Abstract
The common γc subunit molecule is shared among all γc cytokines and clearly involved in T-cell function, but its role in HIV infection and immunity is not well understood. Here, we examined expression and function of γc on T cells during SIV infection in Rhesus macaques. Surface γc distribution was differentially expressed on CD4+ and CD8+ T cells, and CD4+ naive/memory cell populations in various lymphoid tissues of normal macaques. However, surface γc expression was rapidly and significantly down-regulated on T cells in acute infection with pathogenic SIV, compared to infection with a less virulent SHIV or controls and did not recover on CD8+ T cells in the chronic stage. Moreover, the peripheral and CD4+T cell loss was inversely correlated with γc+ CD8+ T cells in individual tissues. γc+ T cells were mainly functional as evidenced by higher cytokine secretion and proliferative capacity. Further in vitro experiments found that surface γc expression could be down-regulated following high level of IL-7 treatment by both internalization and shedding. Down-regulation of γc during early HIV/SIV infection may inhibit T-cell function, particularly of CD8+ T cells, and, may be linked with immune failure and loss of viral containment.—Xu, H., Wang, X., Pahar, B., Alvarez, X., Rasmussen, K. K., Lackner, A. A., Veazey, R. S. Rapid down-regulation of γc on T cells in early SIV infection correlates with impairment of T-cell function.
Keywords: cytokine, CD4+ T cell, HIV, intestine, mucosal immunology
Human immunodeficiency virus (HIV)-1 infection is characterized by rapid CD4+ T-cell loss and progressive immunodeficiency, resulting in impaired T-cell homeostasis with subsequent development of opportunistic infections and viral associated malignancies (1). Highly active antiretroviral therapy (HAART) has been very effective in increasing CD4+ T-cell counts, decreasing viral load, and enhancing survival in the majority of infected patients (2). However, it is also evident that current antiretroviral therapies are unable to completely suppress HIV replication or normalize immune regulation, and drug resistance remains a serious problem (3, 4). Given the known effects of common γ chain (γc; CD132) cytokines on the growth, differentiation, and survival of T cells, cytokine therapy in combination with HAART has been proposed as a strategy to improve immune reconstitution of T cells in patients infected with HIV.
The γc-sharing cytokines include IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, which play vital roles in the survival, proliferation, differentiation, and function of T lymphocytes. IL-2 and IL-15 signal through a trimeric receptor comprised of γc, shared IL-2Rβ chains, and an IL-2- or IL-15-specific α chain (5). IL-7 binds a heterodimeric receptor comprised of γc and IL-7Rα (CD127) (5–7). IL-2 (a.k.a. T-cell growth factor) plays a major role in proliferation and survival of activated T cells and expansion of cytolytic natural killer (NK) cells (8–11). IL-7 is produced mainly by nonhematopoietic cells, such as bone marrow and thymic stromal cells (12–14). Under normal circumstances, the level of constitutively produced IL-7 is appropriate to maintain memory CD4+ T cells and naive CD4 or CD8 T cells, and thymic development (15, 16). IL-15 has major functions pertaining to the development, growth, homeostasis and function of various innate and adaptive immune cells, particularly NK, naive and effective memory CD8+ T cells (17, 18). Because these cytokines share the γc chain, mutations in this region result in severe combined immunodeficiency (SCID), characterized by a marked decrease in the number of circulating T and NK cells (19, 20). Thus, the γc chain appears to play a central role in T-cell development and function. Furthermore, in HIV infection, defects in the cytokine network and their receptors have been linked to T-lymphocyte dysfunction. Indeed, it appears that the signaling network of γc cytokines is both disrupted and exploited by HIV at various stages of infection (21–23).
The simian immunodeficiency virus (SIV) macaque model is closely analogous to HIV infection in humans, duplicating key elements of HIV pathogenesis. In this study, we investigated the potential function and interaction between γc expression on T cells and γc cytokines during SIV infection in Rhesus macaques. The results show that in acute SIV infection, surface γc expression on circulating and mucosal T cells is significantly down-regulated and accompanied by an increase in all γc cytokines in plasma. In chronic infection, expression of γc on CD4+ T cells rebounds but remains depressed on CD8+ T cells. The data suggest that surface γc on T cells could be down-regulated during SIV infection, which might be, to some extent, associated with high levels of IL-7, leading to unresponsiveness to these cytokines. Furthermore, since functional, virus-specific T cells were among the γc+ T-cell subpopulation, down-regulation of the common γc receptor subunit on T cells may contribute to T-cell dysfunction, impairment of immune reconstitution, and the pathogenesis of the AIDS.
MATERIALS AND METHODS
Animals and virus
A total of 72 adult Rhesus macaques (Macaca mulatta), which were negative for SIV, type D retrovirus, and STLV-1 infection, were utilized in this study. All animals were housed at the Tulane National Primate Research Center in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. Of the 72 animals, 9 were uninfected controls, 46 were infected with SIVmac251, and 17 were infected with SHIV162P3. Blood was examined in acute (d 7–28) or chronic (3 mo and later) infection. To examine cells from tissues such as intestine, spleen, etc., macaques infected with SIVmac251 were euthanized for tissue collection in acute (n=7) or chronic infection (SIV infection >3 mo; n=8). The SIVmacv251-infected animals included a subset of 4 “controllers” that, after a high peak viremia, controlled viral replication to undetectable levels (<125 copies/ml) in plasma. All tissue samples were collected at necropsy from all animals except for the controllers, in which only blood was collected, as they remained clinically healthy and were considered important for further study.
Phenotyping blood and tissue mononuclear cells
Mononuclear cells from peripheral blood, lymph node, and intestinal tissues were isolated and processed, as described previously (24). Briefly, tissues were collected from the jejunum and mesenteric lymph nodes within minutes of euthanasia and processed immediately to prepare cell suspensions. Cells were stained with CD3 (clone SP34), Ki67 (clone B56), CD132/γc (clone AG184), CD8 (clone 3B5; Caltag, Carlsbad, CA, USA), CD4 (clone L200), CD45RA (clone 5H9), CCR7 (clone TG8/CCR7; BioLegend; San Diego, CA, USA), anti-phospho-STAT5 (Y694) Pacific Blue (clone 47), BrdU FITC (clone 3D4), TNF FITC (clone MAB11), and IFN-γ PE-Cy7 (clone B27). All antibodies and reagents were purchased from BD Biosciences Pharmingen (San Diego, CA, USA) unless otherwise noted. Stained samples were resuspended in BD Stabilizing Fixative (BD Biosciences) and stored in the dark at 4°C overnight and acquired on a BD FACSAria flow cytometer (BD Biosciences) the next day. Data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA).
Confocal microscopy
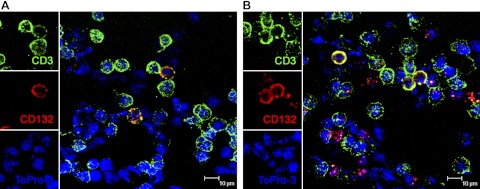
Multilabel confocal microscopy was performed on peripheral blood mononuclear cells (PBMCs) using antibodies to CD3 (rabbit anti-human CD3 polyclonal Ab; Dako Corp., Carpenteria, CA, USA) and γc (mouse anti-human CD132, IgG1, clone AG184) to visualize the distribution of γc in T cells. We examined both surface γc expression and total γc expression by modifying the staining protocols. For total γc expression, including the intracellular form, viable lymphocytes (5–10×103 cells/slide) were spun onto glass slides, dried overnight, and then labeled for CD3 and γc. For surface-only γc detection, lymphocytes were first stained with anti-γc Ab before being spun onto slides, and then fixed and labeled for CD3 expression with anti-CD3 unconjugated primary antibody. The secondary antibodies for primary anti-CD3 and anti-CD132 antibody were conjugated to Alexa Fluor 488 (green) or Alexa Fluor 568 (red) (Molecular Probes, Eugene, OR, USA), respectively. Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with 3 lasers (Leica Microsystems, Exton, PA, USA). NIH Image 1.62 (U.S. National Institutes of Health, Bethesda, MD, USA) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA, USA) were used to assign colors to the channels collected: Alexa Fluor 568 fluoresces when exposed to a 568-nm wavelength laser, and appears red; Alexa Fluor 488 appears green. For individual cells, nuclear staining with ToPro-3 (Molecular Probes; 1 μg/ml for 5 min, washed in PBS) appears blue.
T-cell proliferation assays
To investigate the effects of major γc cytokines on γc+ T cells, fresh PBMCs were added to a 12-well plate at a concentration of 2 × 106/ml complete medium and pulsed with BrdU (BD Pharmingen, San Diego, CA, USA) at a concentration of 10 μM. IL-2 (20 U/ml), IL-7 (20 ng/ml) and IL-15 (20 ng/ml) together was added to experimental groups, and cells with no cytokine stimulation served as negative controls. After 24 h incubation, cells were harvested and surface-labeled with anti-CD3, anti-CD4, and anti-CD8 antibodies as the clones indicated above, then labeled with anti-BrdU FITC (BD Pharmingen), according to the manufacturer's instructions.
PBMC stimulation and intracellular cytokine staining
To further evaluate the γc expression on SIV-specific T cells, purified PBMCs from elite controllers (chronically SIV-infected but undetectable viral loads, n=4) were isolated from heparinized blood and incubated at 37°C in a 5% CO2 environment for 6 h in the presence of RPMI 1640-10% FCS alone (unstimulated), a pool of 15-mer Gag peptides (5 μg/ml each peptide), or staphylococcal enterotoxin B (5 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) as a positive control. All cultures contained brefeldin A (Sigma-Aldrich), as well as 1 μg/ml of anti-CD49d and anti-CD28 costimulatory molecules (BD Biosciences). Cultured cells were stained with monoclonal antibodies specific for surface molecules (CD3, CD4, CD8, and CD132). After being fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences), cells were stained with antibodies specific for IFN-γ and TNF-α and washed by Perm/wash buffer (BD Biosciences). Labeled cells were finally fixed in 1.5% paraformaldehyde, and data were acquired with a FACSAria cytometer (Becton Dickinson, San Jose, CA, USA) and analyzed using FlowJo software (Tree Star). The background level of cytokine staining varied within different samples and different cytokine patterns but was typically <0.01% of the total CD4+ T cells (median, 0%) and <0.05% of total CD8+ T cells (median, 0.01%). Only samples in which the percentage of cytokine-staining cells was at least twice that of background were considered positive.
Detection of cytokines and chemokines in plasma by multiplex microbead immunoassay
Plasma cytokines were analyzed at multiple longitudinal time points from 9 SIVmac251- and 6 SHIV162P3-infected animals. A multiplex suspension immunoassay, containing fluorescent dyed microspheres conjugated with a monoclonal antibody specific for the target protein (Bio-Plex Human Cytokine Assay; Bio-Rad, Hercules, CA, USA), was used for cytokine measurements according to the manufacturer's instructions. Briefly, 50 μl diluted plasma (1:1) was incubated with antibody-coupled beads. Complexes were washed and incubated with biotinylated detection antibody, and then streptavidin-PE. A range of 1.95–32,000 pg/ml of recombinant human cytokines was used to establish standard curves and maximize the sensitivity of the assay. Cytokine levels were determined using a Bio-Plex Workstation (Bio-Rad). Analyte concentrations were calculated using Bio-Plex Manager software (Bio-Rad).
SIVmac251 infection of macaque PBMCs in vitro and γc expression on T cells
PBMCs from SIV-uninfected Rhesus macaques (n=8) were isolated using Ficoll and resuspended in RPMI supplemented with 15% FCS and antibiotics containing recombinant human interleukin-2 (5% v/v hIL-2; Hemagen, Columbia, MD, USA) and 2 μg PHA/ml, stimulated for 48 h at a density of 2 × 106/ml. PBMCs were then cultured for an additional 3 d at 37°C with recombinant human IL-2. PBMCs (2×106) were incubated with medium (negative control) or cell-free supernatant of CEMX174-derived stock of SIVmac251 at a multiplicity of 100TCID50 for 2 × 105 cells for 30 min/1 h at 37°C; unattached virus was removed by 3 washes with PBS. The PBMCs were finally plated in duplicate at 1 × 106 cells/ml in a 24-well tissue culture plate. At preinfection and 1, 3, 5, and 7 d after infection, γc expression levels on CD4+ or CD8+ T cells were monitored by flow cytometry, as described above.
Regulation of γc expression by IL-2, IL-7, and IL-15 treatment in vitro
Purified PBMCs from normal and uninfected animals were isolated from heparinized blood, and 1 × 106/ml PBMCs in R10 medium were treated with human IL-2 (final concentration 20 U/ml; BD Biosciences), human IL-7 (20 ng/ml; R&D Systems, Minneapolis, MN, USA), human IL-15 (20 ng/ml; R&D Systems) alone or combined at 37°C in a 5% CO2 incubator for 30 min. In other experiments, the PBMCs were stimulated by IL-7 (20 ng/ml), and the cells were detected by γc expression on T cells at d 1, 3, 5, and 7. In dose-titration experiments, PBMCs were treated with IL-7 or IL-15 alone for 24 h at 0, 1, 5, 10, and 20 ng/ml; then they were surface stained with anti-CD3, CD4, CD8, and γc. For further investigation of γc regulation, whole blood was stained with two separate groups: anti-CD3, anti-CD4, anti-CD8, and anti-γc; or anti-CD3, anti-CD4, anti-CD8, and isotope controls. After 30 min of incubation, PBMCs were isolated by density gradient centrifugation using Lymphocyte Separation Medium (MP Biomedicals, Solon, OH, USA). PBMCs from both groups were further stimulated by IL-7 or IL-15 for 30 min. For the γc control group, PBMCs were stained with anti-γc antibody alone after cytokine treatment. All samples were resuspended in BD Stabilizing Fixative and stored in the dark at 4°C overnight and acquired the following day.
Statistics
Graphical presentation and statistical analysis of the data were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA). Comparisons between groups were analyzed by a 1-way ANOVA and Mann-Whitney U-test. Values of P < 0.05 were considered statistically significant.
RESULTS
γc-expressing T-cell distribution in normal lymphoid and mucosal tissues
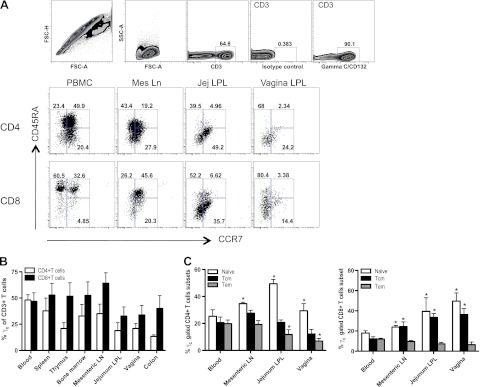
To investigate γc expression by CD4+ and CD8+ T cells in various lymphoid tissues, lymphocyte suspensions from multiple tissues of normal adult Rhesus macaques were analyzed. Both CD4+ and CD8+ T cells expressed γc in all tissues examined, including blood, spleen, thymus, bone marrow, and mucosal tissues (Fig. 1B). However, γc was differentially expressed on naive and memory CD4+, CD8+ T cells in different tissues, as defined by CD45RA and CCR7 expression (refs. 25, 26 and Fig. 1A). For example, CD4+CD45RA+CCR7+ T cells (naive) in mesenteric lymph node, jejunum lamina propria, and vaginal tissues expressed more γc than peripheral blood, whereas in mucosal lymphoid tissues, such as jejunum and vagina, CD4+CD45RA+/CD45RA−CCR7− (effector memory) cells expressed less γc, compared with peripheral blood. Similarly, CD8+ CD45RA+CCR7+ (naive) or CD45RA−CCR7+ (central memory) T cells in other lymphoid tissues expressed more γc than peripheral blood, but γc expression on effector CD8+ T cells from various tissues showed no significant differences. (Fig. 1C).
Figure 1.
Surface expression of γc on T cells in various tissues of normal macaques. A) Representative dot plots showing the gating strategy for identifying γc+ CD3+ T lymphocytes and distribution of γc+ CD4+/CD8+ T-cell subsets, defined here as naïve, CD45RA+CCR7+; effector memory, CD45RA+/CD45RA−CCR7−; and central memory, CD45RA−CCR7+, in different tissues. Mes Ln, mesenteric lymph node; Jej LPL, jejunum lamina propria lymphocyte. B) Frequency of γc+ CD4+ (open bars) or γc+CD8+ (solid bars) as a percentage of gated CD3+ T cells in peripheral blood and various lymphoid tissues from normal Rhesus macaques. C) Group data showing the mean frequency of naive (CD45RA+CCR7+), central memory (Tcm; CD45RA-CCR7+), and effector memory (Tem; CD45RA+/CD45RA−CCR7−) gated γc+ CD4/CD8+ T cells in blood, mesenteric lymph node (LN), jejunum lamina propria lymphocyte (LPL), and vagina (n=7 for each tissue). Error bars represent means ± se. *P < 0.05 vs. peripheral blood.
γc is significantly down-regulated on T cells in acute SIV infection
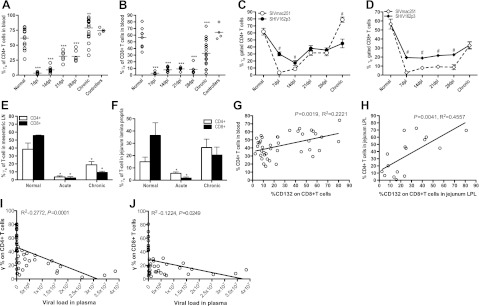
Because of its central role in γc cytokine regulation, γc expression on both CD4+ and CD8+ T cells was examined after SIV infection. It was previously reported that expression of extracellular γc increased on T-cell subsets in patients with chronic HIV-1 infection (27, 28), but this has not been examined in primary infection. Surprisingly, we found markedly decreased percentages of both γc+ CD4+ and γc+ CD8+ T cells in peripheral blood during acute infection (d 7–28), with a nadir at d 7 (Fig. 2A, B). In chronic infection, levels of γc on CD4+ T cells significantly increased, consistent with previous reports. However, γc levels on CD8+ T cells did not completely recover to baseline levels (Fig. 2B). Interestingly, no significant differences between uninfected animals and controllers were detected in γc expression on either circulating CD4+ or CD8+ T cells (Fig. 2A, B). Thus, full recovery of surface γc expression only increased to or above baseline levels on CD4+ T cells (Fig. 2A), and remained decreased on CD8+ T cells in pathogenic chronic SIV infection (Fig. 2B).
Figure 2.
Marked down-regulation of surface γc expression occurs on circulating and mucosal T cells in acute SIV infection of macaques. A, B) Note γc expression on blood CD4+ T cells (A) or CD8+ T cells (B) during SIV infection. Dpi, days postinfection. C, D) Comparison of γc expression on circulating CD4+ (C) or CD8+ (D) T cells between macaques infected with pathogenic SIVmac251 (n=10) or the less pathogenic SHIV162p3 (n=11). E, F) Note γc expression on CD4+ (open bars) or CD8+ (solid bars) T cells in mesenteric lymph node (E) and lamina propria lymphocytes from the jejunum (F) in naive (n=5), acute (n=7), and chronically SIVmac251-infected animals (n=8). G, H) Correlation between CD4+ T-cell loss and γc expression on CD8+ T cells in the peripheral blood (G) or jejunum lamina propia lymphocytes (LPL; H) in naive and SIV-infected macaques. I, J) Correlation of γc expression on CD4+ (I) or CD8+ T cells (J) with viral loads in plasma during SIV infection (n=41). Error bars represent means ± se. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normal group. #P < 0.05 vs. SIVmac251 group.
We next compared γc expression on T cells in blood from macaques infected with SIVmac251 to those infected with the less pathogenic SHIV162p3. Despite similar viral loads in primary infection, macaques infected with SHIV162p3 usually clear virus from plasma (<125 copies/ml) within 60–90 d of infection, and some even acquire protection from subsequent challenge with pathogenic SIVmac251 (29). Although the frequency of γc+ CD4+ and CD8+ T cells in SHIV162p3-infected animals was also markedly decreased in early infection, the frequency of both γc+ CD8+ and CD4+ T cells remained significantly higher than in macaques infected with pathogenic SIVmac251 (Fig. 2C, D). However, in chronic SIVmac251 infection, expression of γc on CD4+ T cells was higher than in controls or macaques infected with SHIV (Fig. 2C). These results suggest that down-regulation of γc on T cells in acute infection could contribute to the impairment of T-cell function, particularly CD8+ cytotoxic T lymphocytes (CTLs).
Similar to blood, percentages of both γc+ CD4+ and γc+ CD8+ T cells from gut-associated lymphoid tissues also markedly decreased during acute [14–21 days postinfection (dpi)] SIV infection (Fig. 2E, F). In contrast, levels of γc on CD4+ T cells did not recover in mesenteric lymph node (Fig. 2E) but did increase in the jejunum (Fig. 2F) in chronic infection. However, levels of γc on CD8+ cells did not completely recover to normal levels in either mesenteric lymph node or jejunum in chronic infection.
In summary, we found significant γc down-regulation on CD8+ T cells in acute infection, which may play an important role in controlling HIV and SIV disease progression. Thus, we examined the correlation of CD4+ T-cell loss with γc+ CD8+ T cells in peripheral blood and jejunum lamina propria in naive cells and at various stages after SIV infection. We found that CD4+ T-cell loss inversely correlated with the frequency of γc+ CD8+ T cells in blood (P=0.0019, R2=0.2221) and in the jejunum (P=0.0041, R2=0.4557) after SIV infection (Fig. 2G, H). In addition, we found a strong indirect correlation between γc expression on either CD4+ or CD8+ T cells with plasma viral loads during SIV infection (CD4+ vs. viral load, P=0.0001, R2=0.2722; CD8+ vs. viral load, P=0.0249, R2=0.1224; Fig. 2I, F). Combined, these data suggest that γc down-regulation on CD8+ T cells might result in CTL impairment and CD4+ T-cell loss in acute SIV infection, which likely contributes to disease progression.
Proliferation of γc+ T cells during SIV infection
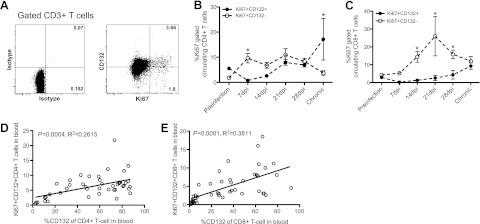
We next examined the dynamics of γc+ CD4 and CD8+ T-cell expansion after SIV infection. The percentage of proliferating (Ki-67+) γc+ CD4+ and γc+ CD8+ T-cell subsets were lower than others at d 7 after SIV infection but gradually increased (Fig. 3B, C). This increased proliferation was consistent with the gradual recovery of γc expression (Fig. 2A, B) and positively correlated with levels of γc on both CD4+ and CD8+ T cells (Fig. 3D, E). Although γc was markedly down-regulated in early infection, proliferation of γc− CD4+ and CD8+ T cells increased during acute SIV infection compared to γc+ T cells (Fig. 3B, C), especially CD8+ T cells. These differences may be associated with γc-independent effects [independent Janus kinase (JAK)-3 signal] and/or viral protein stimulation (30, 31).
Figure 3.
Proliferation (Ki-67+) of γc+ T cells during SIV infection. A) Representative dot plots showing Ki-67 levels on γc+/γc− CD3+ T cells. B, C) Proliferation of γc+ CD4+ or CD8+ T cells compared with γc populations during SIVmac251 infection in macaques (n=21). C, D) Correlation of proliferating (Ki-67+) γc+ T cells with γc levels on CD4+ (D) and CD8+ (E) T cells in the naive and SIV-infected macaques. Error bars represent means ± se. *P < 0.01 vs. γc+ population.
γc+ T cells are functional cytokine-secreting T cells
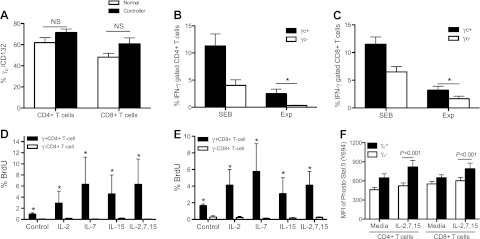
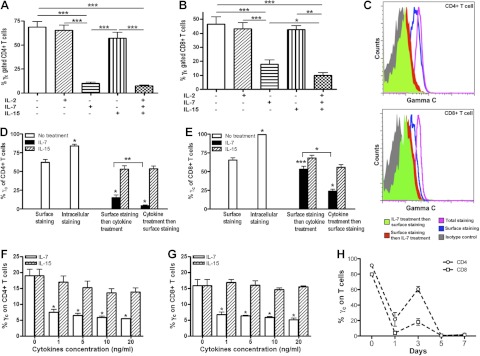
To examine the functional capabilities of γc T cells, PBMCs from 4 SIV-infected controllers were isolated, and SIV-specific cytokine responses were examined. There were no significant differences in γc expression on CD4+ or CD8+ T cells between naive and controller animals (Fig. 4A). Following SIV peptide stimulation, the majority of IFN-γ-producing CD4+ and CD8+ T cells in controllers coexpressed γc (Fig. 4B, C). Of note, the frequency of antigen-specific cytokine-secreting CD4+ T cells among the γc+ population were significantly higher than in the corresponding γc populations (γc+ IFNγ+ CD4+ T cells 2.512±0.8193% vs. γc− IFNγ+ CD4+ 0.318±0.0637%, P=0.0079) following gag peptide pool stimulation. Similarly, γc+ CD8+ T cells also contained a higher percentage of IFN-γ-secreting cells (7.087 vs. 3.647%, P=0.0313) than γc− CD8+ T-cell subsets (Fig. 4B, C). Further, following stimulation with 3 cytokines [IL-2 (20 U/ml), IL-7 (20 ng/ml), and IL-15 (20 ng/ml)] or combination to naive animal-derived PBMCs, the percentage of proliferating γc+ CD4+ and γc+ CD8+ T cells (those that incorporated BrdU and thus were in S-phase division) was significantly increased compared to γc− CD4+ (P<0.001) and CD8+ T cells (P<0.001) after γc cytokine stimulation (Fig. 4D, E). Because the surface γc on T cells was detected after stimulation with cytokines, these data could effectively suggest that γc+ T cells represent a distinctively active population. Likewise, these differences were also observed for γc+ T cells without cytokine pretreatment (Fig. 4D, E). Since γ-chain cytokines primarily activate STAT5 (5), we next assessed the level of STAT5 phosphorylation in T cells. PBMCs from naive animals were stimulated for 30 min with IL-2 (20 U/ml), IL-7 (20 ng/ml), and IL-15 (20 ng/ml) and then stained to detect intracellular STAT5 phosphorylation (pSTAT5) by flow cytometry. The γc+ T cells expressed significantly higher levels of pSTAT5 compared to γc− T cells (Fig. 4F) in response to γc cytokines, which specifically trigger this signaling pathway. In summary, these data show that most proliferating T cells are γc+, suggesting that γc is essential for T-cell proliferation on γc cytokine stimulation in vitro. Down-regulation of γc may thus result in impairment of SIV-specific T-cell responses, especially during acute SIV infection.
Figure 4.
Surface γc expression on functional (cytokine-secreting) SIV-specific CD4+ or CD8+ T cell subsets. A) No significant differences in γc expression on CD4+ or CD8+ T cells were detected between naive (solid bars; n=4) and spontaneous controllers (open bars; n=4) animals. B, C) Note that most SIV-specific CD4+ (B) and CD8+ (C) T cells derived from spontaneous controllers that are producing cytokines in response to SIV peptides coexpress γc+ as compared to the γc− cells. Experimental samples (Exp) represent SIV peptide stimulation. PBMCs were derived from naive (uninfected) or chronically SIV-infected animals that eventually controlled infection to undetectable levels (n=4). D, E) γc+ CD4+ or γc+ CD8+ T cells from naive animals have higher proliferative capacity after treatment with γc receptor-sharing cytokines, including IL-2 (20 U/ml), IL-7 (20 ng/ml), and IL-15 (20 ng/ml) or combination for 24 h in vitro. F) Phosphorylated STAT5 levels in the γc+/γc− T cells from naive animals after combined treatment with IL-2, IL-7, and IL-15 cytokines for 30 min. Data represent means ± sd of ≥3 independent experiments.
γc exists in both intracellular and surface compartments of T cells
Because we observed rapid and profound down-regulation of surface γc expression on both CD4+ and CD8+ T cells in acute SIV infection, we hypothesized that regulation of surface γc expression might be fine-tuned at the protein level. FACS analysis showed significant intracellular γc expression within T cells, including CD4+ (84.43±2.271%) and CD8+ T cells (99.38±0.1499%). Levels of surface γc expression on the same T-cell subsets were ∼52% for CD4+, and 65% for CD8+ T cells in normal macaques. Confocal microscopy also confirmed that γc (CD132) was expressed on CD3+ T cells both in the cytoplasm and on the surface (Fig. 5A, B). A few CD3− lymphocytes also expressed γc receptor subunit, which could be NK or B cells, as these are also reported to coexpress γc (5). Combined, these data suggest that γc could be stored in an intracellular reservoir and rapidly mobilized for surface expression on T cells under specific circumstances.
Figure 5.
Surface and intracellular γc coexist in T cells from normal animals. Confocal microscopy performed on live cells (A) and permeabilized cells (B) confirmed both surface γc (A) or intracellular and surface γc expression (red; B) on CD3+ T cells (green). Images at left of each confocal image are the individual channels shown in the merged image at right. Green, CD3; red, CD132 (γc); blue, ToPro-3 (nuclear stain).
γc cytokines in plasma are elevated in acute SIV infection
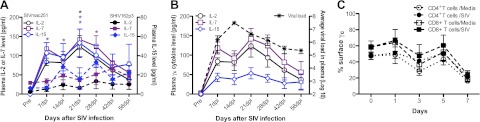
As shown in Fig. 2, surface γc expression on T cells was significantly down-regulated during acute SIV infection. Thus, we next examined levels of γc cytokines in plasma from SHIV162p3- and SIVmac251-infected animals. Levels of IL-2, IL-7, and IL-15 were all significantly increased in plasma at d 7, 14, and 21 after SIVmac251 infection compared with preinfection levels (P<0.05). Plasma IL-7 levels in SIVmac251 infection were also higher than SHIV162p3-infected animals from d 7–28, and for both IL-2 and IL-15 at 21 dpi (P<0.05; Fig. 6). The results indicate that pathogenic SIV infection results in markedly elevated levels of γc cytokines in plasma, especially IL-7.
Figure 6.
γc cytokine levels in plasma in SIV-infected macaques and effects of SIV infection on γc expression on T cells in vitro. A) Dynamics of γc cytokine (IL-2, IL-7, and IL-15) levels in plasma of SHIV162p3- or SIV-infected macaques. These γc cytokines were detected in plasma by cytokine bead array at different time points postinfection in 9 SIVmac251-infected and 6 SHIV162p3-infected macaques. B) Correlation of γc cytokine levels with viral loads in plasma in SIVmac251-infected macaques. C) γc expression on CD4+ or CD8+ T cells after SIVmac251 infection in vitro (n=8). Error bars represent means ± se. *P < 0.05 between SHIV162p3- or SIV-infected macaques.
To determine whether SIV infection altered γc expression on T cells after in vitro infection, PBMCs from naive animals (n=8) were infected with SIVmac251 in vitro, and γc expression on T cells analyzed at different time points after SIV infection. The data showed that γc expression on T cells was slightly reduced at d 3, then increased at d 5, but gradually reduced at 7 d. Notably, levels of γc on either CD4+ or CD8+ T cells did not show significant differences between uninfected (medium) and SIV-infected groups (Fig. 6C). These data further supported the hypothesis that direct effects of SIV infection were not likely responsible and that indirect effects, such as elevated IL-7 levels or other factors, were most likely responsible for the down-regulation of γc on T cells during SIV infection.
Surface γc expression on T cells could be down-regulated by internalization and shedding in response to IL-7 in vitro
To examine the effects of elevated γc cytokine levels on T-cell subsets, we examined changes in surface γc expression on T cells from normal animals following a 30-min stimulation with IL-2 (20 U/ml), IL-7 (20 ng/ml), and IL-15 (20 ng/ml) in vitro. Since signal transduction usually occurs within 15 min of γc cytokine incubation (27), we examined PBMCs before and after 30 min incubation with IL-2, 7 and/or 15 alone, or in combination. The data show that IL-7 alone could markedly down-regulate surface γc expression on T cells (P<0.001), but IL-2 had no significant effect (Fig. 7A). The marked down-regulation of surface γc caused by IL-7 alone could be further enhanced by combined IL-2, IL-7, and IL-15 treatment of CD8+ T cells (P=0.0463) but not CD4+ T cells (P=0.139) (Fig. 7A, B).
Figure 7.
Membrane γc regulation after IL-2, IL-7, and IL-15 treatment in vitro. A, B) Regulation of surface γc expression on CD4+ (A) or CD8+ (B) T cells by IL-2, IL-7, IL-15 alone or combination treatments, as indicated on the x axis. C) Representative FACS histogram of surface γc expression on CD4+ or CD8+ T cells after IL-7 (20 ng/ml) treatment for 30 min in vitro. Black histogram, antibody isotype control; blue histogram, surface γc; purple histogram, total γc; red histogram, surface staining and then IL-7 treatment; green histogram, IL-7 treatment followed by surface staining. D) γc surface and intracellular γc expression on CD4+ T cells after IL-7 (solid bars) or IL-15 alone (striped bars) treatment for 30 min. E) γc surface and intracellular γc expression on CD8+ T cells after IL-7 (solid bars) or IL-15 alone (striped bars) treatment for 30 min. Note that IL-7 promotes both extracellular γc shedding and internalization on T cells. F–H) Surface γc CD4+ (F) or CD8+ (G) expression on T cells was down-regulated in a dose-dependent manner after 24 h or over 7 d (H) treatment with IL-7 in vitro. PBMCs were treated by IL-7 or IL-15 alone at 20 ng/ml. Error bars represent means ± sd. Data are representative of ≥3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether changes in γc expression were the result of internalization or shedding, we performed a series of experiments to compare surface and intracellular expression before and/or after cytokine treatment. First, PBMCs were stained for surface markers and then treated with IL-7 (Fig. 7C) or IL-15, and stained again for both surface and intracellular γc expression (Fig. 7D). To distinguish internalization from shedding, parallel samples were first treated with cytokines and then surface stained. Surface expression of γc after both IL-7 and IL-15 treatment was significantly lower than without cytokine treatment on CD4+ T cells (P<0.05). However, cells that were first surface stained and then treated with IL-7 had significantly higher γc expression than cells treated and then surface stained. This indicated that some surface γc was being internalized from the surface. Further, the difference between control and γc staining before IL-7 treatment indicated that some membrane γc was being shed from T cells (Fig. 7D, E). Combined, these results demonstrate that surface γc expression on T cells is transiently down-regulated by IL-7 by both internalization and shedding. When PBMCs were incubated with IL-7 or IL-15 in varying doses for 24 h, surface γc was also markedly down-regulated by IL-7 treatment in a dose-dependent manner (P<0.05), but IL-15 had no significant effect on either CD4+ or CD8+ T cells (Fig. 7F, G). Finally, disparate results depending on the order of staining and differential responses of CD4+ and CD8+ cells to these cytokines (Fig. 7F, G) indicate this is not an effect of “masking” of the receptor by the cytokines or antibodies used. Besides transient regulation of γc, the effects of IL-7 on surface CD132/γc expression were also monitored on Rhesus macaque-derived T cells after IL-7 treatment over 7 d. Unstimulated T cells (d 0) highly expressed cell-surface γc, IL-7 treatment significantly reduced surface γc level on T cells at d 1, and then its level gradually recovered to some extent at d 3. However, γc on T cells markedly decreased to low levels at d 5 and 7 (Fig. 7H). Combined, these data indicate that overexpressed IL-7, to some extent, or other conditions may have biological effects, such as on overstimulating this pathway.
DISCUSSION
The γc cytokines IL-2, IL-7, and IL-15 are primary regulators of T-cell homeostasis, and thus have been considered prime candidates for immunotherapeutics, either for promoting T-cell proliferation and function and/or for augmenting vaccine-elicited viral-specific T-cell responses. In this study, the γc receptor subunit was found to be differentially expressed on T cells in various lymphoid tissues, rapidly and profoundly down-regulated during acute pathogenic SIV infection, and only fully restored on CD4+, cells in chronically SIV-infected macaques. Further, IL-7, which is markedly elevated in acute SIV infection, could transiently down-regulate extracellular γc expression by both internalization and shedding, possibly in synergy with IL-2 and IL-15 in vitro. Moreover, functional T cells are predominantly represented within this γc+ population. Because the early immune response to HIV infection is likely to be an important factor in determining the clinical course of disease, as later responses are too late to control infection (32), this marked down-regulation of γc in early infection could contribute to impairment of T-cell function in acute SIV infection and may contribute to failure of virus containment and immune reconstitution.
In Rhesus macaques, γc expression on CD8+ T cells is similar to that on CD4+ T cells, differentially expressed on naive and memory T-cell subsets in a tissue-dependent manner (Fig. 1). This differential expression on naive, central, or effector memory T cells might reflect their functions, which are associated with the development, homeostasis, survival, and expansion of T cells. Evidence showed that decreased γc expression on T cells, induced by factors such as viral infection or tumor supernatants, reduced sustained Bcl-2 expression and rendered cells susceptible to apoptotic cell death (7, 33, 34), but regulation of γc expression is still unclear. In this study, we found that SIV infection could result in down-regulation of γc on T cells, especially during acute infection. The down-regulation of γc could be associated with T-cell development and preserved memory and effector T cells in the host immunity.
The γc receptor subunit likely plays a central role in T-cell function. Both IL-2Rβ and IL-7Rα activate and signal through the JAK-1 pathway, whereas their common γ chain signals through JAK-3 (5). Paradoxically, the expression of extracellular γc has been reported to increase (27, 28) or decrease (35) on T-cell subsets in patients infected with HIV-1. However, utilizing the nonhuman primate model in which the earliest events can be documented, we found that extracellular γc on T cells is markedly reduced in acute infection, is only restored on CD4+ T cells months after infection, and never fully recovers on CD8+ T cells (Fig. 2A, C). The latter findings could explain a previous report that chronically stimulated CD8+ T cells were unable to undergo homeostatic proliferation and responded poorly to IL-7 and IL-15 stimulation (36). Although other findings have reported that CD8+ T-cell responses to IL-2 and IL-15 are maintained in patients infected with HIV (35), their role may be limited through JAK-1 signaling due to lack of receptor integrity (γc−). However, other evidence shows that chronically stimulated CD8+ T cells were unable to undergo homeostatic proliferation and responded poorly to IL-7 and IL-15 stimulation (36, 37). Also, the treatment of IL-2, IL-7, and IL-15 from beginning of infection cannot affect the effector or memory CD8+ T-cell population, resulting in no efficacy on treatment of infectious diseases (38, 39). Although possessing cytotoxic ability to some extent during the acute stage, the significant down-regulation of γc on CD8+ T cells might be associated with reduced proliferation and survival of CD8+ T cells in response to viral infection. These findings indicate that expression of γc varies markedly, depending on the stage of HIV infection. Changes in expression of a variety of molecules in addition to the γc cytokines and their receptors may also be involved, including up-regulated PD-1 (40) or down-regulation of IL-2Rα/CD25 and IL-7Rα/CD127 on T cells (data not shown). IL-15 and IL-21 receptors may also be involved, but the lack of cross-reactive antibodies for their specific receptor subunits (IL-15Rα and IL-21R) prevented us from assessing this. Because γc+ T cells represent the dominant responsive population to γc cytokines (Fig. 4D, E), the massive levels of γc down-regulation on T cells during early SIV infection makes it unlikely that T cells responded to these high level of cytokines by signal transduction. Similarly, proliferation of γc+ T cells only gradually increased after SIV infection, accompanied by slight increases in γc expression on T cells in primary SIV infection (Fig. 3B, E). Although γc− T-cell proliferation markedly increased during acute infection, these proliferative responses are likely driven by γc cytokine-independent mechanisms, such as activation by exogenous viral proteins or antigens such as gp41 (30, 31, 41).
Since γc + T cells mainly comprise functional T cells (Fig. 4), down-regulation of extracellular γc in acute SIV/HIV infection may reflect an early impairment of T cells, particularly CTLs, which are dependent on γc cytokines (42). However, the level of γc on T cells from controllers was similar to healthy macaques, as shown no difference between these animals (Fig. 4A). The early immune response to HIV infection is likely to be an important factor in determining the clinical course of disease, as strong responses arising later in infection are too late to control infection (32). Taken together, these findings suggest that early down-regulation of γc on T cells might result in T-cell dysfunction, which could be correlated with virus escape, disease progression, and subsequent failure of immune reconstitution.
Because of their critical role in maintaining immune function, γc cytokines are being considered for AIDS therapeutic candidates in preclinical or clinical trials. Recombinant IL-2 has been tested in a number of clinical trials as a supplement to increase CD4 T-cell counts in patients infected with HIV, and those on treatment experienced fewer AIDS-defining events compared to patients on HAART alone (43, 44). IL-2 also increased numbers of T cells with a classic regulatory T-cell phenotype, yet these cells were not strongly suppressive in direct ex vivo assays (45). IL-15 also appears to be a promising therapeutic adjuvant in patients infected with HIV, given its inductive effects on innate and adaptive immune response (46). The IL-7/IL-7Rα system continues to be intensively studied during HIV infection. Similar to surface γc regulation in our study, it was reported that the IL-7Rα is also down-modulated by IL-7 in vitro (47, 48). In this study, however, IL-7 treatment alone could significantly down-regulate surface γc expression on T cells in vitro (Fig. 7). In vivo elevations of IL-7 in plasma (Fig. 6) were also associated with transient down-regulation of surface γc on T cells during acute SIV infection, confirming the in vivo relevance of this observation. Further, this effect was shown to be dose dependent on IL-7 and occurred through both internalization and shedding of γc (Fig. 7D, E), which is similar to that reported for CD127 (IL-7 Rα) expression on CD8+ T cells in HIV+ patients (28, 49, 50). These findings suggest that down-regulation of γc could, at least in part, be attributed to increased IL-7 levels, which cautions the use of IL-7 for HIV treatment. This notion is supported by data showing that increased serum IL-7 levels are associated with higher viral loads throughout all stages of disease, and inversely correlated with CD4+ T-cell counts (51–54). In addition, transient increases in plasma HIV-RNA levels were observed in most IL-7-treated patients (55). Moreover, IL-7 levels appear to be significantly lower in long-term nonprogressors, and HAART therapy was able to normalize IL-7 levels in patients with primary HIV infection (14). In addition, IL-7 has been found to up-regulate Fas expression and to potentiate Fas-mediated apoptosis in human T-cell subsets (56, 57). A similar phenomenon is observed in pathogenic SIV infection of Rhesus macaques, where the CD4+ T cell pool cannot be restored despite persistently elevated IL-7 levels (58). However, the other factors such as gp120 may also be involved in regulation and inhibition of γc function during HIV/SIV infection (59, 60).
Taken together, the expression of γc molecules is critical for maintaining T-cell function, and their down-regulation during acute SIV infection could contribute to the impairment of T-cell function. These data provide a clue that maintenance of physiological level of γc on T cells could be useful as therapeutic strategy for patients infected with HIV.
Acknowledgments
The authors thank Julie Bruhn, Calvin Lanclos, and Desiree Waguespachek for flow cytometry support and Janell LeBlanc, Maryjane Dodd, Linda Green, and Maury Duplantis for technical support.
This work was supported by U.S. National Institutes of Health grants AI49080, AI084793, U19 AI084793, U19 AI76982, and RR000164, and a Faculty Enhancement grant from Tulane University (New Orleans, LA, USA). The authors declare no conflict of interests.
Footnotes
- γc
- common γ chain (CD132)
- CD132
- common γ chain (γc)
- CTL
- cytotoxic T lymphocyte
- dpi
- days postinfection
- HAART
- highly active antiretroviral therapy
- HIV
- human immunodeficiency virus
- JAK
- Janus kinase
- NK
- natural killer
- PBMC
- peripheral blood mononuclear cell
- SIV
- simian immunodeficiency virus.
REFERENCES
- 1. Hazenberg M. D., Hamann D., Schuitemaker H., Miedema F. (2000) T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1, 285–289 [DOI] [PubMed] [Google Scholar]
- 2. Palella F. J., Jr., Delaney K. M., Moorman A. C., Loveless M. O., Fuhrer J., Satten G. A., Aschman D. J., Holmberg S. D. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 338, 853–860 [DOI] [PubMed] [Google Scholar]
- 3. Grant R. M., Hecht F. M., Warmerdam M., Liu L., Liegler T., Petropoulos C. J., Hellmann N. S., Chesney M., Busch M. P., Kahn J. O. (2002) Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA 288, 181–188 [DOI] [PubMed] [Google Scholar]
- 4. Parkin N. T., Lie Y. S., Hellmann N., Markowitz M., Bonhoeffer S., Ho D. D., Petropoulos C. J. (1999) Phenotypic changes in drug susceptibility associated with failure of human immunodeficiency virus type 1 (HIV-1) triple combination therapy. J. Infect. Dis. 180, 865–870 [DOI] [PubMed] [Google Scholar]
- 5. Rochman Y., Spolski R., Leonard W. J. (2009) New insights into the regulation of T cells by gamma (c) family cytokines. Nat. Rev. Immunol 9, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pahwa S. (2007) Role of common gamma chain utilizing cytokines for immune reconstitution in HIV infection. Immunol. Res. 38, 373–386 [DOI] [PubMed] [Google Scholar]
- 7. Alves N. L., Arosa F. A., van Lier R. A. (2007) Common gamma chain cytokines: dissidence in the details. Immunol. Lett. 108, 113–120 [DOI] [PubMed] [Google Scholar]
- 8. Kim H. P., Imbert J., Leonard W. J. (2006) Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 17, 349–366 [DOI] [PubMed] [Google Scholar]
- 9. D'Souza W. N., Lefrancois L. (2003) IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J. Immunol. 171, 5727–5735 [DOI] [PubMed] [Google Scholar]
- 10. Lenardo M. J. (1991) Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature 353, 858–861 [DOI] [PubMed] [Google Scholar]
- 11. Farag S. S., Caligiuri M. A. (2006) Human natural killer cell development and biology. Blood Rev. 20, 123–137 [DOI] [PubMed] [Google Scholar]
- 12. Bradley L. M., Haynes L., Swain S. L. (2005) IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 26, 172–176 [DOI] [PubMed] [Google Scholar]
- 13. Mazzucchelli R., Durum S. K. (2007) Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 7, 144–154 [DOI] [PubMed] [Google Scholar]
- 14. Sasson S. C., Zaunders J. J., Kelleher A. D. (2006) The IL-7/IL-7 receptor axis: understanding its central role in T-cell homeostasis and the challenges facing its utilization as a novel therapy. Curr. Drug Targets. 7, 1571–1582 [DOI] [PubMed] [Google Scholar]
- 15. Schluns K. S., Kieper W. C., Jameson S. C., Lefrancois L. (2000) Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–432 [DOI] [PubMed] [Google Scholar]
- 16. Goldrath A. W., Sivakumar P. V., Glaccum M., Kennedy M. K., Bevan M. J., Benoist C., Mathis D., Butz E. A. (2002) Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 195, 1515–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carson W. E., Fehniger T. A., Haldar S., Eckhert K., Lindemann M. J., Lai C. F., Croce C. M., Baumann H., Caligiuri M. A. (1997) A potential role for interleukin-15 in the regulation of human natural killer cell survival. J. Clin. Invest. 99, 937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prlic M., Blazar B. R., Farrar M. A., Jameson S. C. (2003) In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 197, 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kovanen P. E., Leonard W. J. (2004) Cytokines and immunodeficiency diseases: critical roles of the gamma (c) -dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev. 202, 67–83 [DOI] [PubMed] [Google Scholar]
- 20. Giliani S., Mori L., de Saint Basile G., Le Deist F., Rodriguez-Perez C., Forino C., Mazzolari E., Dupuis S., Elhasid R., Kessel A., Galambrun C., Gil J., Fischer A., Etzioni A., Notarangelo L. D. (2005) Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol. Rev. 203, 110–126 [DOI] [PubMed] [Google Scholar]
- 21. Sirskyj D., Theze J., Kumar A., Kryworuchko M. (2008) Disruption of the gamma c cytokine network in T cells during HIV infection. Cytokine 43, 1–14 [DOI] [PubMed] [Google Scholar]
- 22. Leone A., Picker L. J., Sodora D. L. (2009) IL-2, IL-7 and IL-15 as immuno-modulators during SIV/HIV vaccination and treatment. Curr. HIV Res. 7, 83–90 [DOI] [PubMed] [Google Scholar]
- 23. Gougeon M. L., Chiodi F. (2010) Impact of gamma-chain cytokines on T cell homeostasis in HIV-1 infection: therapeutic implications. J. Intern. Med. 267, 502–514 [DOI] [PubMed] [Google Scholar]
- 24. Veazey R. S., Mansfield K. G., Tham I. C., Carville A. C., Shvetz D. E., Forand A. E., Lackner A. A. (2000) Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74, 11001–11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Appay V., van Lier R. A., Sallusto F., Roederer M. (2008) Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry. A 73, 975–983 [DOI] [PubMed] [Google Scholar]
- 26. Monteiro M., Evaristo C., Legrand A., Nicoletti A., Rocha B. (2007) Cartography of gene expression in CD8 single cells: novel CCR7- subsets suggest differentiation independent of CD45RA expression. Blood 109, 2863–2870 [DOI] [PubMed] [Google Scholar]
- 27. Juffroy O., Bugault F., Lambotte O., Landires I., Viard J. P., Niel L., Fontanet A., Delfraissy J. F., Theze J., Chakrabarti L. A. (2010) Dual mechanism of impairment of interleukin-7 (IL-7) responses in human immunodeficiency virus infection: decreased IL-7 binding and abnormal activation of the JAK/STAT5 pathway. J. Virol. 84, 96–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sasson S. C., Zaunders J. J., Zanetti G., King E. M., Merlin K. M., Smith D. E., Stanley K. K., Cooper D. A., Kelleher A. D. (2006) Increased plasma interleukin-7 level correlates with decreased CD127 and Increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J. Infect. Dis. 193, 505–514 [DOI] [PubMed] [Google Scholar]
- 29. Xu H., Wang X., Morici L. A., Pahar B., Veazey R. S. (2011) Early divergent host responses in SHIVsf162P3 and SIVmac251 infected macaques correlate with control of viremia. PLoS One 6, e17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi M., Lin T. H., Appell K. C., Berg L. J. (2009) Cell cycle progression following naive T cell activation is independent of Jak3/common gamma-chain cytokine signals. J. Immunol. 183, 4493–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen T., Cohen S. J., Antonovsky N., Cohen I. R., Shai Y. (2010) HIV-1 gp41 and TCRalpha trans-membrane domains share a motif exploited by the HIV virus to modulate T-cell proliferation. PLoS Pathog. 6, e1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McMichael A. J., Borrow P., Tomaras G. D., Goonetilleke N., Haynes B. F. (2010) The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 10, 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li X. C., Demirci G., Ferrari-Lacraz S., Groves C., Coyle A., Malek T. R., Strom T. B. (2001) IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat. Med. 7, 114–118 [DOI] [PubMed] [Google Scholar]
- 34. Chattopadhyay S., Bhattacharyya S., Saha B., Chakraborty J., Mohanty S., Sakib Hossain D. M., Banerjee S., Das K., Sa G., Das T. (2009) Tumor-shed PGE(2) impairs IL2Rgammac-signaling to inhibit CD4 T cell survival: regulation by theaflavins. PLoS One 4, e7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pahwa R., McCloskey T. W., Aroniadis O. C., Strbo N., Krishnan S., Pahwa S. (2006) CD8+ T cells in HIV disease exhibit cytokine receptor perturbation and poor T cell receptor activation but are responsive to gamma-chain cytokine-driven proliferation. J. Infect. Dis. 193, 879–887 [DOI] [PubMed] [Google Scholar]
- 36. Gourley T. S., Wherry E. J., Masopust D., Ahmed R. (2004) Generation and maintenance of immunological memory. Semin. Immunol. 16, 323–333 [DOI] [PubMed] [Google Scholar]
- 37. Wherry E. J., Barber D. L., Kaech S. M., Blattman J. N., Ahmed R. (2004) Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 101, 16004–16009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lazarevic V., Yankura D. J., DiVito S. J., Flynn J. L. (2005) Induction of Mycobacterium tuberculosis-specific primary and secondary T-cell responses in interleukin-15-deficient mice. Infect. Immun. 73, 2910–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maeurer M. J., Trinder P., Hommel G., Walter W., Freitag K., Atkins D., Storkel S. (2000) Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect. Immun. 68, 2962–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu H., Wang X., Pahar B., Moroney-Rasmussen T., Alvarez X., Lackner A. A., Veazey R. S. (2010) Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J. Immunol. 185, 7340–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masse G. X., Corcuff E., Decaluwe H., Bommhardt U., Lantz O., Buer J., Di Santo J. P. (2007) gamma(c) cytokines provide multiple homeostatic signals to naive CD4(+) T cells. Eur. J. Immunol. 37, 2606–2616 [DOI] [PubMed] [Google Scholar]
- 42. Carini C., McLane M. F., Mayer K. H., Essex M. (1994) Dysregulation of interleukin-7 receptor may generate loss of cytotoxic T cell response in human immunodeficiency virus type 1 infection. Eur. J. Immunol. 24, 2927–2934 [DOI] [PubMed] [Google Scholar]
- 43. Mitsuyasu R., Gelman R., Cherng D. W., Landay A., Fahey J., Reichman R., Erice A., Bucy R. P., Kilby J. M., Lederman M. M., Hamilton C. D., Lertora J., White B. L., Tebas P., Duliege A. M., Pollard R. B. (2007) The virologic, immunologic, and clinical effects of interleukin 2 with potent antiretroviral therapy in patients with moderately advanced human immunodeficiency virus infection: a randomized controlled clinical trial–AIDS Clinical Trials Group 328. Arch. Intern. Med. 167, 597–605 [DOI] [PubMed] [Google Scholar]
- 44. Youle M., Emery S., Fisher M., Nelson M., Fosdick L., Janossy G., Loveday C., Sullivan A., Herzmann C., Wand H., Davey R. T., Jr., Johnson M. A., Tavel J. A., Lane H. C. (2006) A randomised trial of subcutaneous intermittent interleukin-2 without antiretroviral therapy in HIV-infected patients: the UK-Vanguard Study. PLoS Clin. Trials 1, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sereti I., Imamichi H., Natarajan V., Imamichi T., Ramchandani M. S., Badralmaa Y., Berg S. C., Metcalf J. A., Hahn B. K., Shen J. M., Powers A., Davey R. T., Kovacs J. A., Shevach E. M., Lane H. C. (2005) In vivo expansion of CD4CD45RO-CD25 T cells expressing foxP3 in IL-2-treated HIV-infected patients. J. Clin. Invest. 115, 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alpdogan O., van den Brink M. R. (2005) IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 26, 56–64 [DOI] [PubMed] [Google Scholar]
- 47. Rose T., Lambotte O., Pallier C., Delfraissy J. F., Colle J. H. (2009) Identification and biochemical characterization of human plasma soluble IL-7R: lower concentrations in HIV-1-infected patients. J. Immunol. 182, 7389–7397 [DOI] [PubMed] [Google Scholar]
- 48. Vranjkovic A., Crawley A. M., Gee K., Kumar A., Angel J. B. (2007) IL-7 decreases IL-7 receptor alpha (CD127) expression and induces the shedding of CD127 by human CD8+ T cells. Int. Immunol. 19, 1329–1339 [DOI] [PubMed] [Google Scholar]
- 49. Paiardini M., Cervasi B., Albrecht H., Muthukumar A., Dunham R., Gordon S., Radziewicz H., Piedimonte G., Magnani M., Montroni M., Kaech S. M., Weintrob A., Altman J. D., Sodora D. L., Feinberg M. B., Silvestri G. (2005) Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174, 2900–2909 [DOI] [PubMed] [Google Scholar]
- 50. Park J. H., Yu Q., Erman B., Appelbaum J. S., Montoya-Durango D., Grimes H. L., Singer A. (2004) Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21, 289–302 [DOI] [PubMed] [Google Scholar]
- 51. Napolitano L. A., Grant R. M., Deeks S. G., Schmidt D., De Rosa S. C., Herzenberg L. A., Herndier B. G., Andersson J., McCune J. M. (2001) Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7, 73–79 [DOI] [PubMed] [Google Scholar]
- 52. Llano A., Barretina J., Gutierrez A., Blanco J., Cabrera C., Clotet B., Este J. A. (2001) Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J. Virol. 75, 10319–10325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fry T. J., Moniuszko M., Creekmore S., Donohue S. J., Douek D. C., Giardina S., Hecht T. T., Hill B. J., Komschlies K., Tomaszewski J., Franchini G., Mackall C. L. (2003) IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 101, 2294–2299 [DOI] [PubMed] [Google Scholar]
- 54. Roberts L., Passmore J. A., Williamson C., Little F., Bebell L. M., Mlisana K., Burgers W. A., van Loggerenberg F., Walzl G., Siawaya J. F., Karim Q. A., Karim S. S. (2010) Plasma cytokine levels during acute HIV-1 infection predict HIV disease progression. AIDS 24, 819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sereti I., Dunham R. M., Spritzler J., Aga E., Proschan M. A., Medvik K., Battaglia C. A., Landay A. L., Pahwa S., Fischl M. A., Asmuth D. M., Tenorio A. R., Altman J. D., Fox L., Moir S., Malaspina A., Morre M., Buffet R., Silvestri G., Lederman M. M. (2009) IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113, 6304–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jaleco S., Swainson L., Dardalhon V., Burjanadze M., Kinet S., Taylor N. (2003) Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J. Immunol. 171, 61–68 [DOI] [PubMed] [Google Scholar]
- 57. Fluur C., De Milito A., Fry T. J., Vivar N., Eidsmo L., Atlas A., Federici C., Matarrese P., Logozzi M., Rajnavolgyi E., Mackall C. L., Fais S., Chiodi F., Rethi B. (2007) Potential role for IL-7 in Fas-mediated T cell apoptosis during HIV infection. J. Immunol. 178, 5340–5350 [DOI] [PubMed] [Google Scholar]
- 58. Muthukumar A., Wozniakowski A., Gauduin M. C., Paiardini M., McClure H. M., Johnson R. P., Silvestri G., Sodora D. L. (2004) Elevated interleukin-7 levels not sufficient to maintain T-cell homeostasis during simian immunodeficiency virus-induced disease progression. Blood 103, 973–979 [DOI] [PubMed] [Google Scholar]
- 59. Bani L., David D., Fevrier M., Pialoux G., Dupont B., Sugamura K., Theze J. (1997) Interleukin-2 receptor beta and gamma chain dysregulation during the inhibition of CD4 T cell activation by human immunodeficiency virus-1 gp120. Eur. J. Immunol. 27, 2188–2194 [DOI] [PubMed] [Google Scholar]
- 60. David D., Bani L., Moreau J. L., Treilhou M. P., Nakarai T., Joussemet M., Ritz J., Dupont B., Pialoux G., Theze J. (1998) Regulatory dysfunction of the interleukin-2 receptor during HIV infection and the impact of triple combination therapy. Proc. Natl. Acad. Sci. U. S. A. 95, 11348–11353 [DOI] [PMC free article] [PubMed] [Google Scholar]