Abstract
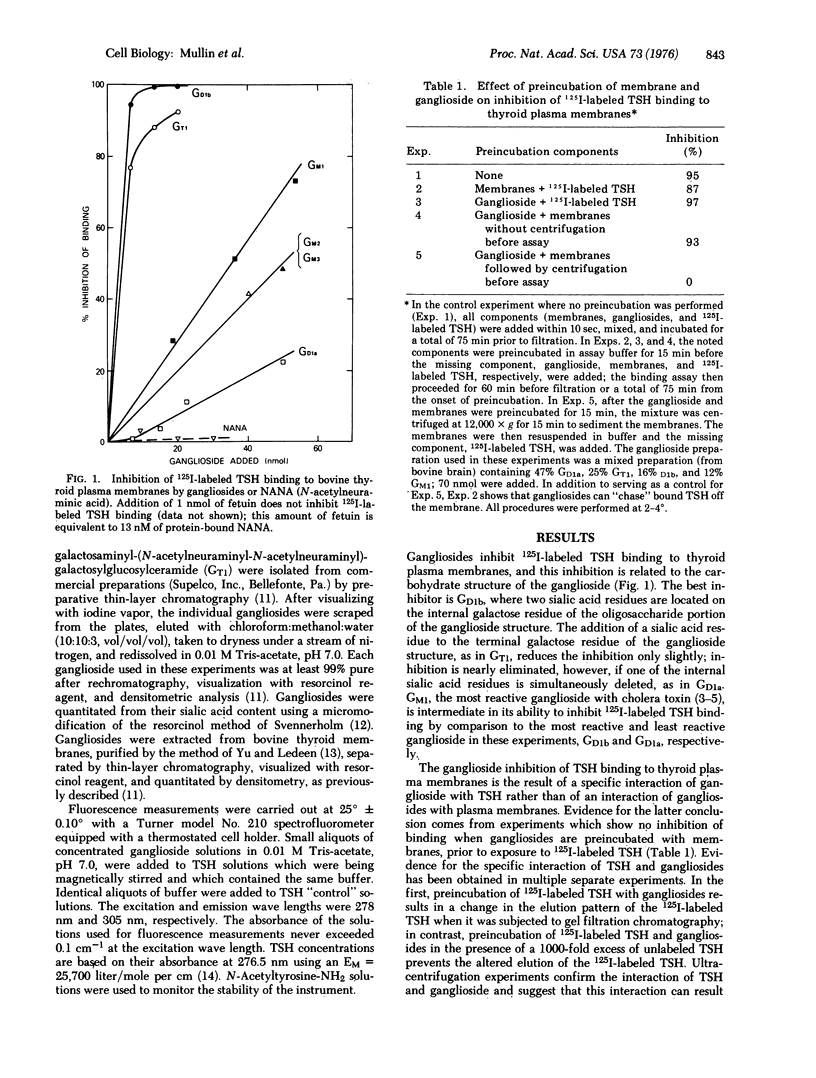
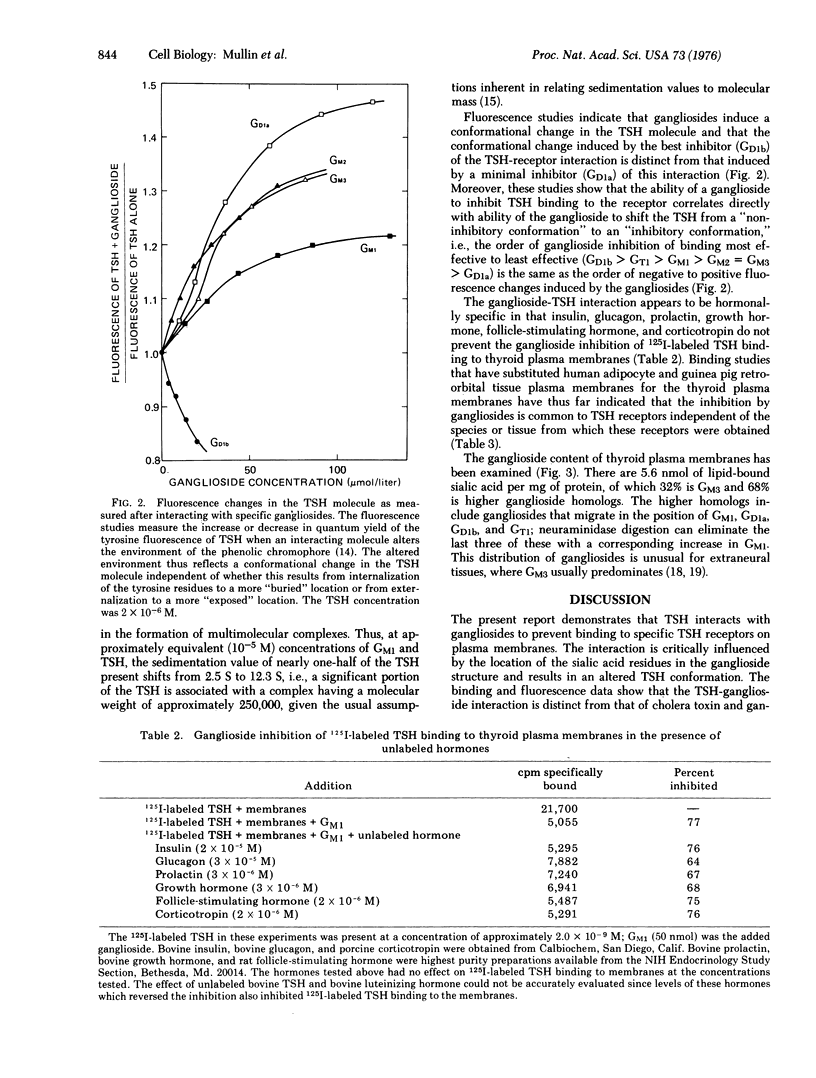
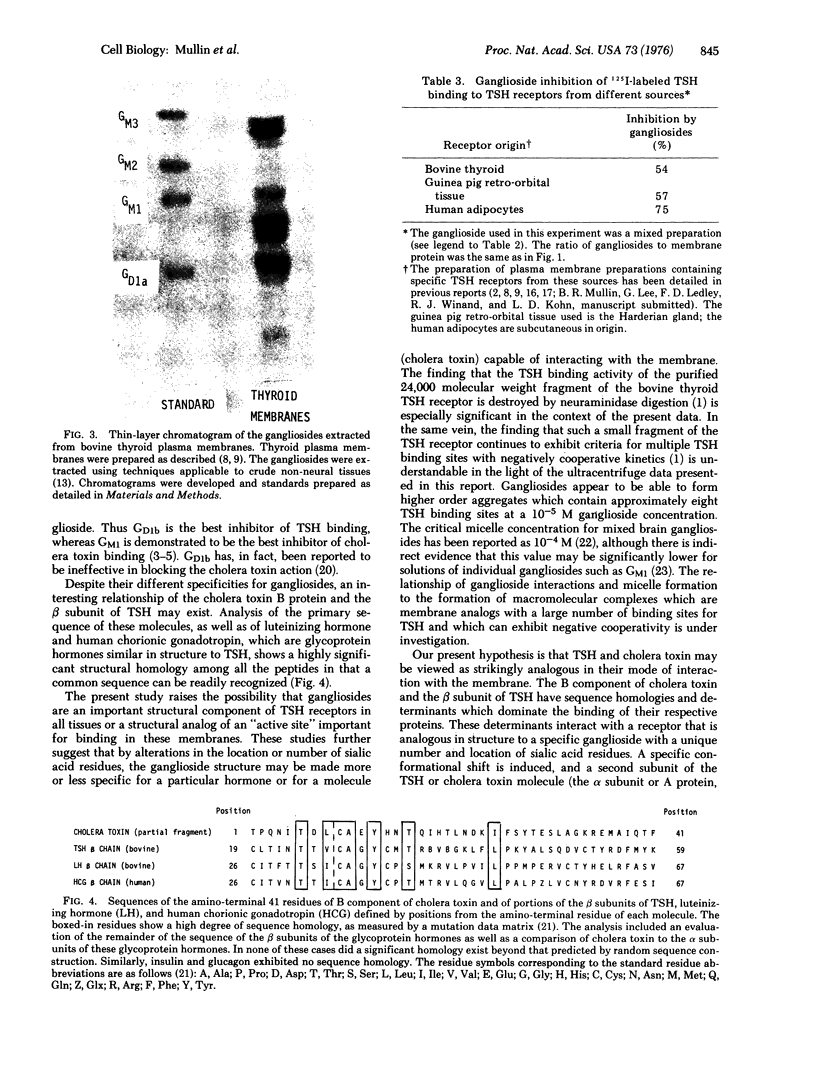
Gangliosides inhibit 125I-labeled thyrotropin binding to the thyrotropin receptors on bovine thyroid plasma membranes, on guinea pig retro-orbital tissue plasma membranes, and on human adipocyte membranes. This inhibition by gangliosides is critically altered by the number and location of the sialic acid residues within the ganglioside structure, the efficacy of inhibition having the following order: GD1b greater than GT1 greater than GM1 greater than GM2 = GM3 greater than GD1a. The inhibition results from the interaction of thyrotropin and gangliosides, rather than the interaction of membrane and gangliosides. Fluorescence studies show that the inhibition is associated with a distinct conformational change of the thyrotropin molecule and that the progression from a "noninhibitory conformation" to an "inhibitory conformation" parallels exactly the order of effectiveness in inhibiting 125I-labeled thyrotropin binding. The ganglioside inhibition of 125I-labeled thyrotropin binding appears to be hormonally specific in that it is not affected by albumin, glucagon, insulin, prolactin, follicle-stimulating hormone, growth hormone, or corticotropin. The possibility that a ganglioside or ganglioside-like structure is a component of the thyrotropin receptor is suggested by the finding that gangliosides more complex than N-acetylneuraminylgalactosylglucosylceramide are present in bovine thyroid membranes in much higher quantities than have been previously found in extraneural tissue. The finding that the B component of cholera toxin, which also interacts with gangliosides, has a peptide sequence in common with the beta subunit of thyrotropin, suggests that thyrotropin and cholera toxin may be analogous in their mode of action on the membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloj S. M., Edelhoch H., Ingham K. C., Morgan F. J., Canfield R. E., Ross G. T. The rates of dissociation and reassociation of the subunits of human chorionic gonadotropin. Arch Biochem Biophys. 1973 Nov;159(1):497–504. doi: 10.1016/0003-9861(73)90480-3. [DOI] [PubMed] [Google Scholar]
- Amir S. M., Carraway T. F., Jr, Kohn L. D., Winand R. J. The binding of thyrotropin to isolated bovine thyroid plasma membranes. J Biol Chem. 1973 Jun 10;248(11):4092–4100. [PubMed] [Google Scholar]
- Bolonkin D., Tate R. L., Luber J. H., Kohn L. D., Winand R. J. Experimental exophthalmos. Binding of thyrotropin and an exophthalmogenic factor derived from thyrotropin to retro-orbital tissue plasma membranes. J Biol Chem. 1975 Aug 25;250(16):6516–6521. [PubMed] [Google Scholar]
- Craig S. W., Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O., Bradley R. M., Aaronson S. A., Todaro G. J. Absence of a specific ganglioside galactosyltransferase in mouse cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1974 Feb;71(2):298–301. doi: 10.1073/pnas.71.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H., McFarland V. W., Mora P. T., Brady R. O. Ganglioside biosynthesis in mouse cells: glycosyltransferase activities in normal and virally-transformed lines. Biochem Biophys Res Commun. 1972 Jul 11;48(1):48–57. doi: 10.1016/0006-291x(72)90342-7. [DOI] [PubMed] [Google Scholar]
- Fishman P. H. Normal and abnormal biosynthesis of gangliosides. Chem Phys Lipids. 1974 Dec;13(4):305–326. doi: 10.1016/0009-3084(74)90006-1. [DOI] [PubMed] [Google Scholar]
- GAMMACK D. B. PHYSICOCHEMICAL PROPERTIES OF OX-BRAIN GANGLIOSIDES. Biochem J. 1963 Aug;88:373–383. doi: 10.1042/bj0880373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973 Aug;8(2):208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfer J. N., Spielvogel C. On the loss of gangliosides by dialysis. J Neurochem. 1973 May;20(5):1483–1485. doi: 10.1111/j.1471-4159.1973.tb00262.x. [DOI] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Winand R. J. Relationship of thyrotropin to exophthalmos-producing substance. Formation of an exophthalmos-producing substance by pepsin digestion of pituitary glycoproteins containing both thyrotropic and exophthalmogenic activity. J Biol Chem. 1971 Nov;246(21):6570–6575. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Tate R. L., Holmes J. M., Kohn L. D., Winand R. J. Characteristics of a solubilized thyrotropin receptor from bovine thyroid plasma membranes. J Biol Chem. 1975 Aug 25;250(16):6527–6533. [PubMed] [Google Scholar]
- Tate R. L., Schwartz H. I., Holmes J. M., Kohn L. D. Thyrotropin receptors in thyroid plasma membranes. Characteristics of thyrotropin binding and solubilization of thyrotropin receptor activity by tryptic digestion. J Biol Chem. 1975 Aug 25;250(16):6509–6515. [PubMed] [Google Scholar]
- Winand R. J., Kohn L. D. Relationships of thyrotropin to exophthalmic-producing substance. Purification of homogeneous glycoproteins containing both activities from [3H]-labeled pituitary extracts. J Biol Chem. 1970 Mar 10;245(5):967–975. [PubMed] [Google Scholar]
- Winand R. J., Kohn L. D. The binding of ( 3 H)thyrotropin and an 3 H-labeled exophthalmogenic factor by plasma membranes of retro-orbital tissue. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1711–1715. doi: 10.1073/pnas.69.7.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., Winand R. J., Kohn L. D. The contribution of subunits of thyroid stimulating hormone to the binding and biological activity of thyrotropin. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3460–3464. doi: 10.1073/pnas.71.9.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972 Sep;13(5):680–686. [PubMed] [Google Scholar]