Abstract
Therapeutic angiogenesis by delivery of vascular growth factors is an attractive strategy for treating debilitating occlusive vascular diseases, yet clinical trials have thus far failed to show efficacy. As a result, limb amputation remains a common outcome for muscle ischemia due to severe atherosclerotic disease, with an overall incidence of 100 per million people in the United States per year. A challenge has been that the angiogenic master regulator vascular endothelial growth factor (VEGF) induces dysfunctional vessels, if expressed outside of a narrow dosage window. We tested the hypothesis that codelivery of platelet-derived growth factor-BB (PDGF-BB), which recruits pericytes, could induce normal angiogenesis in skeletal muscle irrespective of VEGF levels. Coexpression of VEGF and PDGF-BB encoded by separate vectors in different cells or in the same cells only partially corrected aberrant angiogenesis. In marked contrast, coexpression of both factors in every cell at a fixed relative level via a single bicistronic vector led to robust, uniformly normal angiogenesis, even when VEGF expression was high and heterogeneous. Notably, in an ischemic hindlimb model, single-vector expression led to efficient growth of collateral arteries, revascularization, increased blood flow, and reduced tissue damage. Furthermore, these results were confirmed in a clinically applicable gene therapy approach by adenoviral-mediated delivery of the bicistronic vector. We conclude that coordinated expression of VEGF and PDGF-BB via a single vector constitutes a novel strategy for harnessing the potency of VEGF to induce safe and efficacious angiogenesis.—Banfi, A., von Degenfeld, G., Gianni-Barrera, R., Reginato, S., Merchant, M. J., McDonald, D. M., Blau, H. M. Therapeutic angiogenesis due to balanced single-vector delivery of VEGF and PDGF-BB.
Keywords: ischemia, gene therapy, adenoviral vectors
Atherosclerotic coronary artery disease and peripheral vascular disease remain major causes of morbidity and mortality, despite medical and surgical advances, with a prevalence of 15–20% of all people >70 yr of age in the U.S. (1). Therapeutic angiogenesis, the growth of new blood vessels promoted by delivery of vascular growth factors, is well accepted as a strategy that could fill this currently unmet medical need, but to date efforts to achieve this goal have largely failed. Challenges include the need to combine methods for efficient expansion of the microvasculature of ischemic tissue with enlargement of upstream collateral arteries (arteriogenesis) in order to restore blood flow to hypoxic muscle tissue.
Vectors that overexpress vascular endothelial growth factor (VEGF), the master regulator of angiogenesis, can drive robust angiogenesis, but the remarkably narrow dosage range of VEGF expression compatible with normal vessel formation has hindered its efficacious use (2, 3). VEGF binds tightly to the extracellular matrix (4) and induces normal or aberrant vascular growth depending on its localized dosage in the microenvironment (3, 5). Consequently, VEGF has the potential to induce significant adverse effects, such as increased blood vessel permeability with edema (6), and aberrant vascular proliferation with angioma-like growth (7) if even rare “hotspots” of excessive expression occur (3).
Expansion of the microvascular bed following VEGF delivery has been shown to lead to arteriogenesis by increasing blood flow and shear stress (8) and inducing upstream responses through retrograde conduction along vessel walls via intercellular gap junctions (9). However, controlled clinical trials employing a variety of vectors and growth factors have failed to show clear efficacy (10). Indeed, retrospective analyses have shown that when VEGF was delivered at a safe dose, it did not generate sufficient angiogenesis to correct cardiac or skeletal muscle ischemia (11, 12). Therefore, the current conundrum is that increased VEGF levels are required to achieve beneficial effects, yet high expression levels readily lead to a loss of safety.
Why have the approaches used to date failed? Growth of normal blood vessels requires the complex interaction of multiple cell types and growth factors that are coordinated in time and space (13). We postulated that a “well-tempered vessel”—like Bach's “well-tempered clavier”—could be achieved by balancing the effects of exogenously delivered VEGF with endogenous factors (14). Blood vessel stability depends on the coordinated interaction of multiple signaling pathways in the endothelium and pericytes (13). VEGF promotes endothelial cell growth, while platelet-derived growth factor-BB (PDGF-BB) stabilizes blood vessels by recruiting pericytes (13). When blood vessel growth is induced by other proangiogenic factors, such as placental growth factor, fibroblast growth factor-2, and hepatocyte growth factor, VEGF is up-regulated (15). Accordingly, therapeutic strategies that deliver a single angiogenic agent must inevitably depend on the availability of endogenous factors to achieve a balance. Without that balance, the new blood vessels are abnormal, unstable, and inefficient (2, 3, 14, 16, 17). Delivery of VEGF alone can achieve such a balance, but only if its expression is tightly controlled in every transduced cell within a specific range of levels (2, 3, 5), and this control has yet to be achieved with current gene therapy approaches.
Here we test the hypothesis that a combination of factors that promote both endothelial cell proliferation and pericyte recruitment, VEGF and PDGF-BB, can drive the growth of normal blood vessels and reverse muscle ischemia. Notably, we focused on angiogenesis in limb skeletal muscle, because this is the clinically relevant tissue in which ischemia occurs (12). Indeed, the importance of this choice of target tissue is highlighted by the disparate effects of angiogenic growth factors seen by others in nonmuscle tissue microenvironments in which the local factor milieu likely differs (18, 19). Our data suggest that a bicistronic vector encoding VEGF and PDGF-BB in a fixed balanced ratio will constitute an efficacious viral vector-mediated gene therapy strategy for the treatment of debilitating muscle ischemia associated with atherosclerotic vascular disease.
MATERIALS AND METHODS
Cell culture
C57BL/6 mouse primary myoblasts, already transduced to express the β-galactosidase marker gene from a retroviral promoter (20), were further infected at high efficiency, as described previously (7), with retroviruses expressing murine VEGF164, or human PDGFb, or both. Early-passage myoblast clones were randomly isolated using a FacStar cell sorter (Becton Dickinson, San Jose, CA, USA) as described previously (3), and single-cell isolation was confirmed visually. All myoblast populations were cultured in 5% CO2 on collagen-coated dishes as described previously (20).
VEGF and PDGF-BB ELISA measurements
The amounts of mVEGF164 and hPDGF-BB in cell culture supernatants and whole-muscle tissue lysates were quantified using specific ELISA kits (R&D Systems, Abingdon, UK) as described previously (3). Briefly, cell culture supernatants (n=4) were harvested after 4 h incubation with fresh medium supplemented with 10 μg/ml heparin to prevent retention of PDGF-BB on the cell surface. Results for VEGF and PDGF-BB are expressed relative to the number of cells and time of incubation (ng/106 cells/d). The results for tissue lysates were normalized for the total amount of protein, quantified with the BC protein assay (Bio-Rad, Reinach, Switzerland).
In vivo myoblast implantation
Severe combined immunodeficiency (SCID) CB.17 mice (6–8 wk old; Taconic, Germantown, NY, USA) were treated in accordance with the U.S. National Institutes of Health and Swiss federal guidelines for animal welfare, with protocols approved by the Stanford University Administrative Panel on Laboratory Animal Care and the Veterinary Office of the Canton Basel-Stadt. Myoblasts (5×105) in 5 μl of PBS with 0.5% BSA were injected into the tibialis anterior (calf) or the auricularis posterior (dorsal aspect of the external ear) muscles using a 29½-gauge needle.
Recombinant adenovirus production
Recombinant adenoviruses expressing either mouse VEGF164, human PDGF-BB, or both in a bicistronic cassette were produced using the Adeno-X Expression System (Clontech, Saint-Germain-en-Laye, France) according to manufacturer's recommendations. All adenoviral constructs also expressed a truncated version of CD8 as a marker gene. Briefly, target genes were cloned into the pShuttle vector, subcloned into the Adeno-X viral DNA, and used to transfect HEK293 cells with Fugene HD reagent (Roche Applied Science, Basel, Switzerland). After 1 wk, viral particles were collected from transfected cells by repeated freezing–thawing and used for reinfection of fresh HEK293 cells. After 4–5 lysis and infection cycles, viral particles were collected and purified by a double cesium chloride gradient. Viral titer was determined as infectious units after serial infection of HEK293 cells at different multiplicities of infection, as described previously (21). Adenoviral vectors were diluted in physiological solution and injected in ear and hindlimb muscles of SCID mice at the titer of 1 × 108 infectious units/injection.
Tissue staining
The entire vascular network of the ear was visualized by intravascular staining with a biotinylated Lycopersicon esculentum lectin (Vector Laboratories, Burlingame, CA, USA) that binds the luminal surface of all blood vessels, as described previously (3). Mice were anesthetized, lectin was injected intravenously, and 2 min later, tissues were fixed by vascular perfusion of 1% paraformaldehyde and 0.5% glutaraldehyde in PBS (pH 7.4). Ears were removed, bisected in the plane of the cartilage, and stained with X-gal to detect implanted myoblasts. Vessels were stained with avidin-biotin complex-diaminobenzidine histochemistry (Vector Laboratories, Burlingame, CA, USA), dehydrated through an alcohol series, cleared with toluene, and whole-mounted on glass slides with Permount embedding medium (Fisher Scientific, Wohlen, Switzerland).
For tissue sections, mice were sacrificed by cervical dislocation. Tibialis anterior muscles were harvested and frozen in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA). Sections were stained with X-gal or with hematoxylin and eosin (H&E). Immunostaining was performed using the following antibodies and dilutions: rat monoclonal anti-mouse CD31 (BD Biosciences, Basel, Switzerland; 1:100); mouse monoclonal anti-mouse α-smooth muscle actin (αSMA; MP Biomedicals, Basel, Switzerland; 1:400); rabbit polyclonal anti-nerve/glial antigen 2 (NG2; Chemicon International, Chandlers Ford, UK; 1:200); chicken polyclonal anti-laminin (Abcam, Cambridge, UK; 1:200). Fluorescently labeled secondary antibodies (Invitrogen, Basel, Switzerland) were used at 1:100. As areas of effect did not extend to the whole muscle but were limited to the implantation sites, areas of engraftment were unequivocally identified by tracking implanted myoblasts by X-gal staining in serial parallel sections. Images were acquired with an Olympus BX61 epifluorescence microscope (Olympus, Tokyo, Japan) and AnalySIS D acquisition software (Soft Imaging System, Münster, Germany) at the lowest magnification that could adequately reveal the structure of the induced vessels. Composite fluorescent images were generated by overlaying the different channels with Adobe Photoshop CS2 (Adobe Systems, San Jose, CA, USA).
Vessel measurements
Vessel length density (VLD) and diameters were measured in whole mounts of lectin-stained ears as described (3). Vessels were randomly chosen by overlaying microscopic images with a computer-generated grid, and 200–300 total vessel diameters were measured from 4–5 ears/group. VLD was measured on 3–6 fields/ear and 4–5 ears/group by tracing the total length of vessels with AnalySIS D software (Soft Imaging System) and dividing it by the area of the fields. In ischemia experiments VLD was measured on cryosections of the distal thigh muscles (quadriceps femoris and adductor muscles), immunostained for CD31, on 3 randomly acquired images per group. Centerlines of vessels were manually drawn as described previously (3), quantified using calibrated Openlab image analysis software (Improvision, Lexington, MA, USA) and normalized to the number of muscle fibers.
Plasma leakage measurements
Evans blue assays were performed as described previously (6). Briefly, Evans blue dye (30 mg/kg in 100 μl PBS; J. T. Baker, Phillipsburg, NJ, USA) was injected i.v. After 4 h, mice were perfused with 1% paraformaldehyde in 0.05 M citric acid (pH 3.5). Biopsy punches (6 mm) of ears were obtained (Sklar Instruments, West Chester, PA, USA). Evans blue was extracted from tissue with formamide at 55°C overnight and measured with a spectrophotometer at 610 nm. Plasma leakage was measured at 4, 7, and 14 d after myoblast implantation and expressed as nanograms of dye per milligram of tissue wet weight (n=5). Total leakage was also normalized to the total vascular surface induced by the different myoblast populations in similarly injected ears (n=4–5 ears/condition). Total vascular surface was estimated as the product of average vessel perimeter (π × average diameter, measured as described above) and total vessel length (VLD, measured as described above, multiplied by the total area of effect measured on low-magnification microscopic pictures). Normalized leakage was expressed as nanograms of dye per square millimeter of vascular surface in the area of effect (n=5).
Hindlimb ischemia
Male SCID CB.17 mice (16–20 wk old) were treated according to the guidelines of the Stanford University Administrative Panel on Laboratory Animal Care. Mice were maintained under isoflurane anesthesia. Unilateral hindlimb ischemia was induced by ligation and transection of the medial portion of the right superficial femoral artery distal to the deep femoral artery origin as described previously (5). Mice were randomized to receive either vehicle or 8 × 106 total myoblasts suspended in 0.5% BSA in PBS, as 8 injections into the distal thigh muscles (5–7 mice/group).
After 14 d, blood flow was measured using fluorescent microspheres (5). Red fluorescent microspheres (2×105, 15 μm diameter; Invitrogen) were continuously injected over 60 s into the beating left ventricle. The heart was perfused with Tris-HCl buffer containing Na+, Ca2+, Mg2+, and 0.1% adenosine (2 min), followed by 1.5% formaldehyde (2 min). The thigh muscle group (adductor and quadriceps) was excised, cut in midthigh, weighed, and snap-frozen in OCT compound. Microspheres were individually counted by direct fluorescence microscopy on 100-μm cryosections from the entire distal thigh muscles, where the different myoblast populations had also been implanted. These sections were serial to those used for histological analysis of vascular morphology. Microsphere counts were normalized by muscle weight, and the counts from the ischemic leg were expressed as a percentage of those in the contralateral, nonischemic leg. Kidneys were analyzed as reference organs to confirm equivalent bilateral microsphere distribution.
Collateral vessels ≥30 μm in diameter were quantified on cross sections of the proximal adductor muscle by costaining for CD31 and αSMA (5). Damaged muscle was identified on H&E-stained cryosections of the calf muscles as either inflammation (mononucleated cell infiltrates) or necrosis (“ghost fibers” lacking nuclei). Areas were manually drawn on digital images and quantified using calibrated Openlab image analysis software.
Protein isolation and quantification
Whole fresh mouse muscles were disrupted using a Qiagen Tissue Lyser (Qiagen, Hombrechtikon, Switzerland) in 500 μl of PBS + 1% Triton X-100, supplemented with Complete Protease Inhibitor Cocktail (Roche Diagnostics, Rotkreuz, Switzerland), which does not denature proteins or lyse nuclei. After centrifugation, 200-μl aliquots of the lysates were used for protein quantification and ELISA analysis.
Statistics
Data are presented as means ± se. The significance of differences was evaluated using analysis of variance (ANOVA) followed by the Bonferroni test. Values of P < 0.05 were considered statistically significant.
RESULTS
Uncoordinated VEGF and PDGF-BB codelivery results in both normal and aberrant angiogenesis
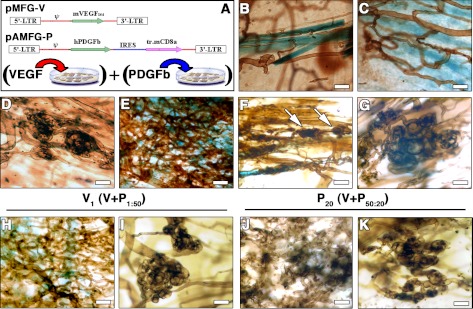
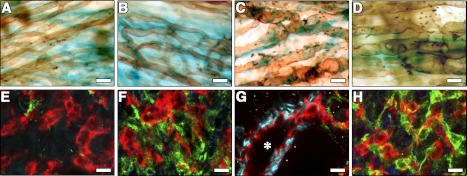
We first investigated the effects of expressing either VEGF or PDGF-BB alone on angiogenesis. For this purpose, we employed a previously described model system entailing primary mouse myoblast-based gene delivery (20), which allows expression of genes in a spatially and temporally controlled manner. β-Galactosidase-expressing cells were further transduced to produce either an isoform of VEGF-A (VEGF164, designated here as VEGF) or PDGF-BB linked to a truncated cell surface marker CD8a, whereas control cells were transduced with CD8a only (V, P, and CD8, respectively, Fig. 1A). In vitro, V cells produced 112 ± 10.4 ng/106 cells/d of VEGF, and P cells secreted 45.7 ± 2.4 ng/106 cells/d of PDGF-BB. Genetically engineered myoblasts were implanted into the posterior auricular muscles of SCID mice, as the ear muscle is thin and particularly advantageous for visualization of the vascular architecture. Vessel morphology was evaluated both 2 and 4 wk after implantation, with equivalent results. Control cells or PDGF-BB alone had no effect on angiogenesis (Fig. 1B, C), while VEGF alone induced abundant aberrant structures (Fig. 1D), which later evolved into large angiomas.
Figure 1.
Uncoordinated expression of VEGF and PDGF-BB leads to a mixture of normal and aberrant vasculature. A) Retroviral constructs used to generate myoblast populations expressing mVEGF164 (pMFG-V) or hPDGF-BB (pAMFG-P). B–K) At 2 wk after implantation in ear muscles (n=7), control cells expressing only CD8 (B) or PDGF-BB (C) induced no angiogenesis. VEGF myoblasts (D) induced aberrant vessels. A 50:50 mixture of the two populations (E–G) induced robust normal angiogenesis (E) but also aberrant vascular structures (F, arrows; G). Vessels are stained brown after intravascular lectin perfusion and implanted myoblasts expressing β-galactosidase are stained blue (X-gal). Different proportions of coimplanted cells (n=4), with a 50:1 excess of PDGF-BB expression (V1) or 50:20 of VEGF expression (P20), did not prevent the development of aberrant angiogenesis (I, K) in neighboring large areas of normal vessels (H, J). Scale bars = 25 μm.
We tested whether coimplantation of a mixture of VEGF- and PDGF-BB-expressing cell populations could prevent aberrant vessel development and lead to normal angiogenesis. Although highly branched networks of short regular capillaries were induced (Fig. 1E), aberrant structures similar to those observed with VEGF alone could always be found (Fig. 1F, G). To determine whether the induction of aberrant angiogenesis depended on the relative dose of VEGF and PDGF-BB, we varied the ratio of the two populations in the implanted mixture. V cells were kept constant at 50%, and P cells were decreased to 20, 10, 5, and 1% (P20, P10, P5 and P1, respectively), with the remainder being made up of control cells. Alternatively, P cells were maintained at 50%, and V cells were similarly decreased to 20, 10, 5, and 1% (V20, V10, V5, and V1, respectively). When PDGF-BB-expressing cells comprised just 1% (P1), only VEGF-induced aberrant structures were detected. However, as the proportion of P cells increased, the proportion of normal capillary networks increased (Fig. 1H, J), but aberrant structures were still always present, even when only 1% of cells expressed VEGF (Fig. 1I, K). Therefore, irrespective of the ratio of cells expressing VEGF to PDGF-BB, aberrant angiogenesis was consistently observed. These findings suggest that normal angiogenesis cannot be reliably achieved when the two factors are randomly localized in the cellular microenvironment due to secretion by distinct muscle cells.
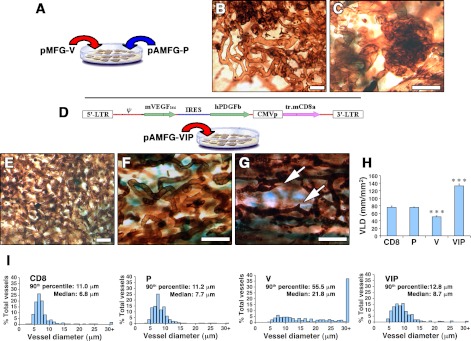
In a second approach designed to ensure colocalization of VEGF and PDGF-BB, we generated cell populations in which each nucleus expressed both factors. Sequential transduction with two independent constructs ensured coexpression of the factors in each cell, but at random ratios due to differential vector uptake (Fig. 2A). The resulting population produced similar amounts of VEGF as V cells (132.7±1.7 ng/106 cells/d) and of PDGF-BB as P cells (54.6±3.4 ng/106 cells/d). After in vivo implantation, coexpression of the two factors at random ratios yielded extensive orderly capillary networks, but aberrant angioma-like structures were also always present (Fig. 2B, C).
Figure 2.
Coordinated expression of VEGF and PDGF-BB induces only homogeneous normal vessels. A) Myoblasts coexpressing VEGF and PDGF-BB in each cell, but at random relative levels, were generated by sequential transduction with two separate retroviral constructs. B, C) At 2 wk after implantation (n=5), coexpression at random relative levels induced some normal capillaries (B) but also aberrant bulbous vascular structures (C). D) The pAMFG-VIP bicistronic construct ensured coordinated expression of VEGF and PDGF-BB at a fixed ratio in the microenvironment around each transduced cell. E–G) Coordinated coexpression induced only robust normal angiogenesis (n=10). Arrows (G) indicate sprouting capillaries. H, I) Amount of angiogenesis, assessed as VLD (H), and distribution of vessel diameters (I) were quantified in areas implanted with control CD8, P, V, or VIPhigh myoblasts (n=4–5). VLD = vessel length (mm)/area of effect (mm2). Scale bars = 25 μm. ***P < 0.001.
Coordinated expression of VEGF and PDGF-BB using a bicistronic vector produces uniformly normal and mature vessels
In a third approach, we aimed to achieve colocalized expression of both factors in fixed relative amounts (coordinated expression). Myoblasts were transduced with a bicistronic construct [designated VIP for VEGF–internal ribosomal entry site (IRES)–PDGF-BB; Fig. 2D], which ensures the translation of both sequences from a single mRNA (22). Two cell populations were generated by different numbers of transduction rounds (VIPlow and VIPhigh). VIPlow cells expressed 58.9 ± 5.8 ng of VEGF and 9.7 ± 0.7 ng of PDGF-BB/106 cells/d. VIPhigh cells expressed 107.9 ± 6.2 ng of VEGF and 26.5 ± 1.6 ng of PDGF-BB/106 cells/d. FACS analysis showed that transgene expression in all populations was heterogeneous on a cell-by-cell basis, as evidenced by staining for the coexpressed cell-surface marker CD8a (data not shown), which has been shown to correlate directly with the amount of transgene production in individual cells (23). Following implantation into muscles, coordinated coexpression by both VIP populations induced a well-structured vasculature, with highly branched networks of homogeneously sized capillaries in all sites (Fig. 2E–G). Remarkably, even though VIPhigh cells expressed heterogeneous VEGF levels similar to the V population, no aberrant structures were ever observed.
As a measure of the amount of vasculature induced, VLD was determined in the implanted muscles. The results for this and subsequent analyses are shown for VIPhigh cells, although VIPlow implantations yielded equivalent findings. PDGF-BB alone did not increase the amount of vasculature. VEGF alone led to somewhat reduced VLD due to the replacement of normal capillaries with hyperfused bulbous structures (ref. 24 and Fig. 2H). By contrast, coordinated coexpression led to a 75% increase in VLD compared with controls. Thus, the extent of vasculature induced was greatly increased by VIP cells.
Vessel diameter distribution is a quantitative measure of vessel size homogeneity, a feature of normal vasculature. As shown in Fig. 2I, normal capillaries in control areas were homogeneous, with a median diameter of 6.8 μm. PDGF-BB alone caused a nonsignificant enlargement (median diameter 7.7 μm, P=n.s.) with no increase in the range of size distribution relative to controls. By contrast, VEGF-induced vessels not only were substantially dilated (median diameter 21.8 μm, P<0.001) but also exhibited a marked increase in size heterogeneity. Notably, coexpression of VEGF and PDGF-BB by VIPhigh cells prevented aberrant dilation and led to only a moderate increase in the median vessel diameter to 8.7 μm (P<0.05), while preserving size homogeneity.
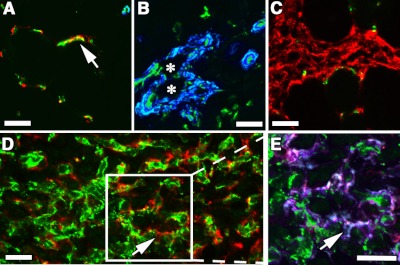
To determine whether the vessels induced by VIP cells were mature, we evaluated whether they acquired pericytes necessary for stabilization (25). Normal muscle capillaries were uniformly covered with pericytes, which expressed NG2 and lacked αSMA (Fig. 3A). By contrast, the aberrant structures induced by VEGF alone lacked typical pericytes and were coated by a thick smooth muscle layer (Fig. 3B). PDGF-BB alone led to the accumulation of NG2-positive pericytes between muscle fibers, in accordance with previous reports (26), but no increase in vasculature (Fig. 3C). By contrast, coordinated expression of both factors by VIP cells led to coverage of new vessels with normal pericytes, which were also found to be embedded in the vascular basement membrane, in close contact with the endothelium (Fig. 3D, E).
Figure 3.
PDGF-BB restores normal pericyte coverage to VEGF-induced vessels. Vessels induced by implantation of control CD8 (A), V (B), P (C), or VIPhigh myoblasts (D, E; n=3) in hindlimb tibialis anterior muscles were immunostained with antibodies against CD31 (endothelium, green), NG2 (pericytes, red), and αSMA (smooth muscle cells, blue in A–D), or laminin (basal lamina, blue in E) on frozen sections. Arrows (A, D, E) indicate pericytes with typical branched processes. Asterisks (B) indicate the lumen of an angioma-like structure devoid of pericytes and covered with smooth muscle cells. Boxed area in D is enlarged in E. P cells caused proliferation of pericytes between muscle fibers, but no angiogenesis (C), while VIP myoblasts induced a network of pericyte-covered branched capillaries (D, E). Scale bars = 25 μm.
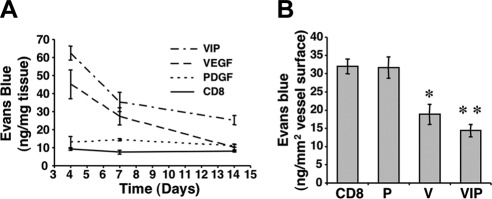
PDGF-BB coexpression via a bicistronic vector does not affect transient induction of vascular leakage by VEGF
An increase in endothelial permeability is an intrinsic property of VEGF-induced angiogenesis. PDGF-BB expression did not increase plasma extravasation above basal levels at any time point (Fig. 4A), whereas, as we previously observed (3), VEGF-induced vascular leakage was transient, peaked at 4 d, and subsided by 2 wk. PDGF-BB coexpression did not inhibit the initial surge in VEGF-induced vascular leakage, which also decreased at 1 and 2 wk. As total plasma leakage depends directly on the surface of induced vasculature, the total amount of extravasated Evans blue (ng) was normalized to the total vessel surface (mm2) in the areas of effect 2 wk after implantation of CD8, P, V, or VIPhigh myoblasts. As shown in Fig. 4B, extravasation from vessels induced by both VEGF alone and together with PDGF-BB was actually less than from normal vessels in areas implanted with control and PDGF-BB-expressing myoblasts (Fig. 4B). These results show that VEGF-induced leakage is transient and is not affected by PDGF-BB coexpression.
Figure 4.
VEGF-induced vascular leakage is transient and not affected by PDGF-BB. A) Vascular leakage was measured 4, 7, and 14 d after implantation of control cells (CD8), P, V, or VIPhigh (VIP) myoblasts in ear muscles and is expressed as nanograms of extravasated Evans blue per milligram of tissue (n=5). Both V and VIP cells caused a transient increase in leakage that peaked at 4 d and returned to baseline by 14 d. B) Total leakage was normalized to the amount of induced vasculature 2 wk after implantation of the same populations (nanograms of extravasated Evans blue per square millimeter of total vessel surface; n=5), showing that both V- and VIP-induced vasculature was less permeable than control vessels. *P < 0.05; **P < 0.01.
PDGF-BB delivered via a bicistronic vector normalizes the remodeling of VEGF-induced vessels
To understand how PDGF-BB prevented the generation of angioma-like structures by uncontrolled VEGF expression, we investigated the early stages of vascular growth after factor delivery. A time course (Fig. 5) revealed that, 4 d after myoblast implantation, the initial response to VEGF overexpression was a marked circumferential enlargement of preexisting vessels, similar to the previously described “mother” vessels (ref. 27 and Fig. 5C), that were almost devoid of pericytes (Fig. 5E) and rapidly remodeled into bulbous smooth muscle-covered structures by 7 d (Fig. 5G). PDGF-BB coexpression did not affect initial vessel enlargement by VEGF (Fig. 5D), but it prevented the early loss of pericytes, which instead accumulated in the interstitial space between activated vessels by 4 d (Fig. 5F), and converted vascular remodeling to a network of mature homogeneous capillaries by 7 d, with pericytes in close contact with the endothelium (Fig. 5H). Parallel tissue samples were analyzed for VEGF concentration per total amount of protein by ELISA at the same time points. Notably, muscle tissues transplanted with VIP myoblasts expressed up to 2-fold more VEGF in vivo than V cells at 4 and 7 d post-transplant (253.0±24.8 vs. 193.8±15.5 pg/mg protein at 4 d and 264.4±8.0 vs. 110.5±16.2 pg/mg protein at 7 d, respectively; P<0.001). These data show that PDGF-BB expressed in a fixed ratio using a bicistronic vector normalizes the effects of VEGF even when expressed at high levels.
Figure 5.
PDGF-BB regulates vascular remodeling by early pericyte recruitment. A–D) Blood vessel morphology was analyzed by whole-mount lectin staining 4 d after implantation with control CD8 (A), P (B), V (C), or VIPhigh myoblasts (D). E–H) Tibialis anterior muscle sections were immunostained 4 d (E, F), and 7 d (G, H) after implantation with V (E, G), or VIPhigh myoblasts (F, H) with antibodies against CD31 (endothelium, red), NG2 (pericytes, green), and αSMA (smooth muscle cells, blue). PDGF-BB coexpression did not affect initial vascular enlargement by VEGF but induced early pericyte recruitment by 4 d, and by 7 d caused remodeling to normal pericyte-covered vessels instead of aberrant structures. Asterisk (G) indicates the lumen of an aberrant angioma-like structure (n=4 in all cases).
The balance between VEGF and PDGF-BB dosage regulates the switch between normal and aberrant angiogenesis
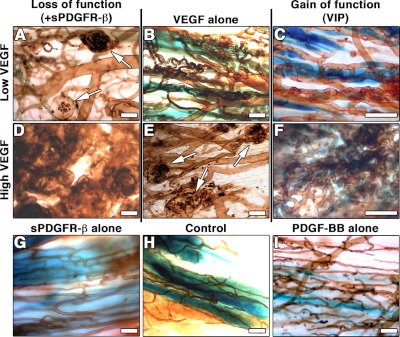
To further assess whether the induction of normal or aberrant angiogenesis by specific VEGF doses depends on PDGF-BB signaling, we performed loss- and gain-of-function experiments (Fig. 6). Specific low and high VEGF levels (70 and 120 ng/106 cells/d, respectively) were homogeneously expressed by clonal populations of transduced myoblasts, in which every cell produced the same amount of factor. These populations induced normal and aberrant angiogenesis respectively (Fig. 6B, E), as described previously (3). In a loss-of-function approach, blockade of endogenous PDGF-BB was achieved by coexpression of a truncated soluble form of PDGF receptor-β (sPDGFR-β; ref. 28). In the presence of low VEGF levels, expression of sPDGFR-β led to many aberrant structures in all implantation sites (Fig. 6A), similar to those induced by high VEGF alone (Fig. 6E). In addition, angioma-like angiogenesis induced by high VEGF levels developed faster and more extensively when sPDGFR-β was expressed (Fig. 6D). In gain-of-function experiments, we used clonal populations derived from the heterogeneous VIP population described in Fig. 2, which expressed similar low and high VEGF levels to those of the V clones (VIPlow: 74 ng VEGF/106 cells/d; VIPhigh: 141 ng VEGF/106 cells/d). Coexpression of exogenous PDGF-BB did not affect normal angiogenesis induced by low VEGF alone (Fig. 6C), but completely prevented the aberrant structures induced by high VEGF alone, yielding uniform branched capillary networks (Fig. 6F), confirming the results seen with the heterogeneous populations (Fig. 2). Expression of sPDGFR-β (Fig. 6G), like control cells (Fig. 6H) or PDGF-BB alone (Fig. 6I), did not affect preexisting vascular networks. Together, these results show that the induction of normal or aberrant angiogenesis depends on the relative dosage of VEGF and PDGF-BB and consequent signaling, rather than on a specific VEGF dose.
Figure 6.
The balance between VEGF and PDGF-BB signaling controls whether angiogenesis is normal or aberrant. Clonal myoblast populations, homogeneously expressing specific low (B) or high (E) VEGF levels, induced normal and aberrant angiogenesis, respectively. Loss-of-function experiments, in which endogenous PDGF-BB was blocked by delivery of soluble PDGFR-β, led to aberrant angiogenesis even in the presence of low VEGF levels (A) and accelerated it in the presence of high VEGF levels (D). Conversely, gain-of-function experiments in which PDGF-BB was coexpressed at coordinated levels by VIP clones did not affect normal angiogenesis by low VEGF (C) but abrogated the development of aberrant vessels by high VEGF (F). Expression of soluble PDGFR-β alone (G) or PDGF-BB alone (I) did not cause any angiogenic effect compared to control cells (H). Arrows (A, E) indicate aberrant angioma-like structures. Scale bars = 25 μm; n = 5/group.
Coordinated expression of VEGF and PDGF-BB by bicistronic delivery leads to functional collaterals and reversal of ischemia
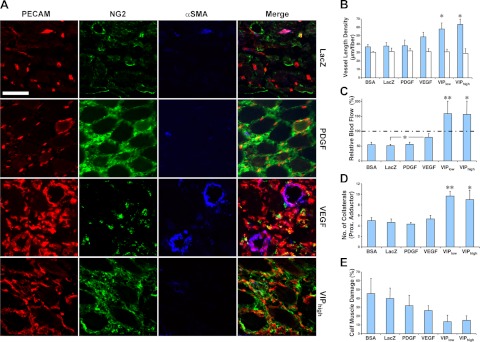
To test the efficacy of balanced coexpression of VEGF and PDGF-BB, we tested the functional effects of VIP cell implantation in a hindlimb ischemia model. The distal thigh muscles of SCID mice were injected with either vehicle or 8 × 106 myoblasts expressing β-galactosidase, VEGF alone, PDGF-BB alone, or the VIP bicistronic vector encoding both factors immediately after induction of ischemia (22) and analyzed 2 wk later. H&E staining of ischemic muscles confirmed the presence of tissue damage, with centralized nuclei in the myofibers and infiltration of inflammatory cells (data not shown). PDGF-BB cells recruited pericytes, but did not induce angiogenesis (Fig. 7A, B), similar to their effects when transplanted into nonischemic muscles. VEGF cells moderately increased vascular density compared to control cells (Fig. 7B), but many vessels were angioma-like, devoid of pericytes, and encompassed by smooth muscle cells (Fig. 7A). By contrast, both VIPlow and VIPhigh cell populations led to significant growth of uniformly normal, pericyte-covered capillaries (Fig. 7A, B).
Figure 7.
Coordinated expression of VEGF and PDGF-BB reverses hindlimb ischemia. Ischemic hindlimbs of SCID mice were treated with vehicle (BSA) or myoblasts expressing only β-galactosidase (LacZ), PDGF-BB, VEGF, or both at coordinated levels (VIPlow and VIPhigh, expressing two different average VEGF levels). Angiogenic response (A, B) and regional blood flow (C) were assessed on the distal thigh muscles (quadriceps femoris and biceps femoris), where cells were injected. Collateral growth (D) was assessed in the proximal adductor muscle, which is anatomically remote from the injection sites, and muscle damage (E) was determined in the calf muscles (tibialis anterior and gastrocnemius), where ischemia was maximal. A) Immunostaining of vessels in implanted muscles with antibodies against endothelial CD31 (PECAM; red), pericyte NG2 (green), or smooth muscle αSMA (blue). Scale bar = 50 μm; n = 5–7. B) VLD quantification (micrometers of vessel length per muscle fiber in the area of effect, n=5–7) in the treated muscles (blue bars) and contralateral nonischemic muscles (white bars). C) Relative blood flow in ischemic muscles (percentage of the contralateral nonischemic leg, n=5–7). Dotted line represents normal flow (100%). D) Number of collaterals in the proximal adductor muscle group of treated legs (n=3). E) Damaged tissue in ischemic calf muscles of treated limbs (percentage area of the total muscle on histological sections, n=5–6). *P < 0.05; **P < 0.01.
Notably, only VIP cells led to significant functional improvement in ischemic muscles. Both efficient angiogenesis and arteriogenesis were detected. Tissue perfusion was restored and damage repaired. Blood flow in ischemic muscles (Fig. 7C) was unaffected by either control or PDGF-BB-expressing cells and only moderately increased by VEGF alone. By contrast, balanced factor coexpression by VIP cells significantly improved perfusion to greater than nonischemic levels. Similarly, collateral growth was substantially increased only when coexpression occurred, and the number of collateral arteries doubled (Fig. 7D). Consistent with these angiogenic effects, while neither VEGF nor PDGF-BB alone greatly altered the percentage of damaged myofibers, coordinated coexpression by VIP cells led to a remarkable reduction in muscle damage by >50% (Fig. 7E). These findings provide compelling evidence that coordinated coexpression of the two factors using the bicistronic VIP vector had a major functional impact on ischemic limb muscles, restoring blood flow and reducing tissue damage.
Gene transfer via adenoviral delivery of a bicistronic vector encoding VEGF and PDGF-BB induces uniformly normal angiogenesis
We sought to broaden our extensive findings using a model myoblast-mediated gene delivery system to a gene delivery system appropriate for clinical translation as a gene therapy approach. Accordingly, we tested whether coordinated expression of VEGF and PDGF-BB from a single bicistronic cassette could lead to normal angiogenesis irrespective of VEGF levels. For this purpose, four different adenoviral vectors were produced, expressing the same cassettes as the retroviral vectors used to generate the CD8, V, P, and VIP myoblast populations described above, and were named Ad-CD8, Ad-VEGF, Ad-PDGF, and Ad-VIP, respectively. Effective growth factor production by the recombinant adenoviruses was confirmed by ELISA on supernatants after in vitro infection of HEK293 cells.
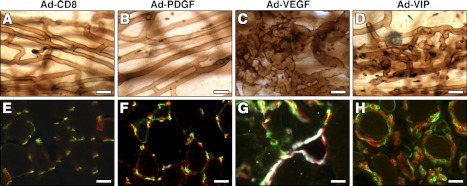
We injected 1 × 108 infectious units of each virus into the posterior auricular muscles of SCID mice. Intravascular lectin staining 2 wk later showed that both Ad-CD8 and Ad-PDGF vectors did not affect the preexisting vascular networks (Fig. 8A, B), whereas Ad-VEGF caused the widespread appearance of aberrant angioma-like structures of heterogeneous sizes and irregular shapes (Fig. 8C), similar to those induced by myoblasts expressing VEGF alone. By contrast, coordinated PDGF-BB expression by the Ad-VIP bicistronic vector prevented all instances of aberrant angiogenesis and induced only the growth of homogeneous normal capillaries (Fig. 8D), in agreement with the myoblast-mediated gene delivery system.
Figure 8.
Adenoviral delivery of a single bicistronic vector leads to coordinated expression of VEGF and PDGF-BB and prevents aberrant angiogenesis. Adenoviruses expressing PDGF-BB (Ad-PDGF), VEGF (Ad-VEGF), or both (Ad-VIP) were injected in ear (A–D) or tibialis anterior muscles (E–H) of immunocompromised mice. Adenovirus expressing only CD8 (Ad-CD8) was used as a control. After 2 wk, blood vessels were visualized in ear whole-mount preparations after intravascular lectin perfusion (in brown; A–D), or after immunofluorescent staining of endothelium (CD31, in red), pericytes (NG2, in green), and smooth muscle cells (α-SMA, in white) in cryosections of hindlimb muscles (E–H). In both locations, aberrant angioma-like structures induced by Ad-VEGF (C, G) were prevented by coexpression of VEGF and PDGF-BB by Ad-VIP, which induced only normal pericyte-covered capillaries (D, H). Scale bars = 20 μm.
These experiments were corroborated in the limb muscles by injection of the viral vectors into the tibialis anterior. Immunostaining of frozen tissue sections showed that the Ad-PDGF vector did not induce any angiogenesis above that of the control Ad-CD8 vector (Fig. 8E, F). Aberrant vasculature was induced by the Ad-VEGF vector (Fig. 8G). By contrast, Ad-VIP vector injection caused the efficient growth of mature, pericyte-covered capillaries (Fig. 8H) and prevented the appearance of aberrant, smooth muscle-covered angioma-like structures. These data lend further support to the finding that gene therapy via adenoviral delivery of a bicistronic vector encoding both VEGF and PDGF-BB to muscles of the leg, which are the targets of ischemia, leads to normal angiogenesis.
DISCUSSION
An unresolved therapeutic challenge is to achieve effective induction of angiogenesis leading to reversal of limb ischemia by growth factor delivery, a problem of major magnitude in the United States, as ∼100,000 limb amputations occur every year due to untreatable peripheral vascular disease (1). Using our myoblast-mediated gene delivery model system, which facilitates testing of variables in a well-controlled manner, we previously showed that low-level VEGF expression gives rise to orderly vasculature, whereas high-level VEGF leads to aberrant structures that eventually evolve into hemangiomas (3, 5). Here we demonstrate that the induction of normal or aberrant angiogenesis depends on the relative expression levels of VEGF and PDGF-BB, rather than on a specific amount of VEGF. Balanced coexpression of VEGF and PDGF-BB in a fixed relative ratio was achieved using a single bicistronic vector and resulted in homogeneously normal angiogenesis, irrespective of VEGF levels. Notably, the results obtained using two independent bicistronic gene delivery platforms were similar. The beneficial effects on angiogenesis of retrovirally transduced myoblasts were corroborated by a clinically relevant gene therapy approach entailing direct injection into muscles of adenoviral vectors.
Taking advantage of the high degree of control afforded by myoblast-based gene transfer, we tested various means of delivering the two factors: high-level expression of VEGF and PDGF-BB in separate populations of juxtaposed cells; different ratios of these cell populations skewed either toward VEGF or PDGF-BB; or high-level coexpression of VEGF and PDGF-BB in each cell by sequential transduction of two different vectors. In each case, although a remarkable network of orderly vasculature resulted, which was far better than that seen with VEGF alone, aberrant angioma-like vessels were also detected. In agreement with our findings with myoblast-mediated gene delivery, codelivery of VEGF and PDGF-BB by two separate adenoviral vectors led to angiogenesis with defective pericyte recruitment (26). We hypothesized that absolute levels of the two factors were not as important as the relative dosage present in the microenvironment of the growing vessels, so that both endothelial cells and pericytes would be exposed to similar balanced growth factor stimulation. A single bicistronic vector, VIP, ensured both colocalization and a fixed ratio of VEGF to PDGF-BB around each cell (coordinated coexpression) when delivered either by myoblasts or by adenoviral vectors. The data presented here show that, irrespective of the markedly different absolute levels of the two factors expressed, two independent VIP cell populations induced a large increase in histologically normal and mature vasculature. Notably, this balanced, spatially and temporally coordinated expression of both growth factors led to an increase in functional blood vessels, efficient collateral arteriogenesis, reduced muscle damage, and correction of muscle ischemia. To ensure that effective muscle perfusion was measured, rather than effects on the superficial skin layer, blood flow was determined by fluorescent microsphere counting in histological sections, as this method represents the gold standard and is routinely used to validate results obtained with new techniques (29).
VEGF has been shown to negatively regulate pericyte function by inhibiting PDGFRβ phosphorylation through the formation of a nonfunctional VEGFR2/PDGFRβ complex (19). Consistent with this report, we found that VEGF expression alone leads to initial vessel enlargement with an almost complete loss of pericytes. Our data extend these findings by demonstrating that the antipericyte effect of VEGF can be completely overcome by balanced coexpression of PDGF-BB in the same cells, preventing pericyte loss at 4 d and producing homogeneous capillary networks at 7 d. Thus, our data highlight that balanced levels and spatial colocalization of VEGF and PDGF-BB are both essential for optimal therapeutic benefit. Furthermore, although in physiological angiogenesis PDGF-BB is produced by endothelial cells (30–32), our results show that this cell source is not essential for normal vessel formation, provided that VEGF and PDGF-BB are produced in spatiotemporally colocalized gradients and balanced amounts. In fact, it should be noted that, contrary to previous observations of inadequate pericyte coverage on codelivery by two separate adenoviral vectors (26), coordinated coexpression from a single bicistronic adenovirus both restored physiological pericyte recruitment and ensured complete normalization of newly induced vasculature. These findings suggest that this balance is necessary and sufficient for effective therapeutic angiogenesis in ischemic muscle.
In the present experiments, VEGF and PDGF-BB were expressed simultaneously by the bicistronic vector. Because of the success of this approach, we did not determine whether sequential expression of the two growth factors was even more efficacious than simultaneous expression. Nonetheless, our findings suggest that early expression of PDGF-BB did not limit VEGF-driven capillary growth. The larger amount of vessel growth found with synchronous expression of the two growth factors, compared to expression of VEGF alone, argues that increased pericyte recruitment within the first 4 d did not have a net negative effect on angiogenesis.
Critical to our findings was the delivery of the angiogenic factors to skeletal muscle, the clinically relevant target for treatment of peripheral ischemia. Others have studied VEGF and PDGF-BB interaction in nonmuscle tissues and observed different effects. Although PDGF-BB is proangiogenic in the chicken chorioallantoic membrane and in Matrigel plug assays (19), it does not induce angiogenesis either in normal or ischemic skeletal muscle (26). Furthermore, PDGF-BB expression in the cornea does not correct the aberrant features of VEGF-induced vessels (18), by contrast with its effects in skeletal muscle. Thus, these growth factors are context-dependent and their effects cannot be readily extrapolated across tissues.
Most striking was the functional impact of coordinated VEGF and PDGF-BB delivery to ischemic limb muscles of mice. The extent and morphology of the induced vasculature caused not only increased angiogenesis but also a doubling of collateral arteries. This, in turn, led to increased tissue perfusion and substantially reduced muscle damage assessed by myofiber integrity. All of these functional changes were observed only with VIP cells, not V, P, or control cells.
Our results have major implications for therapeutic angiogenesis. VEGF gene delivery has a narrow therapeutic window for the treatment of muscle ischemia (16, 17), which has hindered use of gene therapy vectors (2). Based on our findings using adenoviral vectors, we predict that balanced expression of VEGF and PDGF-BB in the same cells using viral delivery of a single bicistronic vector will overcome this problem, enabling safe and efficacious gene therapy for angiogenesis in patients with limb ischemia.
Acknowledgments
The authors thank N. Di Maggio (Basel University Hospital, Basel, Switzerland) for critical comments on the manuscript, P. Lindblom (Karolinska Institute, Stockholm, Sweden) for the hPDGFb cDNA, and L. T. Williams (University of California, San Francisco, CA, USA) for the sPDGFR-β cDNA.
This work was supported by an American Heart Association Scientist Development grant (043031), a Swiss National Science Foundation grant (310030-127426), and the EU FP7 grant ANGIOSCAFF (CP-IP 214402) to A.B.; by a Deutsche-Forschungsgemeinschaft grant (DE 740/1-1) to G.V.D.; by U.S. National Institutes of Health (NIH) grants HL-24136, HL-59157 and CA-082923 to D.M.M.; and by NIH grants AG-009521, HL-065572, AG-020961, and AG-024987 and support from the Baxter Foundation to H.M.B. The authors declare no conflicts of interest.
Footnotes
- αSMA
- α-smooth muscle actin
- H&E
- hematoxylin and eosin
- IRES
- internal ribosomal entry site
- LacZ
- β-galactosidase
- NG2
- nerve/glial antigen 2
- P cell
- PDGF-BB-expressing cell
- PDGF-BB
- platelet-derived growth factor-BB
- SCID
- severe combined immunodeficiency
- sPDGFR-β
- soluble platelet-derived growth factor receptor-β
- V cell
- VEGF-expressing cell
- VEGF
- vascular endothelial growth factor
- VIP cell
- VEGF–IRES–PDGF-BB–expressing cell
- VLD
- vessel length density
REFERENCES
- 1. Norgren L., Hiatt W. R., Dormandy J. A., Nehler M. R., Harris K. A., Fowkes F. G. R. (2007) Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 45(Suppl. S), S5–S67 [DOI] [PubMed] [Google Scholar]
- 2. Banfi A., von Degenfeld G., Blau H. M. (2005) Critical role of microenvironmental factors in angiogenesis. Curr. Atheroscler. Rep. 7, 227–234 [DOI] [PubMed] [Google Scholar]
- 3. Ozawa C. R., Banfi A., Glazer N. L., Thurston G., Springer M. L., Kraft P. E., McDonald D. M., Blau H. M. (2004) Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Invest. 113, 516–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park J. E., Keller G. A., Ferrara N. (1993) The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 4, 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Von Degenfeld G., Banfi A., Springer M. L., Wagner R. A., Jacobi J., Ozawa C. R., Merchant M. J., Cooke J. P., Blau H. M. (2006) Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J. 20, 2657–2659 [DOI] [PubMed] [Google Scholar]
- 6. Thurston G., Rudge J. S., Ioffe E., Zhou H., Ross L., Croll S. D., Glazer N., Holash J., McDonald D. M., Yancopoulos G. D. (2000) Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 6, 460–463 [DOI] [PubMed] [Google Scholar]
- 7. Springer M. L., Chen A. S., Kraft P. E., Bednarski M., Blau H. M. (1998) VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol. Cell 2, 549–558 [DOI] [PubMed] [Google Scholar]
- 8. Rissanen T. T., Korpisalo P., Markkanen J. E., Liimatainen T., R. O. M., Kholová I., de Goede A., Heikura T., Gröhn O. H., Ylä-Herttuala S. (2005) Blood flow remodels growing vasculature during vascular endothelial growth factor gene therapy and determines between capillary arterialization and sprouting angiogenesis. Circulation 112, 3937–3946 [DOI] [PubMed] [Google Scholar]
- 9. Pries A. R., Höpfner M., le Noble F., Dewhirst M. W., Secomb T. W. (2010) The shunt problem: control of functional shunting in normal and tumour vasculature. Nat. Rev. Cancer 10, 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta R., Tongers J., Losordo D. W. (2009) Human studies of angiogenic gene therapy. Circ. Res. 105, 724–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karvinen H., Ylä-Herttuala S. (2010) New aspects in vascular gene therapy. Curr. Opin. Pharmacol. 10, 208–211 [DOI] [PubMed] [Google Scholar]
- 12. Yla-Herttuala S., Markkanen J. E., Rissanen T. T. (2004) Gene therapy for ischemic cardiovascular diseases: some lessons learned from the first clinical trials. Trends Cardiovasc. Med. 14, 295–300 [DOI] [PubMed] [Google Scholar]
- 13. Gaengel K., Genové G., Armulik A., Betsholtz C. (2009) Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 630–638 [DOI] [PubMed] [Google Scholar]
- 14. Blau H. M., Banfi A. (2001) The well-tempered vessel. Nat. Med. 7, 532–534 [DOI] [PubMed] [Google Scholar]
- 15. Korpisalo P., Ylä-Herttuala S. (2010) Stimulation of functional vessel growth by gene therapy. Integr. Biol. (Camb.) 2, 102–112 [DOI] [PubMed] [Google Scholar]
- 16. Schwarz E. R., Speakman M. T., Patterson M., Hale S. S., Isner J. M., Kedes L. H., Kloner R. A. (2000) Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat–angiogenesis and angioma formation. J. Am. Coll. Cardiol. 35, 1323–1330 [DOI] [PubMed] [Google Scholar]
- 17. Yla-Herttuala S., Rissanen T. T., Vajanto I., Hartikainen J. (2007) Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J. Am. Coll. Cardiol. 49, 1015–1026 [DOI] [PubMed] [Google Scholar]
- 18. Cao R., Brakenhielm E., Pawliuk R., Wariaro D., Post M. J., Wahlberg E., Leboulch P., Cao Y. (2003) Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 9, 604–613 [DOI] [PubMed] [Google Scholar]
- 19. Greenberg J. I., Shields D. J., Barillas S. G., Acevedo L. M., Murphy E., Huang J., Scheppke L., Stockmann C., Johnson R. S., Angle N., Cheresh D. A. (2008) A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456, 809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rando T. A., Blau H. M. (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gueret V., Negrete-Virgen J. A., Lyddiatt A., Al-Rubeai M. (2002) Rapid titration of adenoviral infectivity by flow cytometry in batch culture of infected HEK293 cells. Cytotechnology 38, 87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghattas I. R., Sanes J. R., Majors J. E. (1991) The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol. Cell. Biol. 11, 5848–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Misteli H., Wolff T., Fuglistaler P., Gianni-Barrera R., Gurke L., Heberer M., Banfi A. (2010) High-throughput flow cytometry purification of transduced progenitors expressing defined levels of vascular endothelial growth factor induces controlled angiogenesis in vivo. Stem Cells 28, 611–619 [DOI] [PubMed] [Google Scholar]
- 24. Drake C. J., Little C. D. (1999) VEGF and vascular fusion: implications for normal and pathological vessels. J. Histochem. Cytochem. 47, 1351–1356 [DOI] [PubMed] [Google Scholar]
- 25. Benjamin L. E., Hemo I., Keshet E. (1998) A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF- B and VEGF. Development 125, 1591–1598 [DOI] [PubMed] [Google Scholar]
- 26. Korpisalo P., Karvinen H., Rissanen T. T., Kilpijoki J., Marjomaki V., Baluk P., McDonald D. M., Cao Y., Eriksson U., Alitalo K., Yla-Herttuala S. (2008) Vascular endothelial growth factor-A and platelet-derived growth factor-B combination gene therapy prolongs angiogenic effects via recruitment of interstitial mononuclear cells and paracrine effects rather than improved pericyte coverage of angiogenic vessels. Circ. Res. 103, 1092–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pettersson A., Nagy J. A., Brown L. F., Sundberg C., Morgan E., Jungles S., Carter R., Krieger J. E., Manseau E. J., Harvey V. S., Eckelhoefer I. A., Feng D., Dvorak A. M., Mulligan R. C., Dvorak H. F. (2000) Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab. Invest. 80, 99–115 [DOI] [PubMed] [Google Scholar]
- 28. Duan D. S., Pazin M. J., Fretto L. J., Williams L. T. (1991) A functional soluble extracellular region of the platelet-derived growth factor (PDGF) beta-receptor antagonizes PDGF-stimulated responses. J. Biol. Chem. 266, 413–418 [PubMed] [Google Scholar]
- 29. Bamberg F., Hinkel R., Schwarz F., Sandner T. A., Baloch E., Marcus R., Becker A., Kupatt C., Wintersperger B. J., Johnson T. R., Theisen D., Klotz E., Reiser M. F., Nikolaou K. (2012) Accuracy of dynamic computed tomography adenosine stress myocardial perfusion imaging in estimating myocardial blood flow at various degrees of coronary artery stenosis using a porcine animal model. Invest. Radiol. 47, 71–77 [DOI] [PubMed] [Google Scholar]
- 30. Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abramsson A., Lindblom P., Betsholtz C. (2003) Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J. Clin. Invest. 112, 1142–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lindblom P., Gerhardt H., Liebner S., Abramsson A., Enge M., Hellstrom M., Backstrom G., Fredriksson S., Landegren U., Nystrom H. C., Bergstrom G., Dejana E., Ostman A., Lindahl P., Betsholtz C. (2003) Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 17, 1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]