Abstract
Cardiac fibroblasts (CFs) play an essential role in remodeling of the cardiac extracellular matrix. Extracellular nucleotide signaling may provoke a profibrotic response in CFs. We tested the hypothesis that physical perturbations release ATP from CFs and that ATP participates in profibrotic signaling. ATP release was abolished by the channel inhibitor carbenoxolone and inhibited by knockdown of either connexin (Cx)43 or Cx45 (47 and 35%, respectively), implying that hypotonic stimulation induces ATP release via Cx43 and Cx45 hemichannels, although pannexin 1 may also play a role. ATP released by hypotonic stimulation rapidly (<10 min) increased phosphorylated ERK by 5-8 fold, an effect largely eliminated by P2Y2 receptor knockdown or ATP hydrolysis with apyrase. ATP stimulation of P2Y2 receptors increased α-smooth muscle actin (α-SMA) production, and in an ERK-dependent manner, ATP increased collagen accumulation by 60% and mRNA expression of profibrotic markers: plasminogen activator inhibitor-1 and monocyte chemotactic protein-1 by 4.5- and 4.0-fold, respectively. Apyrase treatment substantially reduced the basal profibrotic phenotype, decreasing collagen and α-SMA content and increasing matrix metalloproteinase expression. Thus, ATP release activates P2Y2 receptors to mediate profibrotic responses in CFs, implying that nucleotide release under both basal and activated states is likely an important mechanism for fibroblast homeostasis.—Lu, D., Soleymani, S., Madakshire, R., Insel, P. A. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors.
Keywords: cardiac fibrosis, purinergic signaling, extracellular matrix, α-smooth muscle actin, MAPK/ERK activation
Cardiac fibroblasts (CFs) are the predominant nonmyocyte cell type and are responsible for the homeostatic maintenance of extracellular matrix (ECM) in the heart (1). CFs generate, maintain, and organize the ECM, which is essential for structural organization and correct contraction of the myocardium (1, 2). In addition to producing ECM proteins, including collagen types I and III (3), CFs can couple to adjacent fibroblasts and myocytes to mediate electrical conduction via connexin (Cx) gap junctions (4, 5).
Aging and pathological conditions, such as heart failure and myocardial infarction (MI), can alter function of CFs, which transform into profibrogenic myofibroblasts (6–8). Compared with “resting” fibroblasts, myofibroblasts have increased synthesis of ECM proteins and expression of profibrotic and proinflammatory cytokines, including plasminogen activator inhibitor 1 (PAI-1), monocyte chemotactic protein 1 (MCP-1), connective tissue growth factor (CTGF), and transforming growth factor-β (TGF-β) (9–13). Myofibroblasts also have increased expression of α-smooth muscle actin (α-SMA), which aids in wound closure at sites of injury (5). Activity of CFs is important in cardiac remodeling and wound healing and, if excessive, can lead to cardiac fibrosis and diastolic dysfunction.
Swelling and increased stretch of cells occur in the infarcted or pressure-overloaded myocardium and can increase myocyte hypertrophy and activation of CFs (14–17). Physical stretch or damage to cells triggers cellular release of adenosine triphosphate (ATP) from numerous cell types in the heart, including erythrocytes, cardiac myocytes, and endothelial cells (18–21). In addition to their roles in gap-junction coupling, Cx hemichannels have been implicated in the cellular release of ATP (20, 22, 23).
Release of ATP in the heart may be an important response to myocardial damage through its ability to promote the recruitment of phagocytes (24–26) and initiate profibrotic responses (20, 27). Relatively little is known about the mechanisms of ATP release and signaling in CFs. We previously characterized the P2Y subtypes expressed by rat CFs and found that activation of P2Y2 receptors, the most highly expressed subtype, increases their migration, proliferation, and expression of α-SMA and several profibrotic markers (28). In the current study, we sought to determine whether physical perturbation of CFs (e.g., hypotonicity-induced cell swelling) causes the release of endogenous ATP and, if so, possible mechanisms for this release and whether released ATP participates in profibrotic signaling via activation of P2Y2 receptors.
MATERIALS AND METHODS
Isolation and culture of adult rat CFs
Ethical approval for the care and use of animals for this study was granted by the University of California–San Diego Institutional Animal Care and Use Committee and was in compliance with the guiding principles of the American Physiological Society. CFs were isolated from adult (8–10 wk), male Sprague-Dawley rats, as described previously (29). Briefly, Sprague-Dawley rats were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) via intraperitoneal injection. The heart was excised and digested with collagenase II (Worthington Biochemical, Lakewood, NJ, USA) via a modified reverse-Langendorff apparatus. CFs were separated from cardiac myocytes by gravity separation and grown to confluency in 10-cm culture dishes at 37°C, 10% CO2 in DMEM supplemented with 10% FBS, 1% penicillin, and 1% streptomycin. CFs were then split to appropriate-sized culture dishes, allowed to adhere overnight, and then serum starved in DMEM without FBS for 24 h before treatment.
Hypotonic stimulation
Isotonic medium consisted of standard physiological saline (280–290 mosmol) containing 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES, and 100 mM EGTA. In Ca2+-containing experiments, EGTA was replaced with the appropriate concentration of CaCl2. Hypotonic medium (150–160 mosmol) was prepared by reducing NaCl concentration and KCl concentration to 70 and 2.5 mM, respectively.
Isolated CFs were trypsinized and plated onto 6- or 12-well plates (150,000 and 60,000 cells, respectively) and serum starved. DMEM was then gently aspirated and replaced with hypotonic or isotonic medium. Plates were incubated at 37°C, 10% CO2 for 5 or 10 min, depending on the experiment. In experiments containing pharmacologic inhibitors, CFs in 0% DMEM were pretreated with drugs for 30 min. Inhibitors were added to isotonic or hypotonic medium used for CF stimulation.
Extracellular ATP quantification
ATP-containing isotonic or hypotonic medium (100 μl) was carefully removed by placing a pipette as close to the fluid surface as possible to avoid perturbation of the cells. A luciferase-based ATP assay kit (ENLITEN ATP assay; Promega, Madison, WI, USA) and TD-20/20 luminometer (Turner Biosystems, Sunnyvale, CA, USA) were used according to the manufacturers' instructions. Assays were conducted at room temperature. Separate standard curves of known ATP concentrations were generated with isotonic and hypotonic medium and used to quantify the concentration of ATP in the experimental samples.
siRNA transfection
Targeted siRNA sequences for rat Cx43 (GJA1; S127779), Cx45 (GJA7; S141483), pannexin 1 (Panx1; S163990), P2Y2 (S131806), and negative control siRNA were purchased from Ambion (Grand Island, NY, USA). Cells were transfected with 25 nM siRNA using RNAiMAX (Invitrogen, Grand Island, NY, USA) for 24 h according to the manufacturer's instructions. The medium containing transfection reagent was then replaced with fresh serum-free DMEM.
Quantitative real-time PCR (qPCR)
Total RNA was isolated by TRIzol extraction (Invitrogen), and cDNA was generated using the Superscript III cDNA synthesis system (Invitrogen) according the manufacturer's instructions. qPCR analysis was performed on a DNA Engine Opticon 2 (Bio-Rad, Hercules, CA, USA) using the qScript One-Step qRT-PCR kit (Quanta Biosciences, Gaithersburg, MD, USA). Primers for PCR amplification (Table 1) were designed based on the nucleotide sequences of the respective gene target using Primer3Plus software (General Public License; http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi). P2Y receptor primers were obtained from published literature (30). When possible, each forward and reverse primer set was designed between multiple exons. Amplification efficiency of each primer pair was tested before analysis. Relative gene expression levels were determined using the ΔΔCT method with 18S as the reference gene (31).
Table 1.
Oligonucleotides used for qPCR
| Gene | Forward, 5′–3′ | Reverse, 5′–3′ |
|---|---|---|
| Cx40 | CAGAGCCTGAAGAAGCCAAC | ATGCGGAAAATGAACAGGAC |
| Cx43 | GCTCCACTCTCGCCTATGTC | GAGTTCATGTCCAGCAGCAA |
| Cx45 | TTTGGGTAACGGAGGTTCTG | ACCGCAGTTAGGACAATTCG |
| Panx1 | ACCTGAGAGATGGACCTGGA | AGTACCACCGCAAAGGTCAC |
| P2Y2 | TGCTGGGTCTGCTTTTTGCT | ATCGGAAGGAGTAATAGAGGGT |
| P2Y6 | TGCTGCTACCCCCAGTTTAC | TGGCATAGAAGAGGAAGCGT |
| α-SMA | CATCAGGAACCTCGAGAAGC | TCGGATACTTCAGGGTCAGG |
| PAI-1 | GGAGAAGCGAAACAGGAGTG | TCCAGAAGGGGATATGTTGC |
| MCP-1 | CTTCTGGGCCTGTTGTTCA | TTCCTTATTGGGGTCAGCAC |
| MMP2 | ACACTGGGACCTGTCACTCC | ACACGGCATCAATCTTTTCC |
| MMP9 | TTCGACGCTGACAAGAAGTG | AGGGGAGTCCTCGTGGTAGT |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Immunofluorescent microscopy
CFs were plated onto 12-mm glass coverslips, allowed to adhere overnight, and then serum starved for 24 h. CFs were treated with either 10 μM ATP or 0.67 U/ml apyrase (or corresponding vehicle control) for 24 h. Cells were washed in PBS, fixed in 10% formalin for 10 min, washed with 100 mM glycine (pH 7.4), permeabilized with 0.3% Triton-X/PBS, washed in 0.1% Tween 20/PBS, and blocked in blocking buffer (1% BSA/0.05% Tween 20/PBS). Samples were incubated with primary antibodies to α-SMA (Invitrogen) or Cx43 (Abcam, Cambridge, MA, USA) for 18 h at 4°C. Samples were washed and incubated with fluorescent secondary antibody (Alexa Fluor 488; Invitrogen) for 1 h and then washed and mounted on glass slides with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA). Confocal images were acquired on a Nikon Eclipse laser scanning confocal microscope using Nikon EZ-C1 software (Nikon Instruments Inc., Melville, NY, USA).
Immunoblot analysis
Whole-cell lysates were prepared in 150 mM Na2CO3 buffer (pH 11) and homogenized by sonication. Equal amounts of protein (assayed using a dye-binding reagent; Bio-Rad) were separated by SDS/PAGE using 10% polyacrylamide precast gels (Invitrogen) and transferred to a polyvinylidene difluoride membrane with the iBlot system (Invitrogen). Membranes were blocked in PBS Tween (1%) containing 5% nonfat dry milk and incubated with primary antibody 18 h at 4°C. Bound antibodies were visualized using horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and ECL reagent (Amersham Pharmacia, Pittsburgh, PA, USA). Bands were compared with molecular weight standards to confirm migration of proteins at the appropriate size. Quantification of protein expression densitometry was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). Cx43, Cx45, and GAPDH antibodies were purchased from Abcam, α-SMA from Invitrogen, P2Y2 from Alomone Labs (Jerusalem, Israel), and phosphorylated extracellular signal-regulated kinase (p-ERK) and total ERK from Cell Signaling (Danvers, MA, USA).
Collagenase-sensitive [3H]proline incorporation
Collagenase-sensitive [3H]proline incorporation assays were used to quantify collagen accumulation and were performed as described previously (29). CFs cultured on 12-well plates were serum-starved for 24 h followed by the addition of 1 μCi/ml [3H]proline (PerkinElmer, Waltham, MA, USA; 1 Ci = 37 GBq) along with drugs of interest and incubated at 37°C for 24 h. Cells were lysed in 0.5 M NaOH, and following neutralization with 0.5 M HCl, protein was precipitated overnight with 20% trichloroacetic acid (TCA). Samples were pelleted and washed in 5% TCA, dissolved in 0.2 M NaOH, and neutralized with 0.2 M HCl. Then, 2 mg/ml collagenase II (Worthington) in Tris-CaCl2–N-ethylmaleimide buffer was added to each sample and incubated at 37°C for 1 h. Protein was precipitated in 10% TCA and centrifuged. The radioactive content of the supernatant, corresponding with the amount of [3H]proline incorporated into the digested collagen fraction, was quantified using a liquid scintillation counter.
Reagents
ATP, carbenoxolone (CBX), probenecid (PBC), brefeldin A (BFA), suramin, and U0126 were purchased from Tocris Bioscience (Minneapolis, MN, USA). Apyrase from potato and TGF-β were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Statistical analysis
Calculations and statistics were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). Numerical values are presented as means ± se. Analysis of numerical data from experiments with multiple comparisons was done using ANOVA with Bonferroni's correction. Values of P < 0.05 were considered significant.
RESULTS
CFs release ATP in response to hypotonic stimulation, a response that is inhibited by an increase in extracellular calcium
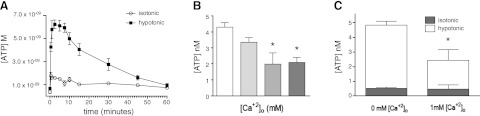
Replacement of growth medium with 50% hypotonic medium rapidly released ATP from rat CFs: released ATP reached a maximal concentration of 6.2 nM in the bulk medium after 2.5 min and decreased over the course of 1 h (Fig. 1A). Up to 1 h incubation of CFs in hypotonic medium did not increase cell death, as verified by trypan blue staining of cells exposed to each experimental condition (data not shown). Thus, ATP is rapidly released from rat CFs into the extracellular space via nonlytic processes in response to hypotonic stimulation and presumably the resultant cell swelling.
Figure 1.
ATP is released into the extracellular space in response to hypotonic stimulation and is sensitive to extracellular calcium ([Ca2+]o). A) Rat CFs were exposed to isotonic and hypotonic conditions for the indicated times. Release of ATP after hypotonic stimulation peaked at 6.2 nM (in bulk medium) after 2.5 min. ATP concentration in the medium of hypotonic-stimulated CFs returned to near baseline after ∼1 h. B) Addition of extracellular Ca2+ dose dependently decreased ATP release in response to 5 min hypotonic stimulation. C) Extracellular Ca2+ (1 mM) reduced hypotonicity-induced ATP release by 50% (P<0.05) but did not affect basal (isotonic) ATP release. Data are presented as means ± se of 3 independent experiments. *P < 0.05.
Prior studies document that ATP release is sensitive to extracellular Ca2+ levels (32, 33). We found that the addition of extracellular Ca2+ decreased swelling-activated ATP release from CFs in a concentration-dependent manner (Fig. 1B); 1 mM Ca2+ blunted ATP release in response to hypotonic stimulation by 50% (P<0.05) but had no effect on ATP release under isotonic conditions (Fig. 1C). Because extracellular Ca2+ can block Cx hemichannel permeability (32, 33), the effect of extracellular Ca2+ on ATP release suggested a role for Cx hemichannels in the release of ATP by CFs.
Cx43 and Cx45 are the most highly expressed subtypes in rat CFs
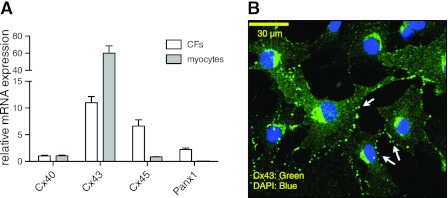
Using quantitative real-time PCR (qPCR), we found that Cx43 mRNA is the most highly expressed Cx subtype in rat CFs and cardiac myocytes; Cx45 mRNA is expressed at somewhat lower levels and Cx40 mRNA expression is ∼10% of that of Cx43 (Fig. 2A) in CFs. Immunofluorescence microscopy showed that while Cx43 mediates gap junction coupling between adjacent CFs in culture, a substantial population of Cx43 hemichannels do not form gap junctions and are exposed to the extracellular space (Fig. 2B). Panx1, another hemichannel protein involved in ATP release (20, 34), is also expressed at low levels in rat CFs (Fig. 2A).
Figure 2.
mRNA expression of Cx and Panx subtypes in rat CFs. A) qPCR revealed that the rank order of mRNA abundance of the 3 most highly expressed Cx isoforms is: Cx43>Cx45>Cx40. Panx1 mRNA expression was detected at 1/5 the relative abundance of Cx43. B) Immunofluorescence microscopy in rat CFs showed that while Cx43 is expressed between adjacent cells to mediate cellular coupling, a substantial population of Cx43-containing hemichannels are exposed to the extracellular space (arrows; green, Cx43; blue, DAPI).
CBX and PBC, but not BFA, inhibit ATP release from CFs
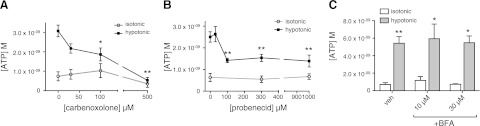
To characterize the role of hemichannels in ATP release, we treated CFs with CBX or PBC, nonspecific gap junction inhibitors: CBX blocks Cx and Panx hemichannels (35–38), while PBC is more specific for Panx than Cx hemichannels (39–41). CBX inhibited ATP release in response to hypotonic stimulation in a concentration-dependent manner (Fig. 3A); PBC also blunted ATP release (Fig. 3B). By contrast, BFA, an inhibitor of exocytosis, had no effect on ATP release produced with 5 min stimulation by hypotonic conditions (Fig. 3B). These results imply that hemichannels and not exocytosis mediate the release of ATP from rat CFs.
Figure 3.
CBX and PBC blunt hypotonicity-induced ATP release in rat CFs; BFA has no such effect. A) CBX dose dependently inhibited ATP release in response to hypotonic stimulation, with maximal inhibition at 500 μM CBX. B) PBC (100 μM) inhibited ATP release (∼40%; P<0.01); greater concentrations had no additional effect. C) BFA had no effect on ATP release. In all experiments, CFs were incubated with vehicle (veh), CBX, PBC, or BFA for 30 min followed by 5 min stimulation with hypotonic medium. Data are presented as means ± se of ≥3 independent experiments. *P < 0.05, **P < 0.01.
siRNA-mediated knockdown of Cx hemichannels inhibits ATP release by CFs
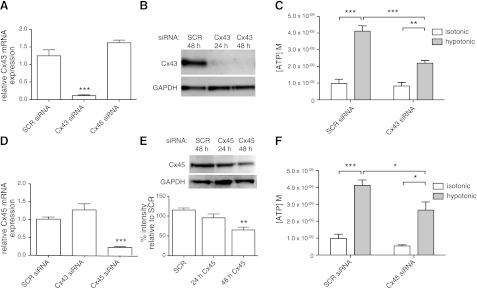
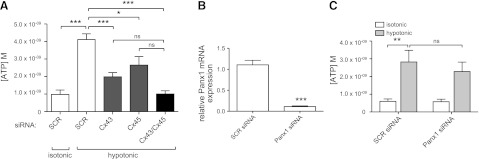
To more precisely define a role for hemichannels and the hemichannel subtypes involved in ATP release from CFs, we used siRNA to knock down expression of the two major Cx subtypes in rat CFs, Cx43 and C45. Cx43 siRNA decreased Cx43 mRNA and protein expression by >90% (P<0.001, assayed 24 and 48 h, respectively, after addition of siRNA; Fig. 4A, B), and at 48 h it decreased the release of ATP by hypotonic stimulation by 47% (P<0.001; Fig. 4C). Cx45 siRNA decreased Cx45 mRNA expression by 79% (P<0.001) after 24 h (Fig. 4D) and Cx45 protein expression by 43% (P<0.01) after 48 h (Fig. 4E). At 48 h, Cx45 knockdown decreased ATP release in response to hypotonic stimulation by 35% (P<0.05; Fig. 4F). The lower abundance of Cx45 expression in rat CFs and the smaller decrease in Cx45 by the siRNA likely explains the more modest effect of this knockdown on ATP release compared with the impact of knockdown of Cx43. Incubation of CFs with siRNAs against both Cx43 and Cx45 decreased ATP release to levels similar to that of isotonic controls (Fig. 5A), implying that both Cx43- and Cx45-containing connexons contribute to ATP release.
Figure 4.
Selective siRNA knockdown of Cx43 and Cx45 expression blunts hypotonicity-induced ATP release from CFs. A) Cx43 siRNA knocked down Cx43 mRNA expression by 91% 24 h after transfection (P<0.001; SCR, scramble siRNA). B) Cx43 protein levels were decreased >90% 24-48 h after transfection with Cx43 siRNA. C) CFs transfected with Cx43 siRNA (assayed 48 h post-transfection) released 47% less ATP than controls after 5 min hypotonic stimulation (P<0.001). D) Cx45 siRNA knocked down Cx45 mRNA expression by 79% (P<0.001) 24 h after transfection. E) Cx45 protein levels were decreased by 43% (P<0.01) 48 h after transfection with Cx45 siRNA. F) CFs transfected with Cx45 siRNA (assayed 48 h post-transfection) released 35% less ATP after hypotonic stimulation for 5 min (P<0.05). mRNA and protein expression data are presented as means ± se of 2 independent experiments; ATP release data are presented as means ± se of 6 (Cx43) or 4 (Cx45) independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.
Simultaneous Cx43/Cx45 knockdown inhibits ATP release, but Panx1 knockdown does not. A) Simultaneous transfection of Cx43 and Cx45 siRNA decreased ATP release to the levels of SCR-transfected isotonic controls. B) Panx1 siRNA decreased Panx1 mRNA expression 90% 24 h post-transfection (P<0.001). C) Knockdown of Panx1 did not significantly blunt ATP release in response to 5 min hypotonic stimulation 48 after siRNA transfection. mRNA expression data are presented as means ± se of 3 independent experiments; ATP release data are presented as means ± se of 4 independent experiments. ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
Panx1 siRNA treatment for 48 h decreased Panx1 mRNA expression by 90% (P<0.001; Fig. 5B) but did not significantly alter ATP release from CFs (Fig. 5C). These results suggest that Cx43 and Cx45 are the major gap junction hemichannel proteins involved in ATP release induced by hypotonic stimulation and cell swelling of rat CFs.
Released ATP participates in MAPK signaling and is sensitive to apyrase
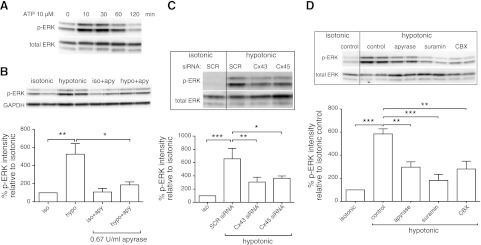
We next tested whether ATP released from CFs affects downstream signaling, in particular the activation of MAP kinase (assessed as ERK phosphorylation). Addition of ATP (10 μM) increased ERK phosphorylation within 10 min (Fig. 6A). Similarly, treatment with hypotonic medium for 10 min increased phosphorylated ERK (p-ERK) levels by 5-fold (P<0.01), but the addition of apyrase, which hydrolyzes nucleotides, substantially blunted this increase (P<0.05; Fig. 6B). ATP released from isolated fibroblasts thus induces downstream signaling, an effect that is blunted by hydrolysis of extracellular nucleotides.
Figure 6.
ATP released from CFs signals via nucleotide receptors to regulate ERK activation. A) Addition of 10 μM ATP induced ERK activation by 10 min. B) p-ERK levels in CFs increased 5-fold after 10 min hypotonic stimulation (P<0.01) and was significantly blunted by addition of 0.67 U/ml apyrase (P<0.05). C) CFs transfected with Cx43 and Cx45 siRNA, which decrease ATP release, showed diminished ERK activation by ∼50% (P<0.05) in response to hypotonic stimulation. D) Hypotonicity-induced ERK activation was decreased 50% by apyrase (0.67 U/ml; P<0.01), 52% by inhibition of ATP release (100 μM CBX; P<0.01), and 69% by P2 receptor blockade (100 μM suramin; P<0.001). In all experiments, cells were treated with the respective compounds for 30 min and then exposed to hypotonic conditions for 10 min. Densitometric quantification data are presented as means ± se of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Inhibition of ATP release from CFs decreased activation of ERK: inhibition of release by CBX or knockdown of Cx43 or Cx45 expression reduced p-ERK levels after 10 min hypotonic stimulation relative to untreated controls by ∼50% (P<0.05; Fig. 6C, D). CFs transfected with Cx43 or Cx45 siRNA had ERK phosphorylation in response to added ATP similar to control CFs incubated with scrambled siRNA (data not shown), indicating that the decrease in p-ERK levels in cells treated with siRNA results from decreased ATP-receptor signaling and not off-target effects of Cx43/45 knockdown. A decrease in ATP released in response to hypotonic stimuli thus diminishes ERK activation in response to autocrine/paracrine signaling by ATP.
Released ATP induces ERK phosphorylation through P2Y2 receptor activation
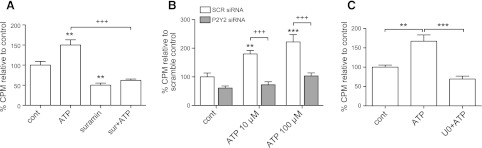
To investigate whether the effects of ATP release occur via P2 receptors, we initially used suramin, a nonspecific P2 receptor inhibitor. Treatment of CFs with 100 μM suramin reduced p-ERK levels following hypotonic stimulation by 70% (P<0.001; Fig. 6D), consistent with P2 receptor activation by release of endogenous ATP from CFs.
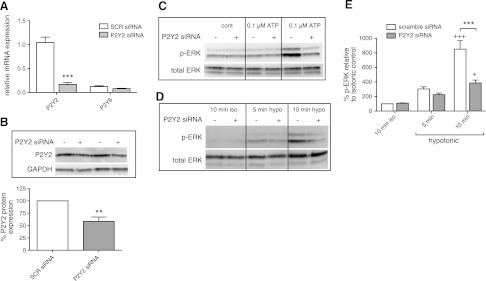
We then sought to define which P2 receptor mediates the activation of ERK by released ATP. Our previous data indicate that P2Y2 receptors are the most highly expressed P2Y receptor in rat CFs (28). Transfection of CFs with P2Y2 targeted siRNA decreased P2Y2 receptor mRNA and protein expression by 84% (P<0.001) and 41% (P<0.01), respectively (Fig. 7A, B), without significantly altering P2Y6 expression. Knockdown of P2Y2 receptors decreased ERK phosphorylation in response to exogenous ATP (Fig. 7C). Furthermore, ERK activation in response to 10 min hypotonic stimulation decreased 55% in CFs treated with P2Y2 receptor siRNA as compared with scramble-treated controls (P<0.001; Fig. 7D, E). The ability of a decrease in P2Y2 receptor expression to prominently decrease ERK activation in response to ATP release implies a key role for those receptors in mediating cellular responses to ATP released from CFs into the extracellular space. In turn, ERK phosphorylation potentially has profibrogenic effects (42), an idea we assessed in subsequent experiments.
Figure 7.
P2Y2 receptor knockdown decreases extracellular ATP-stimulated ERK activation. A, B) P2Y2 siRNA reduced P2Y2 gene expression by 84% (P<0.001; A) and protein expression by 41% (P<0.01; B). C, D) P2Y2 siRNA transfection (+) reduced ERK activation in response to 10 min ATP stimulation (C) and to 10 min hypotonic stimulation by 55% (P<0.001; D), compared with scramble-transfected (−) controls. E) Quantification of 3 independent experiments; data presented as means ± se. **P < 0.01, ***P < 0.001; +P < 0.05, +++P < 0.001 vs. isotonic control.
ATP stimulation of P2Y2 receptors increases profibrotic marker expression and collagen synthesis via ERK activation
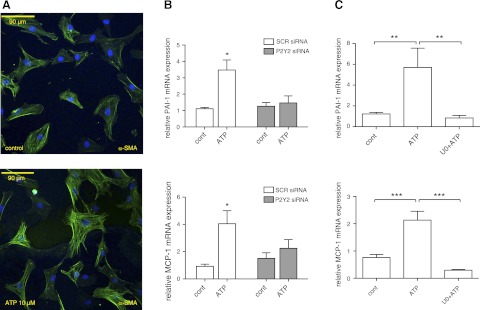
P2Y receptor activation induces profibrotic effects in rat CFs (20, 28). ERK phosphorylation resulting from P2Y activation has been shown to promote proliferation and phenotypic transformation in various cell types (43–46). Treatment of CFs with 10 μM ATP for 24 h increased the expression of α-SMA-positive stress fibers, a response that is a marker of fibroblast-to-myofibroblast transformation (ref. 47 and Fig. 8A). Moreover, treatment with ATP increased the expression of the profibrotic markers PAI-1 and MCP-1 by 3.5- and 4-fold, respectively (P<0.05); P2Y2 knockdown eliminated the up-regulation of PAI-1 and MCP-1 in response to ATP stimulation (Fig. 8B). Thus, consistent with prior results (28), ATP signals via P2Y2 receptors to promote profibrotic responses in CFs. Treatment with U0126, a MAPK/ERK kinase (MEK)/ERK inhibitor, abolished the stimulatory effect of ATP on the expression of PAI-1 and MCP-1, demonstrating that these responses depend on ERK (Fig. 8C).
Figure 8.
ATP increases α-SMA and expression of profibrotic markers PAI-1 and MCP-1. A) Immunofluorescent microscopy shows that incubation with 10 μM ATP for 24 h increased expression of α-SMA-containing stress fibers. B) Treatment with 10 μM ATP for 4 h increased mRNA expression of the profibrotic markers PAI-1 and MCP-1 (3.5- and 4-fold, respectively; P<0.05). siRNA knockdown of P2Y2 expression abolished this effect. C) ERK inhibition with U0126 (U0) eliminated the stimulatory effect of ATP on PAI-1 and MCP-1 expression. Data are presented as means ± se of ≥3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Incubation of CFs with ATP for 24 h increased collagen accumulation by 50–60% (P<0.01, quantified using a collagenase-sensitive [3H]proline incorporation assay; ref. 29), a response that was eliminated by suramin treatment (Fig. 9A). Knockdown of P2Y2 receptor with siRNA (Fig. 9B) or inhibition of ERK activation with U0126 (Fig. 9C) abolished the stimulation of collagen synthesis by ATP. Together, these data show that release of endogenous ATP by CFs in response to hypotonic medium activates the P2Y2-MAPK pathway to stimulate collagen accumulation and expression of the profibrotic markers PAI-1 and MCP-1.
Figure 9.
P2Y2 activation by ATP increases collagen synthesis in CFs and is ERK dependent. Collagen synthesis was measured by a collagenase-sensitive [3H]proline incorporation assay. A) ATP (10 μM for 24 h) increased collagen synthesis by 50% (P<0.01) and was blunted with 50 μM suramin (P<0.001). B) siRNA knockdown of P2Y2 receptor expression reduced ATP-stimulated collagen synthesis (at 10 and 100 μM) over 24 h (P<0.001). C) MEK/ERK inhibition with 10 μM U0126 (U0) prevented ATP-stimulated collagen synthesis (P<0.001). Data are presented as means ± se of 3 independent experiments. **P < 0.01, ***P < 0.001 vs. untreated control; +++P < 0.001.
Nucleotides contribute to basal profibrotic signaling in CFs
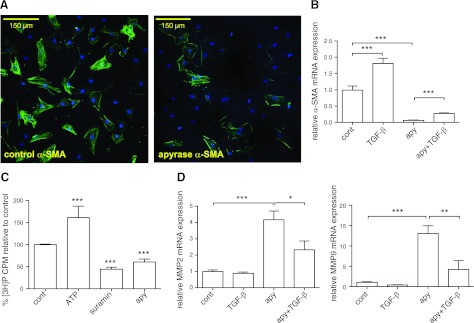
The results shown thus far utilized hypotonic stimuli to release ATP and produce profibrotic effects. Basal ATP concentrations in the bulk medium of unstimulated CFs are low, but the concentrations of released nucleotides near the cell surface may be sufficient to activate adjacent P2Y receptors and contribute to basal signaling (48, 49). To assess the role of basal extracellular ATP concentrations on profibrotic activity, we added apyrase to CFs in culture for 24 h. We found that incubation with apyrase substantially reduces the fibrotic phenotype of CFs (Fig. 10). Immunofluorescent staining for α-SMA revealed that treatment with apyrase decreases the expression of α-SMA-positive stress fibers (Fig. 10A). Treatment with apyrase also decreased α-SMA mRNA expression by >90% (P<0.001; Fig. 10B), but importantly, did not prevent the profibrotic response of CFs to TGF-β (Fig. 10B, D). The decrease in profibrotic activity by nucleotide hydrolysis thus appears to result from a decrease in basal nucleotide signaling.
Figure 10.
Hydrolysis of extracellular ATP decreases profibrotic α-SMA and collagen accumulation and increases MMP expression. A) By immunofluorescent microscopy, apyrase treatment for 24 h decreased expression of α-SMA-containing stress fibers in CFs. B) Apyrase treatment (24 h) decreased α-SMA mRNA expression by >90% (P<0.001), which was partially reversed by cotreatment with 10 ng/ml TGF-β. C, D) Apyrase also decreased collagen accumulation (C) after 24 h by 40% (P<0.001) and increased MMP2 and MMP9 mRNA expression (D) by 4- and 13-fold, respectively (P<0.001). These effects were also partially reversed by TGF-β cotreatment. Data are presented as means ± se of ≥3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Apyrase treatment for 24 h also decreased basal collagen accumulation by 40% (P<0.001; Fig. 10C) and increased the expression of matrix metalloproteinase (MMP)-2 and MMP-9 by 4- and 13-fold, respectively (P<0.001). The apyrase-promoted increases in MMP-2 and MMP-9 were blunted if the CFs were incubated with TGF-β (Fig. 10D), implying that basal levels of those MMPs are regulated by ambient concentrations of extracellular ATP but retain their ability to respond to TGF-β. Based on the results observed in CFs treated with apyrase, we conclude that ATP signaling represents not only a profibrotic mechanism for cells exposed to physical perturbation (e.g., hypotonic stimulation/cell swelling) but also that ATP signaling helps establish the basal level of fibroblast activation. This conclusion is supported by the ability of suramin treatment to also decrease basal levels of collagen formation by 68% (P<0.001; Fig. 10C).
DISCUSSION
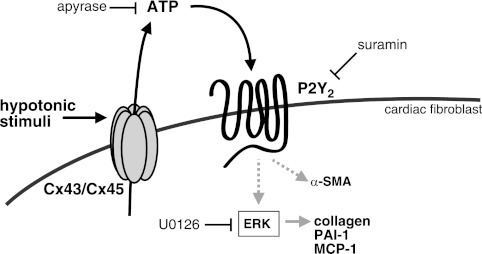
The data presented here demonstrate that ATP is released from rat CFs into the extracellular space in response to hypotonicity (and the presumed swelling of the cells) and that released ATP induces MAPK signaling and profibrotic cellular responses (Fig. 11). Treatment with CBX, a gap junction inhibitor, decreases ATP release; siRNA-mediated knockdown of Cx43 and Cx45 (the two most highly expressed subtypes in rat CFs) also reduces ATP release. Furthermore, because Cx43 and Cx45 can form heteromeric connexons (50, 51), selective knockdown of either Cx subtype conceivably alters the expression and function of these heteromeric channels. Although simultaneous knockdown of both Cx subtypes decreased ATP release to isotonic control levels (Fig. 5A), our data do not rule out the potential contribution of heteromeric connexons.
Figure 11.
Model of autocrine/paracrine signaling caused by ATP release. Hypotonic stimulation induces rapid ATP release in CFs. ATP then signals via P2Y2 receptors to up-regulate PAI-1 and MCP-1 expression and collagen accumulation via ERK activation.
Panx1, the only Panx subtype found in the heart (40, 52, 53) and that is implicated in ATP release from myocytes (20), is not highly expressed in our rat CF cultures. By using siRNA knockdown, we found that Panx1 does not substantially contribute to ATP release from rat CFs; however, since PBC, a Panx-preferring inhibitor (39, 40), partially inhibits ATP release, there may be a role, albeit likely a minor one, of Panx hemichannels in ATP release. Furthermore, because our use of siRNA did not completely eliminate Cx expression, future studies using conditional knockout approaches may be required to precisely define the contributions of each Cx subtype to ATP release from CFs. In addition, because it was necessary to use relatively high concentrations of CBX, its precise site of action cannot be determined in our studies. As such, we cannot unequivocally rule out other pathways for ATP release. Overall, our results indicate that both Cx43 and Cx45 mediate ATP release from CFs.
ATP concentrations near the surface of mechanically stimulated cells are in the micromolar range (49, 54), akin to those that activate P2Y receptors (40). Consistent with the ability of ATP to stimulate P2Y2 receptors and activate MAP kinases (43–45, 55), we observed a rapid increase in p-ERK following hypotonic stimulation. Inhibiting ATP release via CBX or Cx43/C45 knockdown, hydrolysis of extracellular ATP, or blockade of P2Y signaling with suramin or P2Y2 siRNA each reduced ERK activation in response to hypotonic stimulation. Our findings are the first of which we are aware to demonstrate that physical perturbations of CFs (or fibroblasts from any tissue) can activate intracellular signaling events via ATP release, P2Y2 receptor activation, and profibrotic responses. The current studies, including via the use of P2Y2 receptor siRNA, show that ATP stimulation of P2Y2 receptors increases collagen accumulation and PAI-1 and MCP-1 gene expression in an ERK-dependent manner.
Nucleotide signaling by ATP may represent a mechanism for CFs to respond to cellular injury/stress. PAI-1 inhibits plasmin activation and can inhibit fibrinolysis and matrix proteolysis in the heart (11). MCP-1 recruits monocytes and macrophages to sites of tissue injury and assists in the regulation of wound healing in the myocardium (56). The ATP-promoted increase in expression of PAI-1 and MCP-1, along with increases in collagen and α-SMA accumulation in CFs, implies that locally released ATP from stressed or damaged cardiac cells provides a profibrotic stimulus via P2Y2 receptors to aid in wound resolution following myocardial injury, such as myocardial infarction. Of note, ischemia in neurons also induces ATP release, which then signals via P2Y receptors to induce ischemic tolerance and neuronal survival (57). A similar mechanism may help protect against ischemic injury in the heart (58, 59).
Our results also indicate a role for ATP activation of P2Y2 receptors in the basal regulation of the phenotype of CFs. The ability of apyrase to decrease α-SMA and collagen expression and increase the expression of MMP2 and MMP9 indicates that signaling by ambient levels of ATP stimulates the basal production and deposition of ECM. Myocardial infarction can increase Cx43 expression and hemichannel opening (60, 61), and Cx43 deficiency has been correlated with reduced fibrosis in infarcted areas (62).
Extracellular ATP can contribute to profibrotic activity in various settings, including in pulmonary fibrosis (27), airway remodeling (63), and hepatic stellate cell activation (64). Our findings thus are likely more broadly applicable: P2Y receptor activation by nucleotides released in response to cellular stress or apoptosis may be a general component of wound healing and fibrotic response. Especially given our data showing that P2Y2 receptors contribute to both basal and stress-induced regulation of CF phenotype, nucleotide signaling may be a key autocrine/paracrine mechanism that regulates fibroblast activity, which may represent a novel therapeutic target to modulate tissue fibrosis.
Acknowledgments
The authors thank Nakon Aroonsakool for assistance in the isolation of rat CFs and Eveline Sun Arnold for assistance with confocal microscopy.
This work was supported by research grants from the U.S. National Institutes of Health (NIH) and the Ellison Medical Research Foundation and support from the University of California–San Diego Academic Senate (to P.A.I.). D.L. was supported by NIH training grant 1T32HL098062-01A1 and National Research Service Award 1F31AG039992-01. The authors declare no conflicts of interests.
Footnotes
- α-SMA
- α-smooth muscle actin
- ATP
- adenosine triphosphate
- BFA
- brefeldin A
- CBX
- carbenoxolone
- CF
- cardiac fibroblast
- Cx
- connexin
- DAPI
- 4′,6-diamidino-2-phenylindole
- ECM
- extracellular matrix
- ERK
- extracellular signal-regulated kinase
- MCP-1
- monocyte chemotactic protein 1
- MEK
- MAPK/ERK kinase
- MMP
- matrix metalloproteinase
- PAI-1
- plasminogen activator inhibitor 1
- Panx
- pannexin
- PBC
- probenecid
- TGF-β
- transforming growth factor-β
REFERENCES
- 1. Camelliti P., Borg T. K., Kohl P. (2005) Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 65, 40–51 [DOI] [PubMed] [Google Scholar]
- 2. Zeisberg E. M., Kalluri R. (2010) Origins of cardiac fibroblasts. Circ. Res. 107, 1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yokoyama U., Patel H. H., Lai N. C., Aroonsakool N., Roth D. M., Insel P. A. (2008) The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc. Natl. Acad. Sci. U. S. A. 105, 6386–6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kakkar R., Lee R. T. (2010) Intramyocardial fibroblast myocyte communication. Circ. Res. 106, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Souders C. A., Bowers S. L., Baudino T. A. (2009) Cardiac fibroblast: the renaissance cell. Circ. Res. 105, 1164–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Espira L., Czubryt M. P. (2009) Emerging concepts in cardiac matrix biology. Can. J. Physiol. Pharmacol. 87, 996–1008 [DOI] [PubMed] [Google Scholar]
- 7. Khan R., Sheppard R. (2006) Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 118, 10–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van den Borne S. W., Diez J., Blankesteijn W. M., Verjans J., Hofstra L., Narula J. (2010) Myocardial remodeling after infarction: the role of myofibroblasts. Nat. Rev. Cardiol. 7, 30–37 [DOI] [PubMed] [Google Scholar]
- 9. Ahmed M. S., Oie E., Vinge L. E., Yndestad A., Oystein Andersen G., Andersson Y., Attramadal T., Attramadal H. (2004) Connective tissue growth factor–a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J. Mol. Cell. Cardiol. 36, 393–404 [DOI] [PubMed] [Google Scholar]
- 10. Chen M. M., Lam A., Abraham J. A., Schreiner G. F., Joly A. H. (2000) CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J. Mol. Cell. Cardiol. 32, 1805–1819 [DOI] [PubMed] [Google Scholar]
- 11. Ghosh A. K., Vaughan D. E. (2012) PAI-1 in tissue fibrosis. J. Cell. Physiol. 227, 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. LaFramboise W. A., Scalise D., Stoodley P., Graner S. R., Guthrie R. D., Magovern J. A., Becich M. J. (2007) Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am. J. Physiol. Cell Physiol. 292, C1799–C1808 [DOI] [PubMed] [Google Scholar]
- 13. Sobel B. E., Lee Y. H., Pratley R. E., Schneider D. J. (2006) Increased plasminogen activator inhibitor type-1 (PAI-1) in the heart as a function of age. Life Sci. 79, 1600–1605 [DOI] [PubMed] [Google Scholar]
- 14. Lal H., Verma S. K., Foster D. M., Golden H. B., Reneau J. C., Watson L. E., Singh H., Dostal D. E. (2009) Integrins and proximal signaling mechanisms in cardiovascular disease. Front. Biosci. 14, 2307–2334 [DOI] [PubMed] [Google Scholar]
- 15. Manso A. M., Kang S. M., Ross R. S. (2009) Integrins, focal adhesions, and cardiac fibroblasts. J. Investig. Med. 57, 856–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Missan S., Shuba L. M., Zhabyeyev P., McDonald T. F. (2011) Osmotic modulation of slowly activating IKs in guinea-pig ventricular myocytes. Cardiovasc. Res. 91, 429–436 [DOI] [PubMed] [Google Scholar]
- 17. Verma S. K., Lal H., Golden H. B., Gerilechaogetu F., Smith M., Guleria R. S., Foster D. M., Lu G., Dostal D. E. (2011) Rac1 and RhoA differentially regulate angiotensinogen gene expression in stretched cardiac fibroblasts. Cardiovasc. Res. 90, 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Communi D., Janssens R., Suarez-Huerta N., Robaye B., Boeynaems J. M. (2000) Advances in signalling by extracellular nucleotides. the role and transduction mechanisms of P2Y receptors. Cell. Signal. 12, 351–360 [DOI] [PubMed] [Google Scholar]
- 19. Montalbetti N., Leal Denis M. F., Pignataro O. P., Kobatake E., Lazarowski E. R., Schwarzbaum P. J. (2011) Homeostasis of extracellular ATP in human erythrocytes. J. Biol. Chem. 286, 38397–38407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishida M., Sato Y., Uemura A., Narita Y., Tozaki-Saitoh H., Nakaya M., Ide T., Suzuki K., Inoue K., Nagao T., Kurose H. (2008) P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 27, 3104–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamamoto K., Furuya K., Nakamura M., Kobatake E., Sokabe M., Ando J. (2011) Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J. Cell Sci. 124, 3477–3483 [DOI] [PubMed] [Google Scholar]
- 22. Anselmi F., Hernandez V. H., Crispino G., Seydel A., Ortolano S., Roper S. D., Kessaris N., Richardson W., Rickheit G., Filippov M. A., Monyer H., Mammano F. (2008) ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. U. S. A. 105, 18770–18775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eltzschig H. K., Eckle T., Mager A., Kuper N., Karcher C., Weissmuller T., Boengler K., Schulz R., Robson S. C., Colgan S. P. (2006) ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 99, 1100–1108 [DOI] [PubMed] [Google Scholar]
- 24. Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., Park D., Woodson R. I., Ostankovich M., Sharma P., Lysiak J. J., Harden T. K., Leitinger N., Ravichandran K. S. (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792–1795 [DOI] [PubMed] [Google Scholar]
- 26. McDonald B., Pittman K., Menezes G. B., Hirota S. A., Slaba I., Waterhouse C. C., Beck P. L., Muruve D. A., Kubes P. (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 [DOI] [PubMed] [Google Scholar]
- 27. Riteau N., Gasse P., Fauconnier L., Gombault A., Couegnat M., Fick L., Kanellopoulos J., Quesniaux V. F., Marchand-Adam S., Crestani B., Ryffel B., Couillin I. (2010) Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am. J. Respir. Crit. Care Med. 182, 774–783 [DOI] [PubMed] [Google Scholar]
- 28. Braun O. O., Lu D., Aroonsakool N., Insel P. A. (2010) Uridine triphosphate (UTP) induces profibrotic responses in cardiac fibroblasts by activation of P2Y2 receptors. J. Mol. Cell. Cardiol. 49, 362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swaney J. S., Roth D. M., Olson E. R., Naugle J. E., Meszaros J. G., Insel P. A. (2005) Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. U. S. A. 102, 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lugo-Garcia L., Filhol R., Lajoix A. D., Gross R., Petit P., Vignon J. (2007) Expression of purinergic P2Y receptor subtypes by INS-1 insulinoma beta-cells: a molecular and binding characterization. Eur. J. Pharmacol. 568, 54–60 [DOI] [PubMed] [Google Scholar]
- 31. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srinivas M., Calderon D. P., Kronengold J., Verselis V. K. (2006) Regulation of connexin hemichannels by monovalent cations. J. Gen. Physiol. 127, 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stout C. E., Costantin J. L., Naus C. C., Charles A. C. (2002) Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 277, 10482–10488 [DOI] [PubMed] [Google Scholar]
- 34. Shestopalov V. I., Panchin Y. (2008) Pannexins and gap junction protein diversity. Cell. Mol. Life Sci. 65, 376–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Iglesias R., Dahl G., Qiu F., Spray D. C., Scemes E. (2009) Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J. Neurosci. 29, 7092–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iglesias R., Locovei S., Roque A., Alberto A. P., Dahl G., Spray D. C., Scemes E. (2008) P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am. J. Physiol. Cell Physiol. 295, C752–C760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ye Z. C., Oberheim N., Kettenmann H., Ransom B. R. (2009) Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57, 258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ye Z. C., Wyeth M. S., Baltan-Tekkok S., Ransom B. R. (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 23, 3588–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chekeni F. B., Elliott M. R., Sandilos J. K., Walk S. F., Kinchen J. M., Lazarowski E. R., Armstrong A. J., Penuela S., Laird D. W., Salvesen G. S., Isakson B. E., Bayliss D. A., Ravichandran K. S. (2010) Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis. Nature 467, 863–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D'hondt C., Ponsaerts R., De Smedt H., Bultynck G., Himpens B. (2009) Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays 31, 953–974 [DOI] [PubMed] [Google Scholar]
- 41. Silverman W., Locovei S., Dahl G. (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell Physiol. 295, C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caraci F., Gili E., Calafiore M., Failla M., La Rosa C., Crimi N., Sortino M. A., Nicoletti F., Copani A., Vancheri C. (2008) TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol. Res. 57, 274–282 [DOI] [PubMed] [Google Scholar]
- 43. Neary J. T., Kang Y., Willoughby K. A., Ellis E. F. (2003) Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 23, 2348–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stenmark K. R., Gerasimovskaya E., Nemenoff R. A., Das M. (2002) Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest 122, 326S–334S [DOI] [PubMed] [Google Scholar]
- 45. Wilden P. A., Agazie Y. M., Kaufman R., Halenda S. P. (1998) ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am. J. Physiol. 275, H1209–H1215 [DOI] [PubMed] [Google Scholar]
- 46. Ratchford A. M., Baker O. J., Camden J. M., Rikka S., Petris M. J., Seye C. I., Erb L., Weisman G. A. (2010) P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J. Biol. Chem. 285, 7545–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leask A. (2010) Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 106, 1675–1680 [DOI] [PubMed] [Google Scholar]
- 48. Corriden R., Insel P. A. (2010) Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci. Signal. 3, re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beigi R., Kobatake E., Aizawa M., Dubyak G. R. (1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. Cell Physiol. 276, C267–C278 [DOI] [PubMed] [Google Scholar]
- 50. Beyer E. C., Gemel J., Martinez A., Berthoud V. M., Valiunas V., Moreno A. P., Brink P. R. (2001) Heteromeric mixing of connexins: compatibility of partners and functional consequences. Cell. Commun. Adhes. 8, 199–204 [DOI] [PubMed] [Google Scholar]
- 51. Martinez A. D., Hayrapetyan V., Moreno A. P., Beyer E. C. (2002) Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ. Res. 90, 1100–1107 [DOI] [PubMed] [Google Scholar]
- 52. Barbe M. T., Monyer H., Bruzzone R. (2006) Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 21, 103–114 [DOI] [PubMed] [Google Scholar]
- 53. Dubyak G. R. (2009) Both sides now: multiple interactions of ATP with pannexin-1 hemichannels. Focus on “A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP”. Am. J. Physiol. Cell Physiol. 296, C235–C241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Okada S. F., Nicholas R. A., Kreda S. M., Lazarowski E. R., Boucher R. C. (2006) Physiological regulation of ATP release at the apical surface of human airway epithelia. J. Biol. Chem. 281, 22992–23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang L., Cranson D., Trinkaus-Randall V. (2004) Cellular injury induces activation of MAPK via P2Y receptors. J. Cell. Biochem. 91, 938–950 [DOI] [PubMed] [Google Scholar]
- 56. Dewald O., Zymek P., Winkelmann K., Koerting A., Ren G., Abou-Khamis T., Michael L. H., Rollins B. J., Entman M. L., Frangogiannis N. G. (2005) CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96, 881–889 [DOI] [PubMed] [Google Scholar]
- 57. Schock S. C., Leblanc D., Hakim A. M., Thompson C. S. (2008) ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem. Biophys. Res. Commun. 368, 138–144 [DOI] [PubMed] [Google Scholar]
- 58. Erlinge D., Burnstock G. (2008) P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 4, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gunduz D., Kasseckert S. A., Hartel F. V., Aslam M., Abdallah Y., Schafer M., Piper H. M., Noll T., Schafer C. (2006) Accumulation of extracellular ATP protects against acute reperfusion injury in rat heart endothelial cells. Cardiovasc. Res. 71, 764–773 [DOI] [PubMed] [Google Scholar]
- 60. Johansen D., Cruciani V., Sundset R., Ytrehus K., Mikalsen S. O. (2011) Ischemia induces closure of gap junctional channels and opening of hemichannels in heart-derived cells and tissue. Cell. Physiol. Biochem. 28, 103–114 [DOI] [PubMed] [Google Scholar]
- 61. Vasquez C., Mohandas P., Louie K. L., Benamer N., Bapat A. C., Morley G. E. (2010) Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ. Res. 107, 1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang Y., Wang H., Kovacs A., Kanter E. M., Yamada K. A. (2010) Reduced expression of Cx43 attenuates ventricular remodeling after myocardial infarction via impaired TGF-beta signaling. Am. J. Physiol. Heart Circ. Physiol. 298, H477–H487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van der Vliet A., Bove P. F. (2011) Purinergic signaling in wound healing and airway remodeling. Subcell. Biochem. 55, 139–157 [DOI] [PubMed] [Google Scholar]
- 64. Dranoff J. A., Ogawa M., Kruglov E. A., Gaca M. D., Sevigny J., Robson S. C., Wells R. G. (2004) Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G417–G424 [DOI] [PMC free article] [PubMed] [Google Scholar]