Abstract
Insulin-like growth factor binding protein (IGFBP)-3 regulates cell proliferation and apoptosis in esophageal squamous cell carcinoma (ESCC) cells. We have investigated how the hypoxic tumor microenvironment in ESCC fosters the induction of IGFBP3. RNA interference experiments revealed that hypoxia-inducible factor (HIF)-1α, but not HIF-2α, regulates IGFBP3 mRNA induction. By chromatin immunoprecipitation and transfection assays, HIF-1α was found to transactivate IGFBP3 through a novel hypoxia responsive element (HRE) located at 57 kb upstream from the transcription start site. Metabolic labeling experiments demonstrated hypoxia-mediated inhibition of global protein synthesis. 7-Methyl GTP-cap binding assays suggested that hypoxia suppresses cap-dependent translation. Experiments using pharmacological inhibitors for mammalian target of rapamycin (mTOR) suggested that a relatively weak mTOR activity may be sufficient for cap-dependent translation of IGFBP3 under hypoxic conditions. Bicistronic RNA reporter transfection assays did not validate the possibility of an internal ribosome entry site as a potential mechanism for cap-independent translation for IGFBP3 mRNA. Finally, IGFBP3 mRNA was found enriched to the polysomes. In aggregate, our study establishes IGFBP3 as a direct HIF-1α target gene and that polysome enrichment of IGFBP3 mRNA may permit continuous translation under hypoxic conditions.—Natsuizaka, M., Naganuma, S., Kagawa, S., Ohashi, S., Ahmadi, A., Subramanian, H., Chang, S., Nakagawa, K. J., Ji, X., Liebhaber, S. A., Klein-Szanto, A. J., Nakagawa, H. Hypoxia induces IGFBP3 in esophageal squamous cancer cells through HIF-1α-mediated mRNA transcription and continuous protein synthesis.
Keywords: cap-dependent translation, internal ribosome entry site
Hypoxia is one of the defining features of the tumor microenvironment, influencing cancer development and progression (1, 2). Global cellular adaptation to hypoxia is mediated mainly by hypoxia-inducible factor (HIF)-1 and HIF-2, heterodimeric transcription factors comprising an oxygen sensitive α-subunit and a constitutively expressed β-subunit (3, 4). Hypoxia stabilizes the α-subunit to activate HIFs. By contrast, normoxia allows prolyl-hydroxylation of the oxygen-dependent degradation domain of the α-subunit, which leads to rapid proteasomal degradation. HIFs transactivate many hypoxia-sensitive genes by binding to hypoxia-response elements (HREs) in their regulatory regions (5). Classic examples of HIF target genes include carbonic anhydrase 9 (CA9; ref. 6) and vascular endothelial growth factor A (VEGFA; ref. 3–5).
The final step in gene expression is mRNA translation. Translational initiation is a rate-limiting step (7–8), influenced by the availability of nutrients, cellular energy status, hormones, and growth factors. Under physiological conditions, most cellular mRNAs are translated by a cap-dependent mechanism, which involves the assembly of a preinitiation complex containing ribosomes and several eukaryotic initiation factors (eIFs), including eIF4E at the 5′ cap structure of the mRNA. This complex migrates along the 5′ untranslated region (UTR) of mRNA in search of the start codon of the open reading frame to begin elongation of the polypeptide chain (7, 8). The cap-dependent translation is regulated by a serine/threonine kinase mammalian target of rapamycin (mTOR) that phosphorylates eIF4E binding protein-1 (4E-BP1), an inhibitor of eIF4E, facilitating the dissociation of 4E-BP1 from eIF4E. Under hypoxic conditions, mTOR is suppressed to reduce cap-dependent translation (9). Nonetheless, some cellular mRNAs are translated. A possible mechanism is selective enrichment of mRNAs into the polysome, a cluster of ribosomes, allowing efficient cap-dependent translation (10). Alternatively, an internal ribosome entry site (IRES) within specific mRNAs is used for the initiation of cap-independent translation (9). The IRES was originally found in encephalomyocarditis virus (EMCV) and has been detected in the 5′ UTR of certain cellular mRNAs, such as HIF-1α, VEGFA, insulin-like growth factor (IGF)2, and IGF1 receptor (IGF1R) (9).
Insulin-like growth factor binding protein 3 (IGFBP3) is a major carrier protein for IGFs. IGFBP3 regulates cell proliferation and apoptosis in IGF- (or IGF1R)-dependent and independent manners. IGFBP3 has been linked to the pathogenesis of vascular diseases and cancers. IGFBP3 is overexpressed in renal carcinomas (11), non-small-cell lung cancers (12), and esophageal squamous cell carcinoma (ESCC; refs. 13, 14). Hypoxia induces IGFBP3 mRNA in endothelial cells (15), fibroblasts (16), and cancer cell lines of various tissue origins (17–19). Hypoxia-mediated induction of IGFBP3 mRNA is impaired in HIF-1α−/− mouse embryonic stem cells (20) and cancer cells with HIF-1α knockdown (21), suggesting that HIF-1α might be a potential mediator of IGFBP3 induction. However, HREs have yet to be identified in the IGFBP3 gene, and it remains to be elucidated how HIF might regulate IGFBP3 transcription. It is also unclear how IGFBP3 mRNA is translated under hypoxic conditions.
Herein, we present novel data as to how hypoxia induces IGFBP3 in ESCC, which is the eighth most common cancer worldwide, and the sixth most common cause of cancer-related death (22). We find that HIF-1α transactivates IGFBP3 through a novel HRE and cap-dependent translation of IGFBP3 mRNA enriched into the polysome, providing novel mechanistic insights into the hypoxic regulation of IGFBP3.
MATERIALS AND METHODS
Tissue samples
Paired frozen tissues and paraffin blocks containing primary ESCC and adjacent normal tissues were procured as described previously (13, 23). All of the clinical materials were obtained with the informed consent of patients, in accordance with the Institutional Review Board standards and guidelines of Okayama University Hospital and Kitano Hospital.
Cell cultures
ESCC cell lines and 293T cells (American Type Culture Collection, Manassas, VA, USA) were grown as described previously (23). Cells were exposed to hypoxia (0.5% O2) in the Invivo2 300 hypoxia workstation (Ruskinn Technologies, Bridgend, UK) or normoxia (21% O2) for indicated time periods at 37°C. Deferoxamine mesylate (DFO; Sigma-Aldrich, St. Louis, MO, USA), rapamycin (Invitrogen), PP242 (Sigma-Aldrich), and tunicamycin (Sigma-Aldrich) were dissolved in dimethyl sulfoxide (DMSO) as a vehicle. Propidium iodide staining was done to determine cell viability by flow cytometry.
Xenograft transplantation experiments
TE11 cells (5×106) were implanted subcutaneously into the dorsal skin of athymic nu/nu mice (4–6 wk old; Charles River Breeding Laboratories, Wilmington, MA, USA). The mice were sacrificed 8 wk after the inoculation. To determine tumor hypoxia, 100 mg/kg of pimonidazole HCl (hypoxyprobe-1; Chemicon International, Temecula, CA, USA) was injected intraperitoneally 90 min before sacrificing the mice. The tumors were fixed in formalin and embedded in paraffin blocks. All experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
RNA isolation, cDNA synthesis, and real-time polymerase chain reaction (PCR)
RNA isolation, cDNA synthesis and real-time PCR were done as described previously (14). SYBR green reagent (Applied Biosystems, Foster City, CA, USA) was used for IGFBP3 and β-actin as described previously (14) and for VEGF with primers 5′-AGCCTTGCCTTGCTGCTCTAC-3′ and 5′-GCTGCGCTGATAGACATCCATG-3′. TaqMan Gene Expression Assays (Applied Biosystems) were used for CA9 (Hs00154208_m1), HIF-1α (Hs00936371_m1), and HIF-2α (Hs01026149_m1). PrimeTime qPCR Assays (Integrated DNA Technologies, Coralville, IA, USA) were used for ribosomal protein S6 (rpS6; Hs.PT.47.3481453). All PCR reactions were performed in triplicate. The relative expression level of each mRNA was normalized to the β-actin serving as an internal control.
Western blotting
Western blotting was done as described previously (14). Anti-IGFBP3 (DSL-R00536) was purchased from Diagnostic Systems Laboratories, Inc. (Webster, TX, USA); anti-HIF-1α (610958) was from BD Bioscience (San Diego, CA, USA); anti-HIF-2α (NB100–122) was from Novus Biologicals (Littleton, CO, USA); anti-4E-BP1 (cat. no. 9452), anti-phospho-4E-BP1 (2855, Thr37/46; 9451, Ser65), anti-eIF4E (9742), anti-eIF4G (2498), anti-eIF2α (9722), anti-phospho-eIF2α (3398), anti-rpS6 protein (2317) and anti-phospho-rpS6 (2211) were from Cell Signaling Technology Inc. (Danvers, MA, USA); and anti-β-actin (AC74) was from Sigma-Aldrich. β-Actin served as a protein loading control.
Immunohistochemistry
Immunohistochemistry was done as described previously (13). In brief, IGFBP3 was detected by primary anti-human IGFBP-3 mouse monoclonal antibody (clone 84728.111; R&D Systems, Minneapolis, MN, USA) at 1:250 dilution. Pimonidazole was detected according to the manufacturer's protocol. The immunohistochemical staining was assessed independently (S.N. and A.J.K.; who were blinded to the specimens), and the intensity for IGFBP3 was expressed as negative (−), weakly positive (+), moderately positive (++), or strongly positive (+++).
DNA constructs
(eHRE)3-luc (24) carrying EPO HRE was a gift of Dr. Frank S. Lee (University of Pennsylvania). IGFBP3 promoter sequences (−1901 to +55, −3590 to +55, and −5992 to +55) in pGL3-basic (25) was a gift of Dr. David Feldman (Stanford University, Palo Alto, CA, USA). Synthetic concatamers containing 3 copies of potential HREs at −55 bp and −209 bp from the transcription start site and distal (−57 kb) regions of IGFBP3 (see Table 1 and Fig. 4A) were subcloned into pGL3-promoter at the NheI and XhoI sites and designated (BP3HRE)3-a-luc, (BP3HRE)3-b-luc, and (BP3HRE)3-c-luc, respectively. In addition, a 700-bp fragment (nucleotide positions 513–1236 on GenBank accession NG_011508) derived from intron 1 of IGFBP3 and containing 2 potential HREs was amplified by PCR using BAC clone RP11-132L11 (BACPAC Resources Center, Children's Hospital Oakland, Oakland, CA, USA) as a template DNA with primers 5′-GCGTGCTAGCTGCCAGCGCCGCCAGCTC-3′ and 5′-CGGGCTCGAGTTTGAGGTCGGCGCAGGTGC-3′, and subcloned into pGL3-promoter at NheI and XhoI sites, resulting in creation of BP3INT1-luc. pBABE-puro-HIF-1αP402A/P564G, expressing degradation-resistant mutant of HIF-1α, was generated with QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) using pBABE-puro-WT-HIF-1α (13) as template. The 5′ UTR of IGFBP3 (nucleotide positions 1-126 on NG_011508) was amplified by PCR with primers 5′-GCAGAATTCAGATGCGAGCACTGCGGCT-3′ and 5′-GCACCATGGTGCAACCGGGGCACGCTGCT-3′ and ligated into the intercistronic EcoRI and NcoI sites of pR/ΔE/F vector (a gift of Dr. Peter Sarnow, Stanford University; ref. 26), resulting in creation of pR/BP3-5′UTR/F. All constructs were validated by DNA sequencing.
Table 1.
Sequences with HRE motif 5′-(A/G)CGT(G/C)C-3′ subcloned into pGL3-promoter
| Construct | Position | Sequence |
|---|---|---|
| (BP3HRE)3–luc–a | −55 bp | [5′–CCGGGCTCCGGGCGTGCGCACGAGGAGCAGGT–3′] × 3 |
| (BP3HRE)3–luc–b | −209 bp | [5′–GCCGTTCCGGGGCGTGTCCTGGGCCACCCCGG–3′] × 3 |
| (BP3HRE)3–luc–c | −57 kb | [5′–CACTGAGTGAACTCACGTCCACACAGGGTGCG–3′] × 3 |
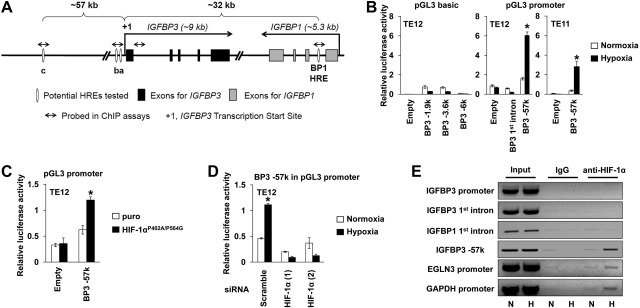
Figure 4.
HIF-1α transactivates the HRE at −57 kb upstream of the IGFBP3 transcription start site. A) Schematic representation of the 100-kb region (chromosome 7, nucleotide positions 45894484–45987396) harboring IGFBP3 and adjacent IGFBP1. IGFBP1 has an HRE (BP1 HRE) within intron 1 (31). Among those predicted within this region were potential IGFBP3 HREs, including HRE-a (nucleotide position 45927451, −55 bp from transcription start site), HRE-b (nucleotide position 45927605, −209 bp), and HRE-c (nucleotide position 45984635, −57 kb). B–D) Luciferase assays were done with indicated cells and vectors following transient transfection and exposure to hypoxia or normoxia for 48 h. B) Hypoxic activation was determined for IGFBP3 promoter using serial deletion constructs (BP3-6k, BP3-3.6k, and BP3-1.9k) or heterologous minimal promoter linked to candidate HRE-c (i.e., BP3-57k) or an IGFBP3 intron1-derived fragment (i.e., BP3 1st intron). *P < 0.05 vs. normoxia (n=3). C) Cells were cotransfected with either pGL3-promoter for empty treatment (minimal promoter only) or (BP3HRE)3-c-luc for BP3-57k along with pBABE-puro-HIF-1αP402A/P564G or pBABE-puro (puro; empty vector control). *P < 0.05 vs. puro (n=3). D) Cells were cotransfected with (BP3HRE)3-c-luc (i.e., BP3-57k) along with indicated siRNA sequences. *P < 0.05 vs. normoxia, #P < 0.05 vs. scramble and hypoxia (n=3). E) ChIP assays determined HIF-1α binding to the indicated gene regions in TE12 cells. Input represents 0.3% of total chromatin, which was precipitated with either anti-HIF-1α antibody or control IgG. PCR products were run on agarose gels and visualized. N, normoxia; H, hypoxia.
In vitro synthesis of bicistronic RNA
The pR/ΔE/F bicistronic reporter constructs were linearized by SalI and subjected to in vitro transcription to generate capped and polyadenylated transcripts using the Ambion mMessage mMachine kit (Applied Biosystems) according to the manufacturer's instruction. The synthesized RNA was purified by Ambion Megaclear kit (Applied Biosystems) for transfection experiments.
RNA interference
Small interfering RNAs (siRNAs) directed against HIF-1α (Silencer Select siRNA; Invitrogen s4698 and s4699), HIF-2α (s6539 and s6541), and nonsilencing negative control (4390843) were purchased. siRNA (5 nM) was transfected into TE12 cells with Lipofectamine RNAi Max (Invitrogen). At 16 h after transfection, cells were exposed to either hypoxia or normoxia for 24 h. When RNAi was coupled with luciferase assays, reporter vectors were transfected 48 h after siRNA transfection, as described below.
Luciferase assays
Lipofectamine LTX and Plus reagents (Invitrogen) were used for DNA transfection, according to the manufacturer's instructions. Briefly, 1 × 105 cells/well were seeded in 24-well plates 24 h before transfection. A reporter vector (200 ng) was transfected along with 200 ng of pBABE-puro-loxP-HIF-1αP402A/P564G or pBABE-puro-loxP (empty vector; ref. 13). phRL-SV40-renilla luciferase vector (5 ng; Promega, Madison, WI, USA) was cotransfected to calibrate the variation in transfection efficiencies. In bicistronic luciferase assays, pR/BP3-5′UTR/F, pR/ΔE/F (empty vector) or pΔEMCV (control) containing an IRES of EMCV was transfected. At 6 h after DNA transfection, cells were exposed to hypoxia or normoxia for 24 h. For RNA transfection, cells were first exposed to normoxia or hypoxia for 24 h. Cells were then transfected with 1 μg RNA with Lipofectamine LTX under either normoxic or hypoxic conditions. At 7 h after RNA transfection, luciferase assays were done. Luciferase activities were determined using Dual-Luciferase reporter assay system and Glomax multidetection system (Promega). The firefly luciferase activity was normalized with the coexpressed Renilla luciferase activity.
Chromatin immunoprecipitation (ChIP) assay
ChIP was done using the EZ-Chip kit (Millipore, Billerica, MA, USA). TE12 cells (1×107) were treated with 1% formaldehyde for 10 min at 37°C and quenched with 0.125 M glycine. The cross-linked chromatin was sheared into 300- to 500-bp DNA fragments with Branson Sonifier 250 (Branson, Danbury, CT, USA) and immunoprecipitated with either anti-HIF-1α (1 μg/106 cells, NB100-105; Novus) or normal mouse IgG (1 μg/106 cells, 12-371B, Millipore) as a negative control. DNA was purified by QIAquick PCR purification kit (Qiagen, Valencia, CA, USA) and analyzed by PCR using the following primers: 5′-GACAGTGTGGTTGTCTGGGCAACA-3′ and 5′-AACACGCACCCTGTGTGGACG-3′ for the −57-kb 5′-upstream region of IGFBP3, 5′-CCCAGGACACGCCCCGGAAC-3′ and 5′-CGAGTCTCGAGCTGCACGCC-3′ for the IGFBP3 promoter region, 5′-ATAGGCGGATTTGGACCAAGG-3′ and 5′-GGCCCGAGTTGTAAGAATGG-3′ for the IGFBP3 1st intron, 5′-TTCTCTGGTGACTGGGGTAGAGAT-3′ and 5′-GAGCCCATGCAATTAGGCACAGTA-3′ for EGLN3, and 5′-TACTAGCGGTTTTACGGGCG-3′ and 5′-TCGAACAGGAGGAGCAGAGAGCGA-3′ for GAPDH.
Metabolic labeling
Metabolic labeling was done as described previously (10). In brief, TE12 cells were first exposed to normoxia or hypoxia for 48 h. Following 1 h starvation under normoxia or hypoxia, cells were labeled for 30 min with 125 μCi of [35S] methionine and [35S] cysteine under normoxia or hypoxia, washed twice with ice-cold phosphate-buffered saline, and lysed in 1× cell lysis buffer (Cell Signaling Technology). 35S-labeled protein in 10 μl cell lysate was precipitated with 10% trichloroacetic acid (TCA; Sigma) and filtrated through a glass microfiber filter (Whatman; GE Healthcare, Piscataway, NJ, USA). Liquid scintillation counting was done to determine the ratio of the radioactivity in the precipitated protein sample to that in 10 μl total cell lysate spotted on the filter.
7-Methyl-GTP (m7GTP)-cap binding assays
Cells were lysed in 1× cell lysis buffer (Cell Signaling Technology) and cleared by centrifugation at 14,000 rpm at 4°C for 10 min. Supernatants were precleared with Sepharose 4B, and then incubated overnight at 4°C with 6 nmol of m7GTP-Sepharose 4B beads (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) per 400 μg of protein in 200 μl of a total reaction volume. Following centrifugation at 2000 rpm at 4°C for 2 min, pelleted beads were washed 4 times with 500 μl of lysis buffer without protease inhibitors and 3 times with 50 mM Tris-HCl and 150 mM NaCl. The beads were suspended in lithium dodecyl sulfate sample loading buffer (Invitrogen), heat-denatured at 70°C for 10 min, and subjected to Western blotting.
Polysome analysis
Polysome analysis was done as described previously (10). Briefly, 1 × 107 cells were lysed and centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was loaded over top of 10–50% linear sucrose gradient. After ultracentrifugation at 38,000 rpm for 90 min at 4°C, 16 fractions were collected for RNA purification and real-time PCR.
Statistical analysis
Data from triplicate experiments are presented as means ± se and were analyzed by 2-tailed Student's t test. Values of P < 0.05 were considered significant.
RESULTS
Hypoxia induces IGFBP3 in primary and xenografted ESCC cells in vivo
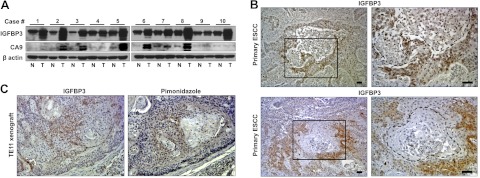
IGFBP3 is up-regulated frequently in primary ESCC tissues compared to adjacent normal mucosa (Fig. 1A), as observed in a distinct set of the samples analyzed previously (14). HIF-α proteins were not detected in the tissue lysates (data not shown), probably due to ex vivo oxygen-dependent degradation on surgical removal of the tumors. However, involvement of hypoxia and HIFs was suspected by concurrent expression of CA9, a canonical HIF-1α target gene (6) in 70% of the tumor samples (Fig. 1A). It is noteworthy that IGFBP3 was found up-regulated in all tumor samples with CA9 up-regulation. Immunohistochemistry of primary ESCC tissues confirmed IGFBP3 up-regulation in tumor cells (75%, n=53). Six samples (11%) displayed focal, yet the strongest level of IGFBP3 expression in tumor cells. In such cases, IGFBP3 was found localized to the area surrounding intratumoral necrotic foci (Fig. 1B). Hypoxia induces keratinization in primary human squamous cell carcinoma (27). In agreement, IGFBP3 was also found in tumor cells at the periphery of well-differentiated keratinized cell nests (Fig. 1B). To further determine the role of hypoxia in IGFBP3 expression in vivo, we probed hypoxic areas within tumors with pimonidazole (i.e., hypoxyprobe-1) using TE11 ESCC cell xenograft tumors. IGFBP3 was colocalized with pimonidazole (Fig. 1C). These results suggest that hypoxia may induce IGFBP3 in ESCC.
Figure 1.
Hypoxic tumor microenvironment may contribute to IGFBP3 up-regulation in ESCC. A) Western blotting determined IGFBP3 and CA9 in primary ESCC tissue lysates. β-Actin served as a loading control. N, adjacent normal mucosa; T, tumor. B) Two representative ESCC cases showing a focal, yet intense IGFBP3 staining in the tumor cells surrounding a necrotic lesion and a well-differentiated keratinized cell nest, respectively (each demarcated by broken lines). Boxed areas in left panels are enlarged in right panels. C) Immunohistochemistry colocalized IGFBP3 and the pimonidazole in tumor cells surrounding a well-differentiated cell nest in TE11 xenograft tumors. Scale bars = 50 μm.
IGFBP3 is a HIF-1α target gene in ESCC cells
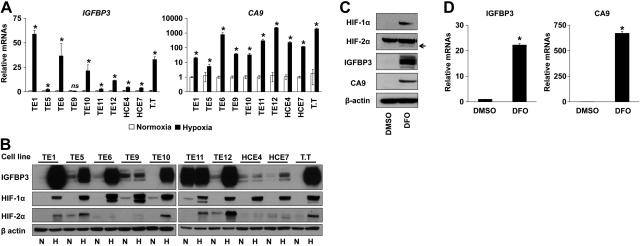
To better define the role of hypoxia in the expression of IGFBP3, we exposed ESCC cell lines to either hypoxia or normoxia for 48 h. Hypoxia induced IGFBP3 at both mRNA and protein levels (Fig. 2A, B). In addition, hypoxia induced CA9 mRNA robustly (Fig. 2A). Hypoxia stabilized HIF-1α protein in all cell lines tested (Fig. 2B). HIF-2α protein was induced in a limited number of cell lines (Fig. 2B). Under hypoxic conditions, transcription factors other than HIFs may also be activated. We next treated TE12 cells with DFO, an iron-chelating agent. Under normoxic conditions, DFO stabilized HIF-α proteins to induce IGFBP3 and CA9 in TE12 cells (Fig. 2C, D), suggesting that they may represent direct HIF target genes.
Figure 2.
Hypoxia stabilizes HIF-α subunits to induce IGFBP3 in ESCC cells. A, B) ESCC cell lines were exposed to either hypoxia (H) or normoxia (N) for 48 h (A, B). TE12 cells were treated with 100 μM DFO or DMSO (vehicle control) for 48 h (C, D). Cells were subjected to real-time RT-PCR (A, D) and Western blotting (B, C). Arrow indicates specific signal for HIF-2α (C). ns, not significant vs. normoxia. *P < 0.05 vs. normoxia or DMSO (n=3).
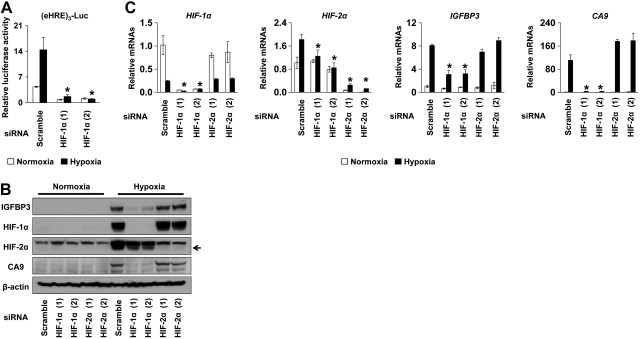
To determine the contribution of HIFs in IGFBP3 expression, we performed RNAi experiments directed against either HIF-1α or HIF-2α in TE12 cells. Suppression of HIF-1α resulted in inhibition of hypoxia-mediated activation of the (eHRE)3-luc HIF-reporter construct (Fig. 3A and ref. 24). RNAi resulted in a suppression of the basal mRNA expression as well as hypoxia-induced protein stabilization of the targeted HIF-α subunits (Fig. 3B). Interestingly, knockdown of HIF-1α, but not HIF-2α, prevented hypoxia from inducing IGFBP3 and CA9 (Fig. 3B, C), suggesting that HIF-1α may have a major role in transcriptional regulation of IGFBP3 and CA9 in ESCC cells under hypoxic conditions.
Figure 3.
HIF-1α knockdown prevents hypoxic induction of IGFBP3. TE12 cells were transfected with two independent siRNA sequences directed against either HIF-1α or HIF-2α [HIF-1α (1), HIF-1α (2), HIF-2α (1), and HIF-2α (2)] or a scrambled nonsilencing control sequence (scramble). Cells were further transfected with (eHRE)3-luc (A; ref. 24). Following transfection, cells were exposed to hypoxia or normoxia for 48 h and subjected to luciferase assays (A), Western blotting (B), and real-time PCR (C). Arrow indicates specific signal for HIF-2α (B). *P < 0.05 vs. scramble under hypoxia (n=3).
Identification of a functional HRE for HIF1α-mediated IGFBP3 transactivation
To search in silico for putative HRE within the IGFBP3 gene regulatory regions, we employed two independent algorithms. The first algorithm is designed to search for HRE consensus sequences within evolutionally conserved noncoding regions (28). This program predicted two candidate HREs within the 5′-promoter region of IGFBP3, located at −63 bp (designated HRE-a) and −215 bp (HRE-b) from the transcription start site, respectively (Fig. 4A). The ECR browser (29), the second algorithm, predicted 99 potential HREs based on the HRE core motif within a 100-kb region spanning a 60-kb 5′-upstream region of the transcription start site for IGFBP3, the entire IGFBP3 gene and its adjacent IGFBP1 located in the 3′-downstream region of IGFBP3 in a head-to-tail orientation (Fig. 4A). Although the ECR browser detected no conserved HREs between rodents and primates within the 5′-upstream region, one of the distal sites at −57 kb, conserved only among primates (HRE-c; Fig. 4A), was included for further analysis as it has been predicted as an only HIF binding site around IGFBP3 in a ChIP sequencing study (30). In addition, both programs predicted an HRE within the first intron of IGFBP1, which has been validated as IGFBP1 HRE (31). Since IGFBP1 and IGFBP3 genes are closely located, the possibility exists that they share the HRE.
To determine the existence of a functional HRE, we transiently transfected TE11 and TE12 cells with serially deleted IGFBP3 promoter-luciferase reporter constructs containing the IGFBP3 proximal promoter region (i.e., −6 kb, −3.6 kb, and −1.9 kb; ref. 25). In addition, we transfected (BP3HRE)3-a-luc, (BP3HRE)3-b-luc, (BP3HRE)3-c-luc, and BP3INT1-luc to test the above candidates HRE-a, b, and c as well as a fragment (0.7 kb) derived from the first intron of IGFBP3 containing 3 HRE core motifs in a heterologous minimal promoter-luciferase reporter system. (BP3HRE)3-c-luc appeared to be the only construct that was activated by hypoxia (Fig. 4B and data not shown), indicating that the distal HRE-c is functional, but no functional HRE may exist within the proximal promoter region or the first intron of IGFBP3. To further validate the HRE-c, we performed cotransfection experiments by expressing HIF-1αP402A/P564G, a degradation-resistant mutant or siRNA directed against HIF-1α. HIF-1αP402A/P564G activated (BP3HRE)3-c-luc under normoxic conditions, while HIF-1α knockdown suppressed hypoxic activation of HRE-c-mediated transactivation (Fig. 4C, D).
We next carried out ChIP assays using TE12 cells exposed to either hypoxia or normoxia to detect HIF-1α binding to the specific DNA regions containing HREs. ChIP with an anti-HIF-1α antibody, but not control IgG, yielded more PCR products under hypoxic conditions for EGLN3 and GAPDH, serving as positive controls with known HREs (Fig. 4E and refs. 5, 28). When the IGFBP3 locus was examined (Fig. 4A), we detected hypoxia-induced binding of HIF-1α to the −57-kb 5′-upstream region of IGFBP3 containing HRE-c, but neither proximal promoter nor the intron 1 region (Fig. 4E), in agreement with the above transfection results (Fig. 4B). Moreover, ChIP did not document HIF-1α binding to the IGFBP1 HRE (Fig. 4E), which was corroborated by only minimal hypoxic induction of IGFBP1 mRNA in TE12 cells (data not shown).
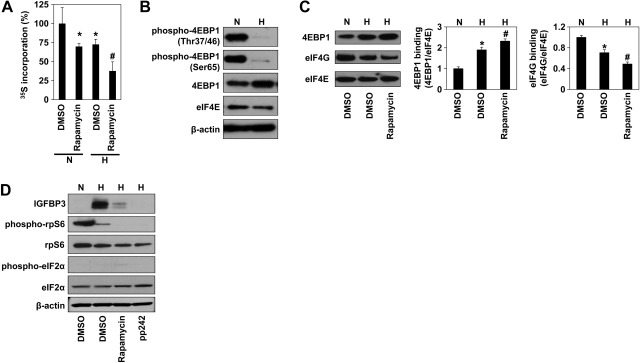
IGFBP3 protein synthesis occurs when mTOR activity and the global translation rate are suppressed under hypoxic conditions
While HIF-1α-mediated transcriptional activation is the first step in IGFBP3 induction, IGFBP3 mRNA needs to be translated when the global translation rate is diminished under hypoxic conditions, as demonstrated by metabolic labeling experiments (Fig. 5A). When we assessed the status of 4E-BP1 in ESCC cells, the nonphosphorylated form of 4E-BP1 was found to be up-regulated under hypoxic conditions. By contrast, the phosphorylated form of 4E-BP1 was significantly reduced (Fig. 5B), suggesting that hypoxia alone may reduce mTOR activity. Interestingly, the global translation rate under hypoxia was decreased further by concurrent rapamycin treatment (Fig. 5A). These observations were corroborated by m7GTP-cap binding assays (Fig. 5C). The additive effects of rapamycin treatment suggest that hypoxia alone may suppress, but not completely abrogate mTOR activity and cap-dependent translation, permitting IGFBP3 protein synthesis without pharmacological mTOR inhibition (Fig. 5D). Reinforcement of such a premise is provided by the finding that phosphorylation of rpS6 was reduced further with another mTOR antagonist, namely PP242 (Fig. 5D). In addition, hypoxia minimally induced eIF2α phosphorylation in our experimental conditions (Fig. 5D and Supplemental Fig. S1). These results suggest that IGFBP3 may be translated under hypoxia in a cap-dependent manner.
Figure 5.
IGFBP3 mRNA may be translated by cap-dependent translation under hypoxic conditions. Global protein synthesis, protein translation regulators, and IGFBP3 were determined in TE12 cells exposed to normoxia (N) or hypoxia (H) for 48 h with or without rapamycin or PP242. DMSO served as a vehicle. A) Global translation rate was measured by 35S-Met/Cys incorporation into the protein. B) Western blotting. C) m7GTP-cap binding assays. Histograms show average densitometric values for 4E-BP1 and eIF4E bound to eIF4E on m7GTP-Sepharose from 3 independent cell samples. D) Western blotting. Whole-cell lysates were used with β-actin as a loading control (B, D). Cell viability was >95% in all above experimental conditions. *P < 0.05 vs. DMSO under normoxia, #P < 0.05 vs. DMSO under hypoxia (n=3).
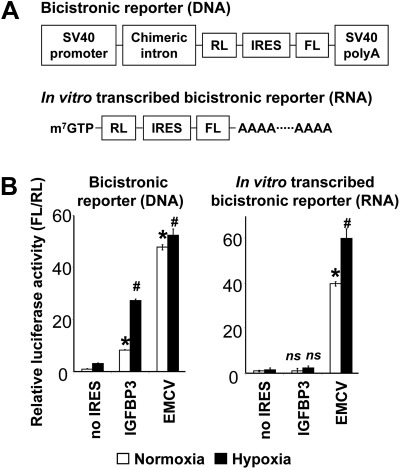
Alternatively, IRES-mediated cap-independent translation may account for hypoxic induction of IGFBP3. To determine whether IGFBP3 mRNA may have an IRES sequence within the 5′ UTR, we performed bicistronic reporter assays to test the potential IRES activity within the 5′ UTR of IGFBP3 (pR/BP3-5′UTR/F) using pR/ΔE/F (empty vector) and pΔEMCV (EMCV IRES) as negative and positive controls, respectively. pR/BP3-5′UTR/F activated the reporter when transfected as plasmid DNA in TE12 and 293T cells compared to pR/ΔE/F, and that was augmented by hypoxia (Fig. 6 and data not shown). However, when transfected as in vitro-transcribed bicistronic RNA, pR/BP3-5′UTR/F did not activate the reporter in response to either hypoxia or normoxia (Fig. 6 and data not shown), indicating that the DNA sequence within the 5′ UTR of IGFBP3 may have a cryptic promoter activity, but not function as an IRES. By contrast, in vitro transcribed pΔEMCV RNA activated the reporter with a significant enhancement by hypoxia (Fig. 6), suggesting hypoxic regulation of EMCV IRES.
Figure 6.
5′ UTR of IGFBP3 mRNA may have no IRES activity. A) Schematic representation of bicistronic vectors and in vitro transcribed RNAs. B) Cells were transfected with either DNA (left panel) or RNA (right panel) for luciferase assays. EMCV IRES served as a positive control. IRES activity was measured as the ratio of firefly luciferase (FL) activity to Renilla luciferase (RL) activity. ns, not significant vs. no IRES.*P < 0.05 vs. normoxia and no IRES, #P < 0.05 vs. hypoxia and no IRES (n=3).
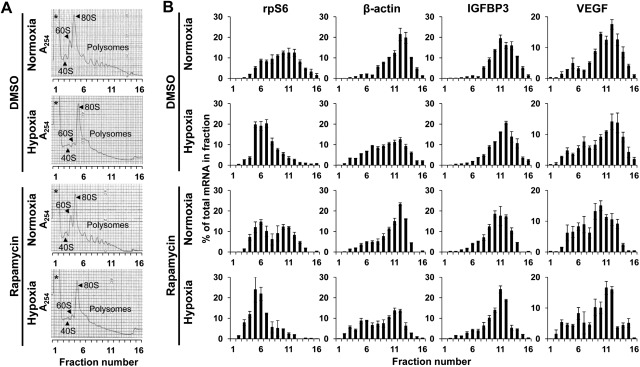
mRNA may be enriched in the polysomes for several hypoxia-responsive genes, thereby allowing continuous translation (10). In the polysome analysis, hypoxia resulted in a general collapse of the polysome gradient as well as the 40S and 60S peaks, with a reciprocal increase in the relative size of the 80S peak (Fig. 7A). The analysis of the mRNA distributions revealed that hypoxia induced a major shift to smaller polysomes for rpS6 and β-actin mRNAs, implying a decrease in translation efficiency (Fig. 7B). By contrast, the active translation profiles for IGFBP3 and VEGF mRNAs were not severely affected by hypoxia (Fig. 7B). This effect of hypoxia appeared to be similar in the presence of DMSO or rapamycin (Fig. 7B), although rapamycin shifted all tested mRNAs toward smaller fractions, with the most notable changes in rpS6 mRNA carrying a 5′ terminal oligopyrimidine tract (TOP) sequence and sensitive to mTOR inhibition (Fig. 7B). These results suggest that IGFPB3 mRNA may remain in the polysomes under hypoxic conditions and that its translation may be less sensitive to mTOR inhibition than other mRNAs including TOP mRNAs.
Figure 7.
IGFBP3 mRNA remains in the polysomes under hypoxic conditions. TE12 cells were exposed to either normoxia or hypoxia for 48 h in the presence or absence of rapamycin. DMSO served as a vehicle. A) Polysome profiles based on sucrose gradient absorbance profile (A254nm). Asterisk indicates messenger ribonucleoprotein. B) RNA from indicated ribosome fractions was subjected to real-time PCR for rpS6, β-actin, IGFBP3, and VEGFA mRNAs. Histograms display percentage of total mRNA in each fraction for indicated mRNA. Cell viability was >95% in all experimental conditions.
In aggregate, our data suggest that hypoxia induces IGFBP3 mRNA through HIF-1α-mediated transactivation through a distal HRE in the 5′-upstream region of IGFBP3 and that polysome enrichment of IGFBP3 mRNA may permit cap-dependent translation under hypoxic conditions.
DISCUSSION
Hypoxia induces IGFBP3 in ESCC via HIF-1α-mediated transcription through a remote, novel HRE
IGFBP3 has been implicated in pathological conditions related to hypoxia, such as oxygen-induced retinopathy (32–34), pulmonary hypertension (35), and tumor angiogenesis (15). IGFBP3 is overexpressed in ESCC (13, 14), which we used as a model system in this study. While very little HIF-α protein is expressed in the normal esophageal epithelium (36), HIF-1α is highly expressed in primary ESCC and associated with the expression of vascular endothelial growth factors, tumor invasion, lymphatic invasion, lymph node metastasis, and poor prognosis (37–40). We have demonstrated previously that hypoxia induces IGFBP3 along with angiogenic factors, such as cyclooxygenase-2 and prostaglandin E synthase (13). In this study, we showed a concurrent expression of IGFBP3 and CA9, the latter HIF-1α target gene determined as a surrogate of HIFs expressed in primary tumors and hypoxic lesions (i.e., pimonidazole localization) within xenograft tumors (Fig. 1). Insulin and IGFs contribute to HIF-1α expression, which, in turn, induces IGF-2 and IGFBP3 (20). Thus, hypoxia-induced IGFBP3 may negatively regulate IGF signaling to promote tumor necrosis by preventing IGF from activating a survival signal. Alternatively, IGFBP3 may bind and enrich hypoxia-induced IGF-2 that stimulates IGF1R-mediated survival signal in the tumor cells surrounding necrotic area, thereby limiting necrosis under hypoxic conditions. Depending on the context of the extracellular matrix, IGFBP3 may also facilitate epidermal growth factor (EGF)-mediated tumor growth in an IGF- (or IGF1R)-independent fashion (41). In addition, IGFBP3 may regulate angiogenesis in ESCC.
Hypoxia stabilized HIFs and IGFBP3 in most ESCC cell lines tested (Fig. 2). RNAi experiments indicated that HIF-1α is a major contributor in hypoxia-mediated induction of IGFBP3 in ESCC cells (Fig. 3). These data are in agreement with previous findings suggesting IGFBP3 as a HIF-1α target gene (20, 21). Additive effects of simultaneous knockdown of HIF-1α and HIF-2α on hypoxic induction of IGFBP3 were observed in MCF7 cells (21). HIF-1α knockdown reduced HIF-2α (Fig. 3). Thus, HIF-1α may indirectly regulate IGFBP3 through HIF-2α. However, HIF-2α knockdown did not suppress IGFBP3 and CA9 (Fig. 3), reinforcing the role of HIF-1α in IGFBP3 expression.
We have for the first time identified a functional HRE for IGFBP3. Located at the 57-kb-upstream region, this HRE is conserved among higher primates, but not rodents. Consistent with our results, this HRE has been detected as an only putative HRE adjacent to the IGFBP3 gene under a high-stringency condition in a recent ChIP sequencing study (30) using MCF7 human breast cancer cells. This study has revealed that 50–70% of genes may have distant HIF binding sites located 2.5 to 100 kb away from core promoter regions (30). The lack of HIF binding to the proximal IGFBP3 promoter region (Fig. 4) has also been corroborated by studies on human cancer cell lines MCF7, MB231, U87 and HepG2 cells using ChIP coupled with promoter microarrays (42, 43). Given other variables, such as cell type-specific transcriptionally active genomic loci (43), it is possible that there may be additional HREs. We considered the involvement of an HRE for IGFBP1 (31), located 32 kb downstream of the transcription start site for IGFBP3. However, HIF-1α was not found binding to this HRE in TE12 cells (Fig. 4E). Surprisingly, the novel −57-kb IGFBP3 HRE was not present in rodents. Rodent Igfbp3 promoter and its first intron contain a few potential HREs. However, they are not conserved in primate IGFBP3. Thus, HREs for IGFBP3 may be localized differently between human and rodents.
A distant HRE may allow a unique regulation of IGFBP3 under hypoxic conditions
IGFBP3 is induced by a number of agents (e.g., chemotherapeutic drugs), hormones (e.g., vitamin D), growth factors (transforming growth factor-β), cytokines (e.g., tumor necrosis factor α) and expressed under conditions related to cellular stresses (e.g., growth factor deprivation and hypoxia) (44). p53 tumor suppressor protein mediates cellular stress responses. P53 transactivates IGFBP3 through specific cis-elements within the proximal promoter region (45–47). However, p53 is inactivated due to mutations in all ESCC cell lines used in this study (48). Therefore, hypoxia induces IGFBP3 in a p53-independent fashion, as suggested previously (18). IGFBP3 may undergo epigenetic silencing through promoter methylation, which may affect p53 binding (49). Although the CpG island of IGFBP3 is heavily methylated in MCF7 cells (50), hypoxia induces IGFBP3 in this cell line (18). Thus, a remote HRE may permit hypoxic induction of IGFBP3 in the presence of promoter methylation. EGF represses IGFBP3 sharply through the Ras-mitogen-activated protein kinase pathway under normoxic conditions (51). However, IGFBP3 is frequently overexpressed in ESCC cells with EGF receptor overexpression (14). Hypoxia induces IGFBP3 in transformed esophageal cells in the presence of EGF (13). Although cis-elements mediating IGFBP3 repression by EGF receptor signaling are not known, our data suggest that hypoxia may overcome such negative regulation by EGF receptor signaling.
mRNA enrichment in the polysomes may allow continuous IGFBP3 protein synthesis under hypoxic conditions
A sharp suppression of IGFBP3 mRNA induction on HIF-1α knockdown (Fig. 3) suggests that hypoxic induction of IGFBP3 depends primarily on HIF-1α-mediated transactivation. Once transcribed, IGFBP3 mRNA needs to be translated into protein, while hypoxia suppresses global protein synthesis to conserve energy. Hypoxia may facilitate a switch from cap-dependent translation to cap-independent IRES-mediated mRNA translation (52). Up-regulation of 4E-BP1 and eIF4G has been implicated in induction of VEGFA and HIF-1α and promotion of tumor angiogenesis in breast cancer cells (53), where bicistronic reporter constructs were used to document 4E-BP1-mediated activation of cap-independent translation. Nevertheless, concerns have been raised over the bicistronic assay system, where cryptic promoters or splice sites within the IRES sequences may permit the translation of the 3′ cistron (54). RNA transfection experiments using bicistronic or uncapped RNAs have documented weak IRES activities for cellular genes such as HIF-1α, VEGF, c-myc, and XIAP, albeit with a very low protein production efficiency that cannot sufficiently compensate for a reduction of cap-dependent translation (55). Our experiments failed to document IRES-mediated activation of IGFBP3 translation under either normoxic or hypoxic conditions (Fig. 6).
Cancer cells may acquire resistance to hypoxia by uncoupling oxygen-responsive signaling pathways from mTOR function, thereby eliminating inhibition of protein synthesis mediated by 4E-BP1 and eukaryotic elongation factor 2 (eEF2; ref. 56). mTOR activity is reduced, but not absent, in esophageal cancer cells under hypoxic conditions (Fig. 5), suggesting that cap-dependent translation may be used for translation of IGFBP3 mRNA enriched in the polysome (Fig. 7).
In summary, our study establishes the functional role of HIF-1α in IGFBP3 mRNA transcription through a novel functional HRE and suggests that translation of IGFBP3 mRNA may occur in a cap-dependent manner under hypoxic conditions.
Supplementary Material
Acknowledgments
This study was supported in part by U.S. National Institute of Health (NIH) grants R01DK077005 (to M.N., S.K., S.O., and H.N.), P01CA098101 (to H.N., M.N., S.K., S.O., and A.J.P.K.), P30-DK050306 (to A.A., H.S., S.C., and K.J.N.), R37-HL65449–MERIT (to S.A.L.); a University of Pennsylvania University Research Foundation Award (to H.N.); Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists B-21790382 (to S.N.); an American Gastroenterological Association (AGA) Research Foundation Student Fellowship Award (to S.C.) and an AGA-Broad Foundation Student Research Fellowship Award (to K.J.N.); and the NIH/National Institute of Diabetes and Digestive and Kidney Diseases Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306) and its core facilities (Molecular Pathology and Imaging, Cell Culture, and Molecular Biology and Gene Expression).
The authors thank James J. Lee and Daniela Budo for technical assistance; Dr. Frank S. Lee (University of Pennsylvania) and Dr. David Feldman and Dr. Peter Sarnow (Stanford University, Palo Alto, CA, USA) for reagents; Dr. Regina M. Young and Dr. Phyllis A. Gimotty (University of Pennsylvania) for discussions; and Dr. Louis del Peso (Universidad Autónoma de Madrid, Madrid, Spain) for in silico screening of IGFBP3 HREs and discussions. The authors are appreciative of discussions with members of the laboratory of Dr. Anil K. Rustgi and his editorial help.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 4E-BP1
- eukaryotic translation initiation factor 4E-binding protein 1
- CA9
- carbonic anhydrase 9
- ChIP
- chromatin immunoprecipitation
- DFO
- deferoxamine
- DMSO
- dimethyl sulfoxide
- EGF
- epidermal growth factor
- eIF
- eukaryotic translation initiation factor
- EMCV
- encephalomyocarditis virus
- ESCC
- esophageal squamous cell carcinoma
- HIF
- hypoxia-inducible factor
- HRE
- hypoxia-response element
- IGF
- insulin-like growth factor
- IGF1R
- insulin-like growth factor1 receptor
- IGFBP3
- insulin-like growth factor binding protein 3
- IRES
- internal ribosome entry site
- mTOR
- mammalian target of rapamycin
- rpS6
- ribosomal protein S6
- TOP
- 5′ terminal oligopyrimidine tract
- VEGFA
- vascular endothelial growth factor A
REFERENCES
- 1. Lin Q., Yun Z. (2010) Impact of the hypoxic tumor microenvironment on the regulation of cancer stem cell characteristics. Cancer Biol. Ther. 9, 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finger E. C., Giaccia A. J. (2010) Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 29, 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wenger R. H. (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 16, 1151–1162 [DOI] [PubMed] [Google Scholar]
- 4. Pugh C. W., Ratcliffe P. J. (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 9, 677–684 [DOI] [PubMed] [Google Scholar]
- 5. Wenger R. H., Stiehl D. P., Camenisch G. (2005) Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, re12. [DOI] [PubMed] [Google Scholar]
- 6. Grabmaier K., de Weijert M. C. A., Verhaegh G. W., Schalken J. A., Oosterwijk E. (2004) Strict regulation of CAIX(G250/MN) by HIF-1alpha in clear cell renal cell carcinoma. Oncogene 23, 5624–5631 [DOI] [PubMed] [Google Scholar]
- 7. Cazzola M., Skoda R. C. (2000) Translational pathophysiology: a novel molecular mechanism of human disease. Blood 95, 3280–3288 [PubMed] [Google Scholar]
- 8. Hay N., Sonenberg N. (2004) Upstream and downstream of mTOR. Genes Dev. 18, 1926–1945 [DOI] [PubMed] [Google Scholar]
- 9. Holcik M., Sonenberg N. (2005) Translational control in stress and apoptosis. Nat. Rev. Mol. Cell. Biol. 6, 318–327 [DOI] [PubMed] [Google Scholar]
- 10. Young R. M., Wang S. J., Gordan J. D., Ji X., Liebhaber S. A., Simon M. C. (2008) Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J. Biol. Chem. 283, 16309–16319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hintz R. L., Bock S., Thorsson A. V., Bovens J., Powell D. R., Jakse G., Petrides P. E. (1991) Expression of the insulin like growth factor-binding protein 3 (IGFBP-3) gene is increased in human renal carcinomas. J. Urol. 146, 1160–1163 [DOI] [PubMed] [Google Scholar]
- 12. Chang Y. S., Kong G., Sun S., Liu D., El-Naggar A. K., Khuri F. R., Hong W. K., Lee H. Y. (2002) Clinical significance of insulin-like growth factor-binding protein-3 expression in stage I non-small cell lung cancer. Clin. Cancer Res. 8, 3796–3802 [PubMed] [Google Scholar]
- 13. Lee J. J., Natsuizaka M., Ohashi S., Wong G. S., Takaoka M., Michaylira C. Z., Budo D., Tobias J. W., Kanai M., Shirakawa Y., Naomoto Y., Klein-Szanto A. J., Haase V. H., Nakagawa H. (2010) Hypoxia activates the cyclooxygenase-2-prostaglandin E synthase axis. Carcinogenesis 31, 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takaoka M., Harada H., Andl C. D., Oyama K., Naomoto Y., Dempsey K. L., Klein-Szanto A. J., El-Deiry W. S., Grimberg A., Nakagawa H. (2004) Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 64, 7711–7723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Jan S., Le Meur N., Cazes A., Philippe J., Le Cunff M., Leger J., Corvol P., Germain S. (2006) Characterization of the expression of the hypoxia-induced genes neuritin, TXNIP and IGFBP3 in cancer FEBS Lett. 580, 3395–3400 [DOI] [PubMed] [Google Scholar]
- 16. Distler J. H., Jungel A., Pileckyte M., Zwerina J., Michel B. A., Gay R. E., Kowal-Bielecka O., Matucci-Cerinic M., Schett G., Marti H. H., Gay S., Distler O. (2007) Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 56, 4203–4215 [DOI] [PubMed] [Google Scholar]
- 17. Koong A. C., Denko N. C., Hudson K. M., Schindler C., Swiersz L., Koch C., Evans S., Ibrahim H., Le Q. T., Terris D. J., Giaccia A. J. (2000) Candidate genes for the hypoxic tumor phenotype. Cancer Res. 60, 883–887 [PubMed] [Google Scholar]
- 18. Grimberg A., Coleman C. M., Burns T. F., Himelstein B. P., Koch C. J., Cohen P., El-Deiry W. S. (2005) p53-Dependent and p53-independent induction of insulin-like growth factor binding protein-3 by deoxyribonucleic acid damage and hypoxia. J. Clin. Endocrinol. Metab. 90, 3568–3574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ragel B. T., Couldwell W. T., Gillespie D. L., Jensen R. L. (2007) Identification of hypoxia-induced genes in a malignant glioma cell line (U-251) by cDNA microarray analysis. Neurosurg. Rev. 30, 181–187; discussion 187 [DOI] [PubMed] [Google Scholar]
- 20. Feldser D., Agani F., Iyer N. V., Pak B., Ferreira G., Semenza G. L. (1999) Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 59, 3915–3918 [PubMed] [Google Scholar]
- 21. Elvidge G. P., Glenny L., Appelhoff R. J., Ratcliffe P. J., Ragoussis J., Gleadle J. M. (2006) Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathways. J. Biol. Chem. 281, 15215–15226 [DOI] [PubMed] [Google Scholar]
- 22. Enzinger P. C., Mayer R. J. (2003) Esophageal cancer. N. Engl. J. Med. 349, 2241–2252 [DOI] [PubMed] [Google Scholar]
- 23. Ohashi S., Natsuizaka M., Naganuma S., Kagawa S., Kimura S., Itoh H., Kalman R. A., Nakagawa M., Darling D. S., Basu D., Gimotty P. A., Klein-Szanto A. J., Diehl J. A., Herlyn M., Nakagawa H. (2011) A NOTCH3-mediated squamous cell differentiation program limits expansion of EMT-competent cells that express the ZEB transcription factors. Cancer Res. 71, 6836–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu F., White S. B., Zhao Q., Lee F. S. (2001) Dynamic, site-specific interaction of hypoxia-inducible factor-1alpha with the von Hippel-Lindau tumor suppressor protein. Cancer Res. 61, 4136–4142 [PubMed] [Google Scholar]
- 25. Peng L., Malloy P. J., Feldman D. (2004) Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol. Endocrinol. 18, 1109–1119 [DOI] [PubMed] [Google Scholar]
- 26. Carter M. S., Sarnow P. (2000) Distinct mRNAs that encode La autoantigen are differentially expressed and contain internal ribosome entry sites. J. Biol. Chem. 275, 28301–28307 [DOI] [PubMed] [Google Scholar]
- 27. Azuma Y., Chou S. C., Lininger R. A., Murphy B. J., Varia M. A., Raleigh J. A. (2003) Hypoxia and differentiation in squamous cell carcinomas of the uterine cervix: pimonidazole and involucrin. Clin. Cancer Res. 9, 4944–4952 [PubMed] [Google Scholar]
- 28. Pescador N., Cuevas Y., Naranjo S., Alcaide M., Villar D., Landazuri M. O., Del Peso L. (2005) Identification of a functional hypoxia-responsive element that regulates the expression of the egl nine homologue 3 (egln3/phd3) gene. Biochem. J. 390, 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ovcharenko I., Nobrega M. A., Loots G. G., Stubbs L. (2004) ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 32, W280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schodel J., Oikonomopoulos S., Ragoussis J., Pugh C. W., Ratcliffe P. J., Mole D. R. (2011) High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117, e207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tazuke S. I., Mazure N. M., Sugawara J., Carland G., Faessen G. H., Suen L. F., Irwin J. C., Powell D. R., Giaccia A. J., Giudice L. C. (1998) Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc. Natl. Acad. Sci. U. S. A. 95, 10188–10193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott A., Fruttiger M. (2010) Oxygen-induced retinopathy: a model for vascular pathology in the retina. Eye (Lond.) 24, 416–421 [DOI] [PubMed] [Google Scholar]
- 33. Chang K. H., Chan-Ling T., McFarland E. L., Afzal A., Pan H., Baxter L. C., Shaw L. C., Caballero S., Sengupta N., Li Calzi S., Sullivan S. M., Grant M. B. (2007) IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc. Natl. Acad. Sci. U. S. A. 104, 10595–10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lofqvist C., Chen J., Connor K. M., Smith A. C., Aderman C. M., Liu N., Pintar J. E., Ludwig T., Hellstrom A., Smith L. E. (2007) IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc. Natl. Acad. Sci. U. S. A. 104, 10589–10594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gharib S. A., Luchtel D. L., Madtes D. K., Glenny R. W. (2005) Global gene annotation analysis and transcriptional profiling identify key biological modules in hypoxic pulmonary hypertension. Physiol. Genomics 22, 14–23 [DOI] [PubMed] [Google Scholar]
- 36. Talks K. L., Turley H., Gatter K. C., Maxwell P. H., Pugh C. W., Ratcliffe P. J., Harris A. L. (2000) The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am. J. Pathol. 157, 411–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katsuta M., Miyashita M., Makino H., Nomura T., Shinji S., Yamashita K., Tajiri T., Kudo M., Ishiwata T., Naito Z. (2005) Correlation of hypoxia inducible factor-1alpha with lymphatic metastasis via vascular endothelial growth factor-C in human esophageal cancer. Exp. Mol. Pathol. 78, 123–130 [DOI] [PubMed] [Google Scholar]
- 38. Kimura S., Kitadai Y., Tanaka S., Kuwai T., Hihara J., Yoshida K., Toge T., Chayama K. (2004) Expression of hypoxia-inducible factor (HIF)-1alpha is associated with vascular endothelial growth factor expression and tumour angiogenesis in human oesophageal squamous cell carcinoma. Eur. J. Cancer 40, 1904–1912 [DOI] [PubMed] [Google Scholar]
- 39. Kurokawa T., Miyamoto M., Kato K., Cho Y., Kawarada Y., Hida Y., Shinohara T., Itoh T., Okushiba S., Kondo S., Katoh H. (2003) Overexpression of hypoxia-inducible-factor 1alpha(HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br. J. Cancer 89, 1042–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsuyama T., Nakanishi K., Hayashi T., Yoshizumi Y., Aiko S., Sugiura Y., Tanimoto T., Uenoyama M., Ozeki Y., Maehara T. (2005) Expression of hypoxia-inducible factor-1alpha in esophageal squamous cell carcinoma. Cancer Sci. 96, 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McIntosh J., Dennison G., Holly J. M., Jarrett C., Frankow A., Foulstone E. J., Winters Z. E., Perks C. M. (2010) IGFBP-3 can either inhibit or enhance EGF-mediated growth of breast epithelial cells dependent upon the presence of fibronectin. J. Biol. Chem. 285, 38788–38800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mole D. R., Blancher C., Copley R. R., Pollard P. J., Gleadle J. M., Ragoussis J., Ratcliffe P. J. (2009) Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J. Biol. Chem. 284, 16767–16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xia X., Kung A. L. (2009) Preferential binding of HIF-1 to transcriptionally active loci determines cell-type specific response to hypoxia. Genome Biol. 10, R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Firth S. M., Baxter R. C. (2002) Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23, 824–854 [DOI] [PubMed] [Google Scholar]
- 45. Buckbinder L., Talbott R., Velasco-Miguel S., Takenaka I., Faha B., Seizinger B. R., Kley N. (1995) Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 377, 646–649 [DOI] [PubMed] [Google Scholar]
- 46. Grimberg A. (2000) P53 and IGFBP-3: apoptosis and cancer protection. Mol. Genet. Metab. 70, 85–98 [DOI] [PubMed] [Google Scholar]
- 47. Bourdon J. C., Deguin-Chambon V., Lelong J. C., Dessen P., May P., Debuire B., May E. (1997) Further characterisation of the p53 responsive element–identification of new candidate genes for trans-activation by p53. Oncogene 14, 85–94 [DOI] [PubMed] [Google Scholar]
- 48. Jia L. Q., Osada M., Ishioka C., Gamo M., Ikawa S., Suzuki T., Shimodaira H., Niitani T., Kudo T., Akiyama M., Kimura N., Matsuo M., Mizusawa H., Tanaka N., Koyama H., Namba M., Kanamaru R., Kuroki T. (1997) Screening the p53 status of human cell lines using a yeast functional assay. Mol. Carcinog. 19, 243–253 [DOI] [PubMed] [Google Scholar]
- 49. Hanafusa T., Shinji T., Shiraha H., Nouso K., Iwasaki Y., Yumoto E., Ono T., Koide N. (2005) Functional promoter upstream p53 regulatory sequence of IGFBP3 that is silenced by tumor specific methylation. BMC Cancer 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tomii K., Tsukuda K., Toyooka S., Dote H., Hanafusa T., Asano H., Naitou M., Doihara H., Kisimoto T., Katayama H., Pass H. I., Date H., Shimizu N. (2007) Aberrant promoter methylation of insulin-like growth factor binding protein-3 gene in human cancers. Int. J. Cancer 120, 566–573 [DOI] [PubMed] [Google Scholar]
- 51. Takaoka M., Smith C. E., Mashiba M. K., Okawa T., Andl C. D., El-Deiry W. S., Nakagawa H. (2006) EGF-mediated regulation of IGFBP-3 determines esophageal epithelial cellular response to IGF-I. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G404–G416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silvera D., Formenti S. C., Schneider R. J. (2010) Translational control in cancer. Nat. Rev. Cancer 10, 254–266 [DOI] [PubMed] [Google Scholar]
- 53. Braunstein S., Karpisheva K., Pola C., Goldberg J., Hochman T., Yee H., Cangiarella J., Arju R., Formenti S. C., Schneider R. J. (2007) A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol. Cell. 28, 501–512 [DOI] [PubMed] [Google Scholar]
- 54. Kozak M. (2005) A second look at cellular mRNA sequences said to function as internal ribosome entry sites. Nucleic Acids Res. 33, 6593–6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bert A. G., Grepin R., Vadas M. A., Goodall G. J. (2006) Assessing IRES activity in the HIF-1alpha and other cellular 5′ UTRs. RNA 12, 1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Connolly E., Braunstein S., Formenti S., Schneider R. J. (2006) Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol. Cell. Biol. 26, 3955–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.