels in the blood by feeding differing amounts of the ester.
Given the increased prevalence of obesity, which occurs because of an imbalance in energy intake and expenditure, it is of interest to evaluate whether elevated blood ketone levels have any effect on energy intake and expenditure. Studies in humans have indicated that low-carbohydrate KG diets are associated with reduced weight gain or weight loss (14). However, it is not clear whether this effect is due to reduced caloric intake or increased energy expenditure. In mice, a KG diet increases total energy expenditure (TEE) but does not affect caloric intake (13, 15). The effects of a KG diet on locomotor activity have been divergent, with one study reporting reduced activity in rats (16) and another finding no change in mice (13). Thus, it is clear that the increased energy expenditure with consumption of a KG diet is not due to increased activity levels but perhaps to changes in the cellular makeup of some tissues involved in energy expenditure, such as activation of peroxisome proliferator activated receptor (PPAR) transcription factors and uncoupling proteins (UCPs).
One tissue that has been suggested to be important for energy homeostasis is brown adipose tissue (BAT; refs. 17, 18), which plays an important role in maintenance of body temperature through nonshivering thermogenesis (19, 20). BAT produces heat by uncoupling electron transport from ATP synthesis through a brown fat-specific UCP, UCP1, which is also present in certain cells in white adipose tissue (WAT; ref. 21). Reduced BAT UCP1 levels have been shown to be associated with an increase susceptibility of mice fed a high-fat diet to obesity, type 2 diabetes, and hyperlipidemia (22). It has also been proposed that activation of brown fat could be a viable treatment for obesity (23, 24). Whether increased brown fat activation and increased UCP1 levels are sufficient to increase whole-body energy expenditure and regulate body weight and fat is, however, a matter of dispute (25).
Both high-fat non-KG diets (26) and high-fat KG diets (15) increase UCP1 in brown fat. It has been presumed that the increase in UCP1 resulted from the activation of the PPAR system of transcription factors. Whether ketosis plays any role in the elevation of UCP1 independent of elevation of blood FFAs could not be examined before the development of ketone esters (KEs).
MATERIALS AND METHODS
Diets and preparations
Powdered Lieber-DeCarli diets were purchased from Bio-Serv (products F1258SP and F1259SP; Bio-Serv, Frenchtown, NJ, USA). The liquid control diet was prepared by adding warm water to the Lieber-DeCarli control diet powder. The KE diet was prepared by adding warm water and charcoal-filtered KE as an equicaloric replacement for maltodextrin. A sweetener (acesulfame K at 0.3% w/v; TCI America, Portland OR, USA) and commercially available mint flavor (at 0.03% v/v; McCormick, Sparks, MD, USA) were added to the liquid diets. The macronutrient composition of the diets is given in Table 1. The two liquid diets contained equal amounts of fat and protein as well as same number of total calories per gram.
Table 1.
Composition of the diets
| Component | Ctrl | KE |
|---|---|---|
| Protein (kcal/L) | 180 | 180 |
| Fat (kcal/L) | 350 | 350 |
| Carbohydrate (kcal/L) | 470 | 186 |
| Ketones (kcal/L) | 0 | 287 |
| Total (kcal/L) | 1000 | 1003 |
Mice and feeding
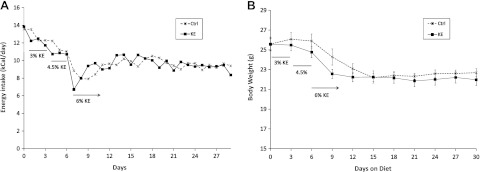
All experimental procedures were approved by the animal care and use committee of the U.S. National Institutes of Health. Eight-week-old male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Animals were housed in temperature (21°C)- and humidity (relative humidity 70%)-controlled rooms with a 12-h light-dark cycle. Animals were acclimated for 4 d before the experimental diets were started. The mice were trained to eat new diets for 3 d, by providing the diets in addition to chow, before switching over completely to the new diets. Fresh diets were provided every evening, <30 min before the start of dark cycle. The food intake was measured daily, and the body weight of animals was measured every third day. Mice fed the KE diet were trained on a diet containing 2.5–3% (w/v) KE and were fed this diet for a further 3 d after training. The KE concentration in the diet was increased to 4.5–5% w/v after 3 d and to 6% after 6 d. The 6% KE diet was used for the remainder of the study. The control (Ctrl) animals were pair-fed to the KE group, in that the control group was given the average amount of liquid diet consumed by the KE group the day before.
Measurement of blood glucose and ketone levels
Blood glucose and βHB levels were measured using a Precision Xtra meter (Abbott Laboratories, Abbott Park, IL, USA) and corresponding glucose and ketone sticks. Animals were placed in a restrainer, and their tail ends were snipped. Glucose and ketone levels were measured directly from the blood thus obtained.
Blood plasma and tissue collection
At the end of the treatment, the animals were anesthetized, and blood was collected in heparinized syringes after cardiac puncture. Blood was kept on ice and centrifuged within 30 min. Plasma was obtained by centrifuging at 4000 g for 10 min at 4°C. Animals were sacrificed by cervical dislocation and decapitation. Interscapular brown adipose tissue (IBAT) and epididymal fat pads were removed, placed in a tube, frozen in liquid nitrogen, and stored at −80°C.
Hematoxylin and eosin (H&E) staining
After the mice were fed the diets for 3 wk, their IBAT was harvested. One hemi-IBAT was immersion-fixed in 4% paraformaldehyde in PBS. The fixative was replaced after ∼1 h with fresh fixative, and the tissues were stored in the fixative at 4°C until further analysis. Routine H&E staining was performed by a histology services company (Histoserv Inc., Gaithersburg, MD, USA).
Transmission electron microscopy (TEM) of IBAT
After dissection of BAT, the tissue pieces were cut into small pieces (∼1–2 mm) and immersed in 0.1 M phosphate buffer containing 2% paraformaldehyde and 2.5% glutaraldehyde (fixing buffer). The fixing buffer was removed and replaced with fresh fixing buffer after 1 h, and the tissues were postfixed for 24 h. Then, the fixing buffer was removed, and the tissue was washed in 0.1 M cacodylate buffer 3 times and stored in this buffer at 4°C. Tissue was processed as described in ref. 27 with minor modifications. After postfixation in 1% osmium tetroxide for 1 h and en bloc staining in 0.5% uranyl acetate for 1 h, the samples were dehydrated in a graded series of 35, 50, 70, and 100% ethanol and exchanged to propylene oxide. The tissue samples were then infiltrated at 1:1 propylene oxide and epoxy resin overnight, allowing for evaporation of propylene oxide, and embedded in 100% epoxy resin the next day. Polymerization of resin was performed for 3 d at 55°C. Thin sections of 70–90 nm were cut on an ultramicrotome (Leica UC6; Leica Microsystems, Buffalo Grove, IL, USA), stained with uranyl acetate and lead citrate, lightly carbon coated, and imaged in a Hitachi 7650 transmission electron microscope (Hitachi High-Tech, Schaumburg, IL, USA) operating at 80 kV. Images were taken with an AMT digital camera (Advanced Microscopy Techniques, Woburn, MA, USA).
Quantitative insulin-sensitivity check index (QUICKI) and leptin measurement
QUICKI measurements were performed after feeding of the diets for 3 wk. Mice were unfed for 12 h before blood samples were collected through cardiac puncture in EDTA-containing tubes. Fasting blood glucose was measured using glucose sticks in a Precision Xtra glucose meter. The blood samples were immediately spun at 4000 g for 10 min to collect plasma. Leptin concentrations were measured in the plasma using a Leptin Mouse/Rat ELISA kit (Alpco Diagnostics, Salem, NH, USA). Plasma insulin concentrations were measured using a Mouse Ultrasensitive Insulin ELISA kit (Alpco Diagnostics), using 25 μl of plasma samples in duplicate. The QUICKI (28) was calculated using fasting blood glucose (Gf, mg/dl) and fasting plasma insulin (If, μU/ml) as
| (1) |
FFA measurement
FFAs were measured in the plasma samples that were collected in EDTA-containing tubes. An HR Series NEFA-HR(2) kit (Wako Chemicals USA, Richmond, VA, USA) was used to measure FFAs in plasma drawn from mice after 12 h without food. Measurements were performed according to the instructions from the manufacturer for the microplate assay.
Western blotting
Ten volumes (10 μl/mg) of ice-cold lysis buffer (20 mM HEPES, pH 7.4; 100 mM NaCl; 1% Triton X-100; 1% SDS; 1% sodium deoxycholate; 5 mM nicotinamide; and 10 mM sodium butyrate) containing protease and phosphatase inhibitor cocktails (Pierce Chemicals, Rockford, IL, USA) were added to frozen IBAT. The tissue was then minced and sonicated on ice. After incubation on ice for 1 h, lysates were centrifuged at 10,000 g for 15 min at 4°C, and clear portions of supernatants were collected. Protein concentration in the lysates was measured by a detergent-compatible (DC) protein assay (Bio-Rad Laboratories, Hercules, CA, USA). To prepare samples for electrophoresis, 3× loading buffer (Cell Signaling Technology, Danvers, MA, USA) containing DTT was added to equal protein amounts from different samples. Novex 4–20% Tris-glycine gels (15 wells × 1 mm) were run under denaturing conditions in Tris-glycine-SDS buffer. Proteins were blotted onto 0.45-μm PVDF membranes (Immobilon-P; Millipore, Billerica, MA, USA) using Towbin transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol, pH 8.3). The tank containing the assembly was filled with cold water. After transfer, blots were blocked for 1 h at room temperature with 5% milk powder in TBS-T, washed with TBS-T, and exposed to primary antibodies diluted in Superblock-TBS buffer (Pierce Chemicals). Blots were incubated in primary antibodies overnight at 4°C. After three 10-min washes with TBS-T, blots were exposed to corresponding secondary antibodies in TBS-T for 1–2 h at room temperature. After 3 washes with TBS-T, blots were developed using SuperSignal West Dura Substrate (Pierce Chemical) and imaged using a VersaDoc imager (Bio-Rad Laboratories). Protein (5 μg) was loaded onto each lane for determination of mitochondrial electron transport chain (ETC) proteins, as well as UCP1, PPAR-γ, and PPAR-γ coactivator 1 α (PGC-1α). For all other proteins, 50 μg of protein was loaded onto each lane. Primary antibodies to cytochrome c (CytC), cytochrome c oxidase subunit IV (Cox-IV), and cAMP response element-binding protein (CREB) were from Cell Signaling Technology. Antibodies to succinate dehydrogenase (complex 2) 70-kDa subunit A (SDHa), AMPK, and PPAR-γ were from Abcam (Cambridge, MA, USA). Succinate dehydrogenase (complex 2) 30-kDa subunit B (SDHb) and NADH:ubiquinone oxidoreductase (complex 1) 70-kDa iron-sulfur subunit 1 (Ndufs1) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to mammalian homolog of silent information regulator 2 (Sir2) 1 and 3 (Sirt1 and Sirt3) were from Millipore (Bedford, MA, USA). UCP1 antibody was from Alpha Diagnostics (San Antonio, TX, USA), and the PGC-1α antibody was from Calbiochem/Millipore. MS-604 antibody cocktail was purchased from Mitosciences (Eugene, OR, USA).
IBAT cAMP measurement
IBAT cAMP was measured using a kit from Enzo Life Sciences/Assay Designs (Plymouth Meeting, PA, USA) using the manufacturer's instructions. In brief, the tissue was ground and lysed in 10 volumes of cold 0.1 M HCl. Tissue was homogenized using a pestle. Then, another 10 volumes of 0.1 M HCl was added and the solution was mixed, followed by brief (20-s) centrifugation at 4°C to settle debris. The supernatant was carefully decanted into a new tube and centrifuged again. The clear supernatant was diluted 2.5-fold with 0.1 M HCl to measure tissue cAMP levels, according to the manufacturer's instructions. Protein concentration was measured using a DC protein assay.
[18F]-Fluorodeoxyglucose (FDG)–positron emission tomography (PET) imaging and biodistribution study
Mice were fed the diets for 3 wk before imaging. Four mice in each group were imaged. After 4 h of food withdrawal, mice were injected intraperitoneally with 3.7 MBq (100 μCi) of [18F]-FDG purchased from the Nuclear Pharmacy of Cardinal Health (Dublin, OH, USA) and reconstituted with sterile saline. Mice were kept awake at room temperature during the uptake period (29). At 1 h after the injection, mice were anesthetized with isoflurane inhalation for the imaging. PET scans and image analysis were performed using an Inveon MicroPET scanner (Siemens Medical Solutions, Washington, DC, USA). Static scans (5 min) were acquired, and the images were reconstructed using a 2-dimensional ordered-subset expectation maximum algorithm. No correction was applied for attenuation or scatter.
Immediately after PET imaging, the mice were sacrificed, and their IBAT was dissected and weighed. The radioactivity in the wet IBAT was measured with a γ counter (Packard Instrument, Meriden, CT, USA). The results are expressed as percentage of injected dose per gram of tissue for a group of 4 mice. Values are expressed as means ± sd (n=4/group).
Indirect calorimetry
Mice were fed for 3 wk and then transferred to the metabolic cages (Oxymax; Columbus Instruments, Columbus, OH, USA). Mice were housed individually in the metabolic cages, and a similar feeding regimen was continued. The animals were acclimated to the chambers for 1 d, and O2 consumption (Vo2) and CO2 production (Vco2) rates were measured over the next 24 h as described previously (30). The chambers were also equipped with 2-dimensional infrared beam sensors (Opto-M3; Columbus Instruments) to measure locomotor activity. TEE over a period of 24 h was calculated as Vo2 × (3.815 + 1.232 × RQ), where RQ is the respiratory quotient (the ratio Vco2/Vo2). To measure the resting energy expenditure, the energy expenditure data from the 12-h period of least ambulatory activity (6:00 AM to 6:00 PM) was used. Data are presented on a per mouse basis and are not normalized to body weight (31, 32).
Data analysis
Results are presented as means ± se of n = 6 animals/group unless specified otherwise. Statistical analysis was conducted in Excel (Microsoft, Redmond, WA, USA) using a Student's t test to identify significant differences at values of P < 0.05.
RESULTS
Food intake and body weight
The caloric intake of mice fed the KE diet decreased as the concentration of KE in the diet was increased (Fig. 1A), and stabilized after 1 wk. The profile of their body weight mirrored that of the caloric intake. After an initial loss of 12–15% of initial body weight with the KE diet, weight stabilized at a new lower level (Fig. 1B). There was no difference in the weights of the KE and Ctrl groups. Owing to the design of the diets, there was no difference in the protein, fat, or micronutrient concentrations of the diets. Because the animals were pair-fed, those components as well as caloric intake were similar between the two groups (Table 2).
Figure 1.
Caloric intake (A) and body weights (B) of mice fed different diets. Ctrl, pair-fed control group; KE; ad libitum ketone ester-fed group.
Table 2.
Daily caloric intake as protein, fat, carbohydrate, and ketone
| Component | Ctrl | KE |
|---|---|---|
| Protein | 1.69 | 1.69 |
| Fat | 3.29 | 3.28 |
| Carbohydrate | 4.42 | 1.74 |
| Ketone | 0.00 | 2.69 |
| Total | 9.41 | 9.40 |
Blood ketones and plasma FFA and leptin levels
Blood ketone levels were higher during the dark phase (∼7 mM βHB), compared with the light phase (∼4 mM) in the KE group (Fig. 2A). The ketone levels are similar to those found in humans during prolonged fasting (33, 34) and are 3- to 5-fold higher than the levels reported for mice fed KG diets (13, 15). Mice fed the KE diet had ∼2-fold the levels of plasma FFAs as the pair-fed controls (Fig. 2B). The results of elevated FFAs are different from those in our previous study in rats (7), in which no effect of KE on FFA levels was seen, indicating a species-specific response. Plasma leptin levels of the mice in the KE group were >2-fold higher than those in the Ctrl group (Fig. 2C).
Figure 2.
A) Blood βHB levels in mice 4 h into dark or light phases. Data are presented as means ± se of n = 12 mice. B) Plasma FFA level. C) Plasma leptin levels. Data are presented as the means ± se of n = 6 mice. *P < 0.05 vs. Ctrl group.
Tissue weights, IBAT appearance, and TEM analysis
IBAT from mice fed the KE diet weighed significantly less (Table 3) but was significantly darker than the IBAT from the Ctrl group (Fig. 3A). In mitochondria-rich tissues, the color of the tissue is associated with mitochondrial levels of CytC (35). The darker color of IBAT in the KE group suggested increased mitochondrial content in the tissue. H&E staining of the IBAT revealed that the intracellular lipid content in the IBAT from the KE group was much less than that in the pair-fed controls (Fig. 3B).
Table 3.
Fat pad weights, absolute and as a percentage of total body weight
| Parameter | Ctrl | KE |
|---|---|---|
| IBAT (mg) | 118 ± 14 | 88 ± 17* |
| Relative IBAT (% body weight.) | 0.52 ± 0.05 | 0.40 ± 0.05 |
| eWAT (mg) | 251 ± 60 | 193 ± 24 |
| Relative eWAT (% body weight.) | 1.07 ± 0.25 | 0.84 ± 0.09 |
P < 0.05 vs. Ctrl.
Figure 3.
Representative IBAT images. A) IBAT from KE animals was much darker than that from the Ctrl animals (n=12). B) H&E staining images (×40 view) showing reduced lipid content in the IBAT from KE-treated mice. C) TEM images of the IBAT showing reduced size of lipid droplets and much greater density of mitochondria in IBAT from KE-fed mice. Scale bars = 2 μm. N, nucleus; L, lipid droplets; M, mitochondria; C, capillary; E, erythrocyte.
TEM analysis showed increased mitochondrial content in the BAT of animals treated with KE. In agreement with the H&E staining results, significantly less lipid content was observed in TEM images, too. Volumetric analysis revealed that in the KE group, mitochondria occupied 53% of the cell volume and lipids occupied 22%, whereas in the Ctrl group, mitochondria occupied 16% of the volume and lipids occupied 61% (Fig. 3). The average number of mitochondria per cell in the KE group was ∼1.7-fold that in the Ctrl group, and the average mitochondrial size was ∼2.2-fold that of the Ctrl group. Thus, there is an increase not only in the number of mitochondria but also in the average size of the mitochondria. Although the number of lipid droplets was greater (∼2-fold) in the KE group, the average size of lipid droplets was ∼5 times smaller. Thus, the TEM analysis indicated mitochondrial biogenesis and increased multilocular droplets in the BAT of KE-treated mice. The significantly reduced lipid content of the tissue, perhaps due to an increase in mitochondrial mass, could explain the overall reduction in mass of the BAT seen in KE-treated mice.
The relative epididymal white adipose tissue (eWAT) from mice fed the KE diet was borderline significantly (P=∼0.06) less than eWAT from the Ctrl group (Table 3).
Changes in IBAT mitochondrial ETC protein levels and UCP1
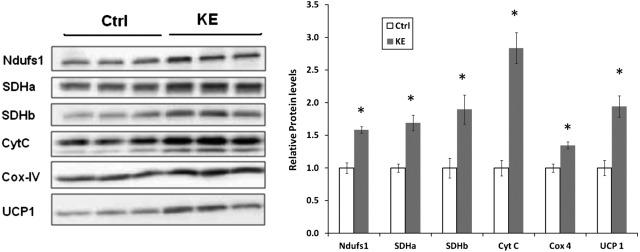
To estimate the increase in mitochondrial content and function, levels of various mitochondrial ETC proteins were measured. The IBAT mitochondrial ETC proteins increased anywhere from 30 to 200% in the KE group (Fig. 4). Similar results were obtained when Western blotting was performed with a cocktail of antibodies against mitochondrial proteins (MS604; MitoSciences) but are not shown. In addition, the levels of brown fat-specific UCP1 were also ∼2-fold those in controls, in proportion to the increase in mitochondrial proteins.
Figure 4.
Changes in the levels of mitochondrial proteins. Levels of various mitochondrial proteins were measured by Western blotting and corresponding densitometric analyses. First 3 lanes correspond to the Ctrl group; last 3 lanes correspond to the KE group. *P < 0.05 vs. Ctrl group.
Effect on IBAT cAMP and CREB levels
BAT has been shown to be regulated by the action of sympathetic nerves, which elevates cAMP levels in the tissue. Indeed, the IBAT from mice fed the KE diet had significantly elevated cAMP levels (Fig. 5A), suggesting sympathetic stimulation of IBAT. Elevated cAMP activates CREB, which regulates transcription of a variety of genes including UCP1, CytC, and PGC-1α (36–39). Mice fed test diets had an ∼2-fold increase in CREB (Fig. 5B).
Figure 5.
IBAT cAMP and CREB levels. cAMP levels (A) and CREB (B) levels in IBAT measured by Western blot and corresponding densitometric analysis. *P < 0.05 vs. Ctrl group.
Changes in mitochondrial biogenesis-related proteins in IBAT
PPAR-γ and its coactivator PGC-1α have been shown to regulate IBAT differentiation and expression of UCP1 (40). PGC-1α has been suggested to be the master regulator of mitochondrial biogenesis in a variety of tissues (41). Feeding the KE diet increased the levels of PPAR-γ and PGC-1α (Fig. 6).
Figure 6.
Western blots for PPAR-γ, PGC-1α, Sirt1, Sirt3, and AMPK. First 3 lanes correspond to the Ctrl group; last 3 lanes correspond to the KE group. *P < 0.05 vs. Ctrl group.
Sirt1 has been suggested as a plausible regulator of brown adipocyte differentiation in that the genes that are increased during differentiation of brown preadipocytes are the same genes that are increased when Sirt1 is overexpressed in myoblasts (42). Feeding the KE diet significantly increased the levels of Sirt1 in brown fat (Fig. 6). These results indicate that elevation of blood ketone bodies, independent of elevation of blood FFAs, increased the differentiation of brown preadipocytes. The process of differentiation is also associated with an increase in mitochondrial protein levels in these cells (19).
Sirt3 overexpression has been shown to induce PGC-1α and UCP1 expression in the brown preadipocyte cell line HIB-1B (43). Feeding the KE diet did not change the levels of Sirt3 in brown fat (Fig. 6). Activation of AMPK has been shown to mediate mitochondrial biogenesis in skeletal muscle (44, 45). Mice fed the KE diet had elevated AMPK levels in BAT (Fig. 6).
PET imaging of [18F]-FDG uptake
PET imaging revealed that mice fed the KE diet had significantly higher uptake of [18F]-FDG in the IBAT (Fig. 7A). Measurement of FDG uptake in excised IBAT with a γ counter agreed well with the imaging results (Fig. 7B). Mice fed the KE diet had ∼2-fold greater FDG uptake in IBAT compared with the Ctrl group (Fig. 7B).
Figure 7.
[18F]-FDG PET imaging and biodistribution analysis. A) Representative PET images of coronal (top panel) and transaxial sections around IBAT (bottom panel). Arrows indicate IBAT. B) γ-Counter measurement of radioactivity in IBAT. Percentage of the initial dose of [18F]-FDG accumulated per gram of IBAT (%ID/g) was calculated by measuring the radioactivity in excised IBAT. Data are presented as means ± se of n = 4 animals. *P < 0.05 vs. Ctrl group.
Increased insulin sensitivity with the KE diet
Although the fasting blood glucose levels were not altered (P=0.08), the levels of insulin were significantly lower in the KE-treated mice (Table 4). The QUICKI, which is a surrogate marker of insulin sensitivity, was significantly higher in the KE group.
Table 4.
Fasting blood glucose, plasma insulin, and QUICKI values
| Parameter | Ctrl | KE |
|---|---|---|
| Fasting blood glucose (mg/dl) | 206 ± 11 | 174 ± 16 |
| Fasting plasma insulin (ng/ml) | 0.13 ± 0.03 | 0.02 ± 0.00* |
| QUICKI | 0.37 ± 0.01 | 0.64 ± 0.11* |
P < 0.05 vs. Ctrl.
UCP1 levels in eWAT
The KE diet also increased the UCP1 levels in the eWAT tissue (Fig. 8). However, the levels of mitochondrial proteins were not changed in this tissue (not shown).
Figure 8.
Western blot and densitometric analysis of UCP1 levels in the eWAT. *P < 0.05 vs. Ctrl group.
Indirect calorimetry
There was no statistically significant difference in the locomotor activity between the two groups, in part because of the large variation in the data (Fig. 9A). The Vo2 of the mice in the KE group was higher for most of the day, except near the time food was provided, when there was an increase in the Vo2 of Ctrl group mice (Fig. 9B). The profile of Vco2 mirrored that of Vo2, being higher in the KE group for most of the day, except near the time food was provided (Fig. 9C). The RQ values of the pair-fed Ctrl group were close to 0.7 for a large part of the day (Fig. 9D), indicating that the oxidation of fat predominates during this period. However, the RQ increased to ∼1 after food was provided, indicating oxidation of carbohydrate postmeal. This behavior of the RQ is in good agreement with a previous study of calorie-restricted mice (46). That study showed that under calorie restriction, the postprandial period is associated with initial fat synthesis, which is oxidized in the later part of the day. Thus, the RQ of mice under calorie restriction goes to ∼1 postmeal when glucose oxidation and lipid synthesis occur and falls to ∼0.7 4–6 h postmeal as the switch to fatty acid oxidation occurs. The theoretical RQ for βΗΒ oxidation is 0.89. The RQ for the KE group was close to 0.7, varying from 0.70 to 0.76. This indicates that fatty acid oxidation was the predominant mode of energy production in this group too, although some amount of other substrates was also used. The increased fatty acid oxidation in the KE group perhaps indicates a glucose-sparing effect of the ketone bodies as seen in our previous studies in isolated perfused hearts (47).
Figure 9.
Ambulatory activity (A), Vo2 (B), Vco2 (C), RQ (D), and daily resting and total energy expenditure (E) of mice. Vertical arrow at ∼1815 h represents the time food was provided to the mice. *P < 0.05 vs. Ctrl group.
There was no difference in the 24-h energy expenditure between the two groups (Fig. 9E). Thus, the body weight data showed no difference between the two groups. However, the KE diet increased the resting energy expenditure (Fig. 9E), in agreement with the activation of brown fat. The resting energy expenditure for the KE group was ∼14% greater than that for the Ctrl group.
DISCUSSION
Because ketone bodies can serve as an alternative fuel for brain and KG diets have been demonstrated to be useful in treating drug-resistant epilepsy, most studies have investigated the effects of elevated ketone levels on brain. Here we show that IBAT is also a ketone-responsive tissue and elevated ketone levels cause significant changes in IBAT physiology. Because the fat, protein, and nutrient contents of the test and the control diets were the same, the changes in IBAT could be attributed to elevated blood ketone levels.
Rodent newborns have larger relative (body weight normalized) amounts of brown fat and higher levels of circulating ketone bodies than adults. The large amount of brown fat in newborns is supposed to play a role in thermoregulation. It is likely that the ketone bodies are used as fuel in the brown fat of newborns and have a role in maintaining brown fat. As the animals grow and their ketone levels drop, there is a reduction in the brown fat levels too. Our results show that the brown fats from adult animals retain their responsiveness to ketones.
The KE diet reduced the ad libitum caloric intake in the mice, which is consistent with an increase in plasma leptin levels found in this group. Blood leptin levels are reported to increase with increasing body fat. However, in the case of KE feeding, the leptin levels were elevated without an increase in body weight or fat levels. The increase in plasma leptin levels would reduce food intake (48) by increasing hypothalamic malonyl-CoA (49) and enhance insulin-stimulated glucose metabolism and induce UCP1 in brown fat (50). Increased leptin levels also potentiate the sympathetic activity to brown fat (51). However, the increased leptin levels with the KE diet in mice are opposite of what we found with rats (7). There are 3 reasons for this divergent response: the difference in species (mice vs. rats); the difference in feeding (blood was collected postmeal in the rat study, compared with ad libitum feeding in the present study); and the difference in ketone levels. In the present study, the ketone levels are elevated throughout the day, whereas in rat study, the ketone levels were increased after the meal and decrease ∼6 h after meal and remain low for the remainder of the day. Further, the ketone levels in mice were always greater than the peak levels in the rat study. Whether elevated ketone levels and the level of this elevation can affect leptin levels in the rat needs further investigation. A previous study reported that a high-fat KG diet does not increase serum leptin in mice (15).
Our results of increased cAMP and CREB levels in the IBAT of mice treated with KE show that this treatment increases sympathetic stimulation to this tissue. The levels of UCP1 in brown fat as well as white fat have been shown to be increased with a sympathetic agonist (52), although the activation of PPAR-γ may be sufficient for brown fat recruitment in WAT independent of sympathetic stimulation (53). In addition, the TEM analyses demonstrated reduced lipid content in the IBAT from KE-treated mice. This observation is also in line with sympathetic stimulation, which increases lipolysis through activation of PKA. The reduced lipid content could also be the reason for a reduction in the weight of this tissue in KE-treated mice. Our results of increased sympathetic activity in the ketone group, however, do not agree with previous work (54), which suggested that βHB suppressed sympathetic nervous system activity by antagonizing GPR41 in vitro and reduced heart rate in vivo. In their study, under in vitro conditions, the ketone bodies suppressed the propionate-induced increase in the G-protein-coupled receptor 41 activity but did not have any effect in the absence of propionate on pERK/ERK. The ketone body also suppressed the reduction in cellular cAMP levels caused by propionate. In addition, in their in vivo studies, a reduction in heart rate was observed as the ketone levels were going down. In our own studies with isolated perfused heart, no reduction in heart rate was observed with ketone bodies. In fact, the cardiac output, hydraulic work, oxygen consumption, and efficiency increased with ketone bodies (8). Our results agree with Kolanowski et al. (55), who have shown increased sympathetic stimulation, as measured by norepinephrine turnover in IBAT on feeding of d(−)-3-hydroxybutyrate. The underlying cause of increased sympathetic activity to brown fat in the KE group animals is not clear. We measured core body temperature with a rectal probe after 2 wk on the diet, and it was not different between the groups.
Mice in the KE group had significantly elevated plasma FFA levels. This is in agreement with increased lipolysis in brown fat and somewhat reduced epididymal white fat levels observed in these animals. It has also been shown that fat depots (56), and to a lesser extent liver (57), could use ketone bodies to synthesize lipids. Perhaps because of the increased sympathetic activity, the FFAs synthesized from ketone bodies are not stored but secreted. However, the result of increased plasma FFAs in mice is different from that in our previous study in rats (7), in which elevated ketone bodies did not increase plasma FFAs. Thus, there is a species-specific effect of ketone bodies on FFA levels.
The Vo2 and Vco2 of mice in the KE group were higher for most of the day. The increase in UCP1 in the brown fat occurred to the same extent as the increase in mitochondrial proteins. Thus, it is likely that the increase in UCP1 reflects the increased mitochondrial content, and there is no change in UCP1 levels per mitochondrion. Our data suggest an increase in mitochondria and not increased uncoupling. In contrast, the level of UCP1 in WAT increased without a concomitant increase in mitochondrial content. This result probably reflects an increase in the recruitment of brown adipocytes in the WAT depot. In agreement with increased IBAT mitochondrial and UCP1 levels in brown fat, as well as increased UCP1 in the epididymal white fat depot, the KE group had significantly greater resting energy expenditure. However, the total daily energy expenditure was not different between the two groups. Thus, it appears that even though the brown fats from KE-fed animals are quite capable of dissipating energy, they are not doing it perhaps because of reduced caloric intake.
The IBAT from the KE group weighed ∼20% less than that of the control group, probably due to the decrease in IBAT fat stores. The reduction in weight was more than compensated for by an ∼2-fold increase in mitochondrial proteins and significantly increased glucose uptake. Our result of increased IBAT FDG uptake by the KE diet group is opposite to the reduced FDG uptake in IBAT of mice treated with a KG diet (13). These results show that the reduced FDG uptake with a KG diet is not due to elevated ketones but perhaps is due to insulin resistance developed in IBAT because of the high-fat KG diet. Our results of increased QUICKI values in the KE-treated group demonstrate that elevated ketone levels without an elevation of fat may, in fact, increase overall insulin sensitivity.
Therefore, our results of reduced voluntary food intake, increased insulin sensitivity, increased resting energy expenditure, and brown fat activity in the KE-treated group demonstrate the utility of this compound as a potential antiobesity supplement.
Acknowledgments
The authors thank Dr. Pal Pacher (National Institute on Alcohol Abuse and Alcoholism, U.S. National Institutes of Health, Bethesda, MD, USA) for unrestricted access to the imager for developing the Western blots.
The intellectual property rights to the ketone esters are owned by the National Institutes of Health and Oxford University with R.L.V. and K.C. eligible to obtain royalty payments in accordance with the rules of the various institutions, should royalty payments occur.
Footnotes
- BAT
- brown adipose tissue
- βHB
- d-3-hydroxybutyrate
- CREB
- cAMP response element-binding protein
- Cox-IV
- cytochrome c oxidase subunit IV
- Ctrl
- control
- CytC
- cytochrome c
- DC
- detergent compatible
- ETC
- electron transport chain
- eWAT
- epididymal white adipose tissue
- FDG
- fluorodeoxyglucose
- FFA
- free fatty acid
- H&E
- hematoxylin and eosin
- KE
- ketone ester
- KG
- ketogenic
- IBAT
- interscapular brown adipose tissue
- Ndufs1
- NADH:ubiquinone oxidoreductase (complex 1) 70-kDa iron-sulfur subunit 1
- PET
- positron emission tomography
- PGC-1α
- PPARγ-coactivator 1α
- PPAR
- peroxisome proliferator-activated receptor
- QUICKI
- quantitative insulin-sensitivity check index
- RQ
- respiratory quotient
- SDHa
- succinate dehydrogenase (complex 2) 70-kDa subunit A
- SDHb
- succinate dehydrogenase (complex 2) 30-kDa subunit B
- Sirt1
- mammalian homolog of silent information regulator 2 (Sir2) 1
- Sirt3
- mammalian homolog of silent information regulator 2 (Sir2) 3
- TEE
- total energy expenditure
- TEM
- transmission electron microscopy
- UCP
- uncoupling protein
- Vco2
- volumetric carbon dioxide evolution rate
- Vo2
- volumetric oxygen consumption rate
- WAT
- white adipose tissue
REFERENCES
- 1. Balietti M., Casoli T., Di Stefano G., Giorgetti B., Aicardi G., Fattoretti P. (2010) Ketogenic diets: an historical antiepileptic therapy with promising potentialities for the aging brain. Ageing Res. Rev. 9, 273–279 [DOI] [PubMed] [Google Scholar]
- 2. Vanitallie T. B., Nonas C., Di Rocco A., Boyar K., Hyams K., Heymsfield S. B. (2005) Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology 64, 728–730 [DOI] [PubMed] [Google Scholar]
- 3. Prins M. L. (2008) Cerebral metabolic adaptation and ketone metabolism after brain injury. J. Cereb. Blood Flow Metab. 28, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henderson S. T., Vogel J. L., Barr L. J., Garvin F., Jones J. J., Costantini L. C. (2009) Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. (Lond.) 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bough K. J., Wetherington J., Hassel B., Pare J. F., Gawryluk J. W., Greene J. G., Shaw R., Smith Y., Geiger J. D., Dingledine R. J. (2006) Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann. Neurol. 60, 223–235 [DOI] [PubMed] [Google Scholar]
- 6. Dahlin M., Elfving A., Ungerstedt U., Amark P. (2005) The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 64, 115–125 [DOI] [PubMed] [Google Scholar]
- 7. Kashiwaya Y., Pawlosky R., Markis W., King M. T., Bergman C., Srivastava S., Murray A., Clarke K., Veech R. L. (2010) A ketone ester diet increases brain malonyl-CoA and uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar rat. J. Biol. Chem. 285, 25950–25956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato K., Kashiwaya Y., Keon C. A., Tsuchiya N., King M. T., Radda G. K., Chance B., Clarke K., Veech R. L. (1995) Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 9, 651–658 [DOI] [PubMed] [Google Scholar]
- 9. Dashti H. M., Mathew T. C., Khadada M., Al-Mousawi M., Talib H., Asfar S. K., Behbahani A. I., Al-Zaid N. S. (2007) Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell. Biochem. 302, 249–256 [DOI] [PubMed] [Google Scholar]
- 10. Veech R. L., Chance B., Kashiwaya Y., Lardy H. A., Cahill G. F., Jr. (2001) Ketone bodies, potential therapeutic uses. IUBMB Life 51, 241–247 [DOI] [PubMed] [Google Scholar]
- 11. Rogovik A. L., Goldman R. D. (2010) Ketogenic diet for treatment of epilepsy. Can. Fam. Physician 56, 540–542 [PMC free article] [PubMed] [Google Scholar]
- 12. Kwiterovich P. O., Jr., Vining E. P., Pyzik P., Skolasky R., Jr., Freeman J. M. (2003) Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 290, 912–920 [DOI] [PubMed] [Google Scholar]
- 13. Jornayvaz F. R., Jurczak M. J., Lee H. Y., Birkenfeld A. L., Frederick D. W., Zhang D., Zhang X. M., Samuel V. T., Shulman G. I. (2010) A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am. J. Physiol. Endocrinol. Metab. 299, E808–E815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samaha F. F., Foster G. D., Makris A. P. (2007) Low-carbohydrate diets, obesity, and metabolic risk factors for cardiovascular disease. Curr. Atheroscler. Rep. 9, 441–447 [DOI] [PubMed] [Google Scholar]
- 15. Kennedy A. R., Pissios P., Otu H., Roberson R., Xue B., Asakura K., Furukawa N., Marino F. E., Liu F. F., Kahn B. B., Libermann T. A., Maratos-Flier E. (2007) A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739 [DOI] [PubMed] [Google Scholar]
- 16. Murphy P., Likhodii S. S., Hatamian M., Burnham W. M. (2005) Effect of the ketogenic diet on the activity level of Wistar rats. Pediatr. Res. 57, 353–357 [DOI] [PubMed] [Google Scholar]
- 17. Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., Kawai Y., Tsujisaki M. (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cannon B., Nedergaard J. (2004) Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 [DOI] [PubMed] [Google Scholar]
- 20. Himms-Hagen J. (2001) Does brown adipose tissue (BAT) have a role in the physiology or treatment of human obesity? Rev. Endocr. Metab. Disord. 2, 395–401 [DOI] [PubMed] [Google Scholar]
- 21. Madsen L., Pedersen L. M., Lillefosse H. H., Fjaere E., Bronstad I., Hao Q., Petersen R. K., Hallenborg P., Ma T., De Matteis R., Araujo P., Mercader J., Bonet M. L., Hansen J. B., Cannon B., Nedergaard J., Wang J., Cinti S., Voshol P., Doskeland S. O., Kristiansen K. (2010) UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS One 5, e11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamann A., Flier J. S., Lowell B. B. (1996) Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 137, 21–29 [DOI] [PubMed] [Google Scholar]
- 23. Stephens M., Ludgate M., Rees D. A. (2011) Brown fat and obesity: the next big thing? Clin. Endocrinol. (Oxf.) 74, 661–670 [DOI] [PubMed] [Google Scholar]
- 24. Vernochet C., McDonald M. E., Farmer S. R. (2010) Brown adipose tissue: a promising target to combat obesity. Drug News Perspect. 23, 409–417 [DOI] [PubMed] [Google Scholar]
- 25. Kozak L. P. (2010) Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 11, 263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rothwell N. J., Stock M. J., Warwick B. P. (1985) Energy balance and brown fat activity in rats fed cafeteria diets or high-fat, semisynthetic diets at several levels of intake. Metabolism 34, 474–480 [DOI] [PubMed] [Google Scholar]
- 27. Nagashima K., Zheng J., Parmiter D., Patri A. K. (2011) Biological tissue and cell culture specimen preparation for TEM nanoparticle characterization. Methods Mol. Biol. 697, 83–91 [DOI] [PubMed] [Google Scholar]
- 28. Katz A., Nambi S. S., Mather K., Baron A. D., Follmann D. A., Sullivan G., Quon M. J. (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 85, 2402–2410 [DOI] [PubMed] [Google Scholar]
- 29. Fueger B. J., Czernin J., Hildebrandt I., Tran C., Halpern B. S., Stout D., Phelps M. E., Weber W. A. (2006) Impact of animal handling on the results of 18F-FDG PET studies in mice. J. Nucl. Med. 47, 999–1006 [PubMed] [Google Scholar]
- 30. Tam J., Vemuri V. K., Liu J., Batkai S., Mukhopadhyay B., Godlewski G., Osei-Hyiaman D., Ohnuma S., Ambudkar S. V., Pickel J., Makriyannis A., Kunos G. (2010) Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Invest. 120, 2953–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butler A. A., Kozak L. P. (2010) A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59, 323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cannon B., Nedergaard J. (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253 [DOI] [PubMed] [Google Scholar]
- 33. Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr. (1967) Brain metabolism during fasting. J. Clin. Invest. 46, 1589–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Owen O. E., Felig P., Morgan A. P., Wahren J., Cahill G. F., Jr. (1969) Liver and kidney metabolism during prolonged starvation. J. Clin. Invest. 48, 574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cha S. H., Rodgers J. T., Puigserver P., Chohnan S., Lane M. D. (2006) Hypothalamic malonyl-CoA triggers mitochondrial biogenesis and oxidative gene expression in skeletal muscle: role of PGC-1α. Proc. Natl. Acad. Sci. U. S. A. 103, 15410–15415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rim J. S., Kozak L. P. (2002) Regulatory motifs for CREB-binding protein and Nfe2l2 transcription factors in the upstream enhancer of the mitochondrial uncoupling protein 1 gene. J. Biol. Chem. 277, 34589–34600 [DOI] [PubMed] [Google Scholar]
- 37. Vercauteren K., Pasko R. A., Gleyzer N., Marino V. M., Scarpulla R. C. (2006) PGC-1-related coactivator: immediate early expression and characterization of a CREB/NRF-1 binding domain associated with cytochrome c promoter occupancy and respiratory growth. Mol. Cell. Biol. 26, 7409–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Z., Huang X., Feng Y., Handschin C., Gullicksen P. S., Bare O., Labow M., Spiegelman B., Stevenson S. C. (2006) Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc. Natl. Acad. Sci. U. S. A. 103, 14379–14384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Franko A., Mayer S., Thiel G., Mercy L., Arnould T., Hornig-Do H. T., Wiesner R. J., Goffart S. (2008) CREB-1α is recruited to and mediates upregulation of the cytochrome c promoter during enhanced mitochondrial biogenesis accompanying skeletal muscle differentiation. Mol. Cell. Biol. 28, 2446–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nedergaard J., Petrovic N., Lindgren E. M., Jacobsson A., Cannon B. (2005) PPARγ in the control of brown adipocyte differentiation. Biochim. Biophys. Acta 1740, 293–304 [DOI] [PubMed] [Google Scholar]
- 41. Scarpulla R. C. (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 1813, 1269–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Timmons J. A., Wennmalm K., Larsson O., Walden T. B., Lassmann T., Petrovic N., Hamilton D. L., Gimeno R. E., Wahlestedt C., Baar K., Nedergaard J., Cannon B. (2007) Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. U. S. A. 104, 4401–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi T., Wang F., Stieren E., Tong Q. (2005) SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 280, 13560–13567 [DOI] [PubMed] [Google Scholar]
- 44. Reznick R. M., Shulman G. I. (2006) The role of AMP-activated protein kinase in mitochondrial biogenesis. J. Physiol. 574, 33–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zong H., Ren J. M., Young L. H., Pypaert M., Mu J., Birnbaum M. J., Shulman G. I. (2002) AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. U. S. A. 99, 15983–15987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bruss M. D., Khambatta C. F., Ruby M. A., Aggarwal I., Hellerstein M. K. (2010) Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am. J. Physiol. Endocrinol. Metab. 298, E108–E116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kashiwaya Y., Sato K., Tsuchiya N., Thomas S., Fell D. A., Veech R. L., Passonneau J. V. (1994) Control of glucose utilization in working perfused rat heart. J. Biol. Chem. 269, 25502–25514 [PubMed] [Google Scholar]
- 48. Halaas J. L., Gajiwala K. S., Maffei M., Cohen S. L., Chait B. T., Rabinowitz D., Lallone R. L., Burley S. K., Friedman J. M. (1995) Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 [DOI] [PubMed] [Google Scholar]
- 49. Wolfgang M. J., Cha S. H., Sidhaye A., Chohnan S., Cline G., Shulman G. I., Lane M. D. (2007) Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc. Natl. Acad. Sci. U. S. A. 104, 19285–19290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cusin I., Zakrzewska K. E., Boss O., Muzzin P., Giacobino J. P., Ricquier D., Jeanrenaud B., Rohner-Jeanrenaud F. (1998) Chronic central leptin infusion enhances insulin-stimulated glucose metabolism and favors the expression of uncoupling proteins. Diabetes 47, 1014–1019 [DOI] [PubMed] [Google Scholar]
- 51. Rahmouni K., Morgan D. A. (2007) Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49, 647–652 [DOI] [PubMed] [Google Scholar]
- 52. Ghorbani M., Claus T. H., Himms-Hagen J. (1997) Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem. Pharmacol. 54, 121–131 [DOI] [PubMed] [Google Scholar]
- 53. Petrovic N., Shabalina I. G., Timmons J. A., Cannon B., Nedergaard J. (2008) Thermogenically competent nonadrenergic recruitment in brown preadipocytes by a PPARγ agonist. Am. J. Physiol. Endocrinol. Metab. 295, E287–E296 [DOI] [PubMed] [Google Scholar]
- 54. Kimura I., Inoue D., Maeda T., Hara T., Ichimura A., Miyauchi S., Kobayashi M., Hirasawa A., Tsujimoto G. (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. U. S. A. 108, 8030–8035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kolanowski J., Young J. B., Landsberg L. (1994) Stimulatory influence of d(−)3-hydroxybutyrate feeding on sympathetic nervous system activity in the rat. Metabolism 43, 180–185 [DOI] [PubMed] [Google Scholar]
- 56. Geelen M. J., Lopes-Cardozo M., Edmond J. (1983) Acetoacetate: a major substrate for the synthesis of cholesterol and fatty acids by isolated rat hepatocytes. FEBS Lett. 163, 269–273 [DOI] [PubMed] [Google Scholar]
- 57. Endemann G., Goetz P. G., Tomera J. F., Rand W. M., Desrochers S., Brunengraber H. (1987) Lipogenesis from ketone bodies in the perfused rat liver: effects of acetate and ethanol. Biochem. Cell Biol. 65, 989–996 [DOI] [PubMed] [Google Scholar]