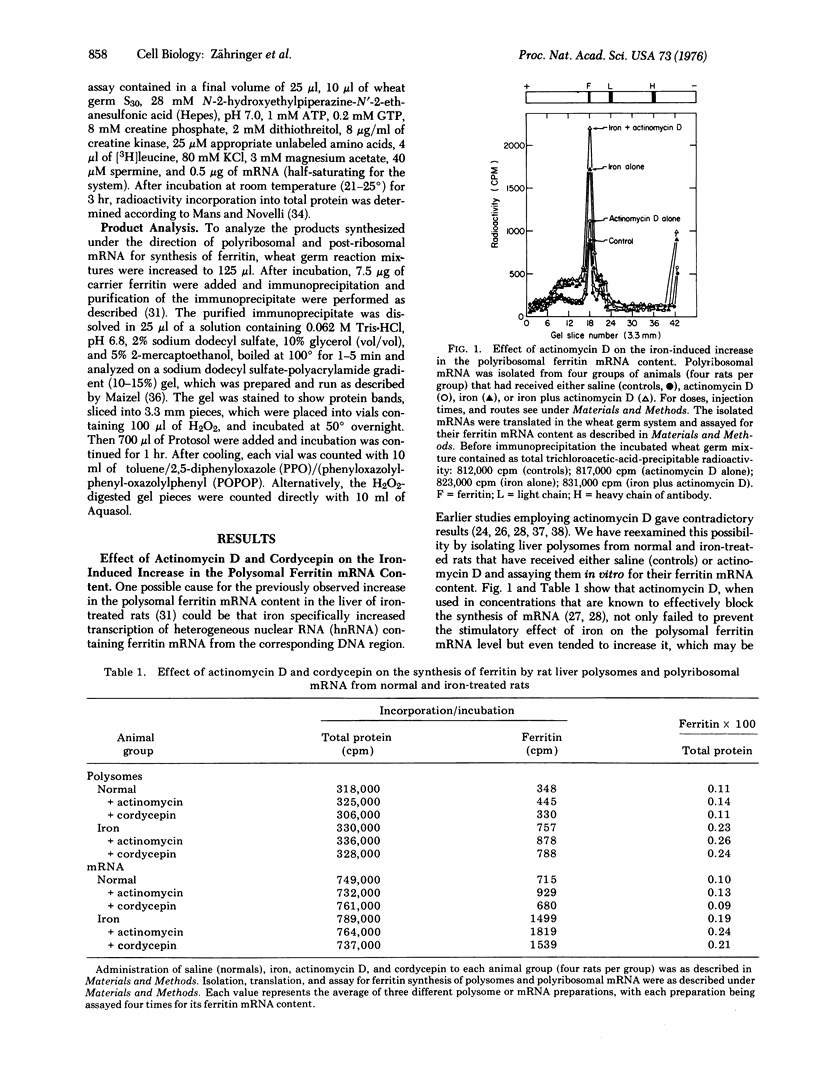
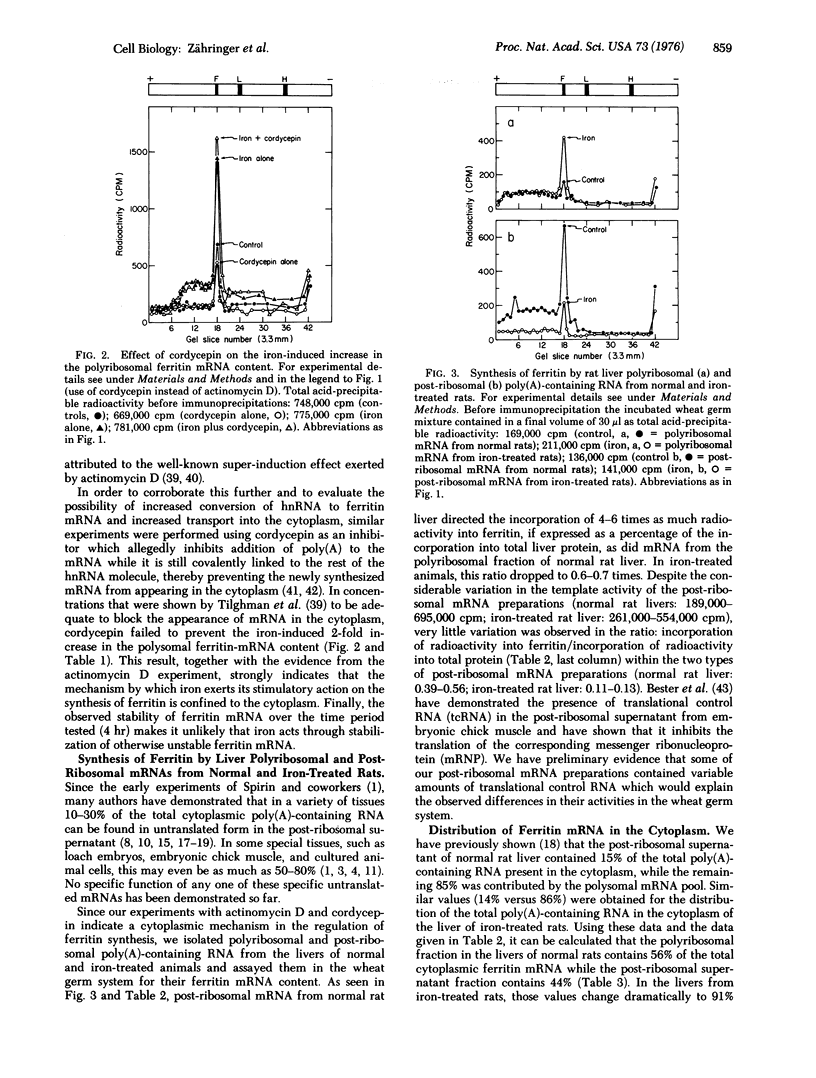
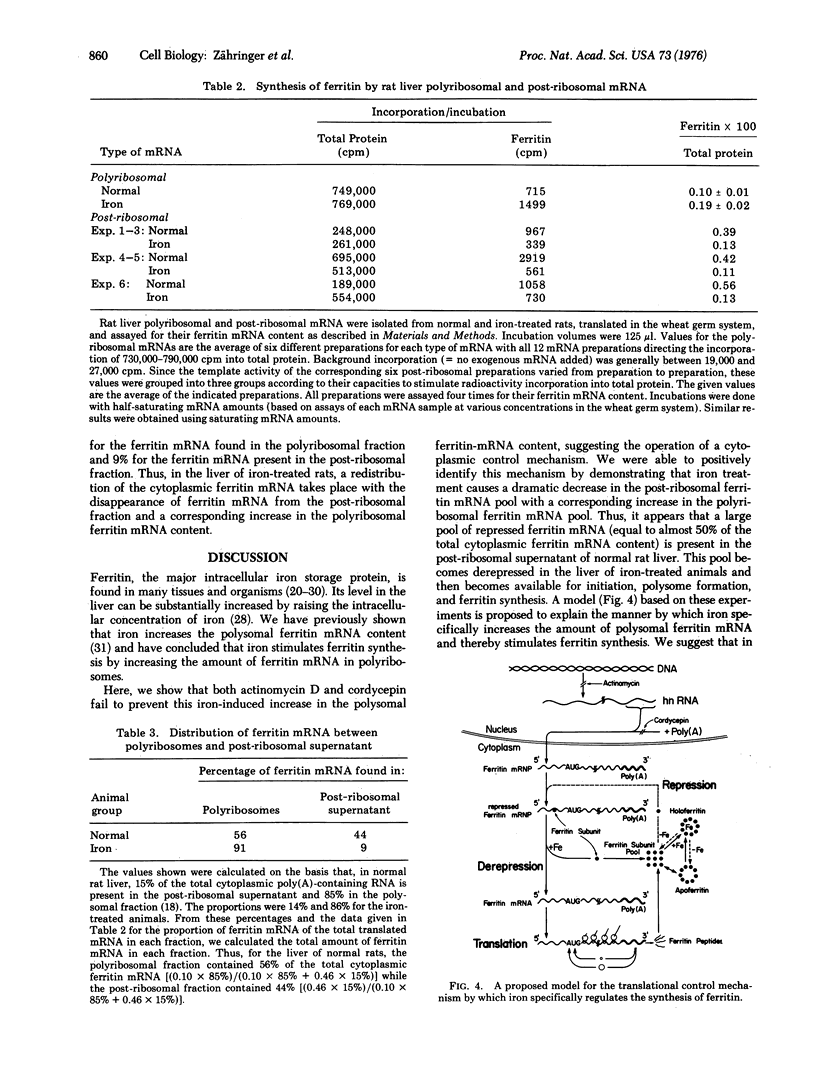
Abstract
Poly(A)-containing RNA was isolated from the polyribosomal and post-ribosomal fractions of the livers of normal and iron-treated rats. These RNA fractions were then translated in a wheat germ system to provide a measure of the amount of ferritin mRNA present in each fraction. Following iron administration, there was a 2-fold increase in the amount of ferritin mRNA in the polyribosomal fraction. This increase was not inhibited by prior treatment of the rats with actinomycin D or cordycepin, suggesting a cytoplasmic control mechanism. In normal rats, the post-ribosomal fraction contained an amount of ferritin mRNA equal to that in the polyribosomes. When iron was administered, this untranslated ferritin mRNA became reduced to negligible quantities, thus accounting for the doubling of the ferritin mRNA content of the polyribosomal fraction. A scheme is proposed in which translation of the ferritin mRNA in the post-ribosomal fraction is prevented by adhering ferritin subunits. Iron administration removes this inhibition of the translation of ferritin mRNA by promoting aggregation of these subunits into ferritin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975 May 1;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag J., Sarkar S. Cytoplasmic nonpolysomal messenger ribonucleoprotein containing actin messenger RNA in chicken embryonic muscles. Biochemistry. 1975 Aug 26;14(17):3800–3807. doi: 10.1021/bi00688a012. [DOI] [PubMed] [Google Scholar]
- Baliga B. S., Pronczuk A. W., Munro H. N. Regulation of polysome aggregation in a cell-free system through amino acid supply. J Mol Biol. 1968 Jul 14;34(2):199–218. doi: 10.1016/0022-2836(68)90247-7. [DOI] [PubMed] [Google Scholar]
- Bester A. J., Kennedy D. S., Heywood S. M. Two classes of translational control RNA: their role in the regulation of protein synthesis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1523–1527. doi: 10.1073/pnas.72.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanou-Tzedaki S. A., Pragnell I. B., Arnstein H. R. Presence of haemoglobin messenger RNA in the postribosomal supernatant of rabbit reticulocytes and conditions necessary for its translation. FEBS Lett. 1972 Oct 1;26(1):77–82. doi: 10.1016/0014-5793(72)80546-5. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Gros F. The use of metrizamide to separate cytoplasmic ribonucleoprotein particles in muscle cell cultures: a method for the isolation of messenger RNA, independent of its poly A content. FEBS Lett. 1975 May 15;53(3):355–359. doi: 10.1016/0014-5793(75)80054-8. [DOI] [PubMed] [Google Scholar]
- Chu L. L., Fineberg R. A. On the mechanism of iron-induced synthesis of apoferritin in HeLa cells. J Biol Chem. 1969 Jul 25;244(14):3847–3854. [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Drysdale J. W., Shafritz D. A. In vitro stimulation of apoferritin synthesis by iron. Biochim Biophys Acta. 1975 Feb 24;383(1):97–105. doi: 10.1016/0005-2787(75)90250-6. [DOI] [PubMed] [Google Scholar]
- Endo Y., Tominaga H., Natori Y. The state of messenger ribonucleic acid and ribosomes in the cytoplasm of ethionine-treated rat liver. Biochim Biophys Acta. 1975 Mar 21;383(3):305–315. doi: 10.1016/0005-2787(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Enger M. D., Campbell E. W., Hanners J. L. Appearance of cytoplasmic informosomes in cultured Chinese hamster cells in the absence of protein synthesis. FEBS Lett. 1975 Jul 15;55(1):194–197. doi: 10.1016/0014-5793(75)80990-2. [DOI] [PubMed] [Google Scholar]
- FINEBERG R. A., GREENBERG D. M. Ferritin biosynthesis. II. Acceleration of synthesis by the administration of iron. J Biol Chem. 1955 May;214(1):97–106. [PubMed] [Google Scholar]
- Gander E. S., Stewart A. G., Morel C. M., Scherrer K. Isolation and characterization of ribosome-free cytoplasmic messenger-ribonucleoprotein complexes from avian erythroblasts. Eur J Biochem. 1973 Oct 18;38(3):443–452. doi: 10.1111/j.1432-1033.1973.tb03078.x. [DOI] [PubMed] [Google Scholar]
- Hemminki K. Labelling kinetics of RNA containg poly(A) in liver subcellular fractions. Mol Cell Biochem. 1975 Aug 30;8(2):123–128. doi: 10.1007/BF02116240. [DOI] [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S., Bester A. J. Stored myosin messenger in embryonic chick muscle. FEBS Lett. 1975 Apr 15;53(1):69–72. doi: 10.1016/0014-5793(75)80684-3. [DOI] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Baglioni C. Messenger RNA for globin in the postribosomal supernatant of rabbit reticulocytes. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1425–1428. doi: 10.1073/pnas.69.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Kaempfer R. Translational repression of a viral messenger RNA by a host protein. J Biol Chem. 1975 Aug 10;250(15):5749–5755. [PubMed] [Google Scholar]
- Knöchel W., Tiedemann H. Rapidly labelled ribonucleic acid in chicken embryos. Evidence for heterogeneous ribonucleoprotein particles. Biochim Biophys Acta. 1972 Apr 26;269(1):104–117. doi: 10.1016/0005-2787(72)90078-0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Lee S. S., Schlesinger K. J., Richter G. W. Production of ferritin by rat hepatoma cells in vitro. Demonstration of protein subunits and ferritin by immunofluorescence. Am J Pathol. 1975 Aug;80(2):235–248. [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Initiation of polysome formation in mouse sarcoma 180 ascites cells. Utilization of cytoplasmic messenger ribonucleic acid. Biochemistry. 1971 Mar 2;10(5):895–900. doi: 10.1021/bi00781a026. [DOI] [PubMed] [Google Scholar]
- Linder-Horowitz M., Ruettinger R. T., Munro H. N. Iron induction of electrophoretically different ferritins in rat liver, heart and kidney. Biochim Biophys Acta. 1970 Mar 31;200(3):442–448. doi: 10.1016/0005-2795(70)90100-5. [DOI] [PubMed] [Google Scholar]
- Linder M., Munro H. N., Morris H. P. Rat ferritin isoproteins and their response to iron administration in a series of hepatic tumors and in normal and regenerating liver. Cancer Res. 1970 Aug;30(8):2231–2239. [PubMed] [Google Scholar]
- Millar J. A., Cumming R. L., Smith J. A., Goldberg A. Effect of actinomycin D, cycloheximide, and acute blood loss of ferritin synthesis in rat liver. Biochem J. 1970 Oct;119(4):643–649. doi: 10.1042/bj1190643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H. Determination of the amount of small messenger ribonucleic acid-containing particles free in liver cytoplasm. Biochem J. 1975 Oct;152(1):51–56. doi: 10.1042/bj1520051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER G. W. Activation of ferritin synthesis and induction of changes in fine structure in HeLa cells in vitro: implications for protein synthesis. Nature. 1961 Apr 29;190:413–415. doi: 10.1038/190413a0. [DOI] [PubMed] [Google Scholar]
- Richter G. W. Comparison of ferritins from neoplastic and non-neoplastic human cells. Nature. 1965 Aug 7;207(997):616–618. doi: 10.1038/207616a0. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman F., Shatkin A. J., Perry R. P. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974 Nov;3(3):197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- Saddi R., von der Decken A. The effect of iron administration on the incorporation of [14C] leucine into ferritin by rat-liver systems. Biochim Biophys Acta. 1965 Nov 15;111(1):124–133. doi: 10.1016/0304-4165(65)90478-2. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Perry R. P. Characterization of the messenger RNA released from L cell polyribosomes as a result of temperature shock. J Mol Biol. 1972 Feb 14;63(3):577–590. doi: 10.1016/0022-2836(72)90449-4. [DOI] [PubMed] [Google Scholar]
- Schultz G. A., Chen D., Katchalski E. Localization of a messenger RNA in a ribosomal fraction from ungerminated wheat embryos. J Mol Biol. 1972 May 28;66(3):379–390. doi: 10.1016/0022-2836(72)90421-4. [DOI] [PubMed] [Google Scholar]
- Spirin A. S. The second Sir Hans Krebs Lecture. Informosomes. Eur J Biochem. 1969 Aug;10(1):20–35. doi: 10.1111/j.1432-1033.1969.tb00651.x. [DOI] [PubMed] [Google Scholar]
- Spohr G., Granboulan N., Morel C., Scherrer K. Messenger RNA in HeLa cells: an investigation of free and polyribosome-bound cytoplasmic messenger ribonucleoprotein particles by kinetic labelling and electron microscopy. Eur J Biochem. 1970 Dec;17(2):296–318. doi: 10.1111/j.1432-1033.1970.tb01168.x. [DOI] [PubMed] [Google Scholar]
- Spohr G., Kayibanda B., Scherrer K. Polyribosome-bound and free-cytoplasmic-hemoglobin-messenger RNA in differentiating avian erythroblasts. Eur J Biochem. 1972 Nov 21;31(1):194–208. doi: 10.1111/j.1432-1033.1972.tb02519.x. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. "Superinduction" of tyrosine aminotransferase by actinomycin D: a reevaluation. Cell. 1975 May;5(1):29–35. doi: 10.1016/0092-8674(75)90088-4. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Hanson R. W., Reshef L., Hopgood M. F., Ballard F. J. Rapid loss of translatable messenger RNA of phosphoenolpyruvate carboxykinase during glucose repression in liver. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1304–1308. doi: 10.1073/pnas.71.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU F. L., FINEBERG R. A. BIOSYNTHESIS OF FERRITIN BY RAT LIVER SLICES. J Biol Chem. 1965 May;240:2083–2087. [PubMed] [Google Scholar]
- Yoshino Y., Manis J., Schachter D. Actinomycin D and the regulation of apoferritin synthesis in rat liver. Nature. 1966 Apr 30;210(5035):538–539. doi: 10.1038/210538b0. [DOI] [PubMed] [Google Scholar]
- Yoshino Y., Manis J., Schachter D. Regulation of ferritin synthesis in rat liver. J Biol Chem. 1968 Jun 10;243(11):2911–2917. [PubMed] [Google Scholar]
- Zähringer J., Konijn A. M., Baliga B. S., Munro H. N. Mechanism of iron induction of ferritin synthesis. Biochem Biophys Res Commun. 1975 Jul 22;65(2):583–590. doi: 10.1016/s0006-291x(75)80186-0. [DOI] [PubMed] [Google Scholar]



