Abstract
Endothelial responses to stressors are non-uniform and follow the rules of stress-induced hormesis. Responses to the same stressor, depending on its intensity, can range from pro-regenerative to pro-lethal. Exposure to sub-lethal stressors induces a programmed response that results in stress resistance, whereas lethal level of a stressor accelerates cell demise. Diverse stressors turn on several default programs within the cells; such programs tend to induce anti-oxidative defenses, anti-inflammatory and pro-survival systems, whereas others tend to switch on pro-apoptotic systems. The response of the kidney endothelium to various forms of acute kidney injury follows these general principles. It is characterized by a pro-inflammatory pattern which includes up-regulation of different adhesion molecules promoting endothelial-leukocyte interactions, generation of reactive oxygen species with formation of oxidative and nitrosative stress and mitochondrial damage. Simultaneously, a series of adaptive mechanisms, both local and systemic, are ignited. Stressed endothelial cells broadcast distress signals systemically; these signals can be directed towards the restoration of homeostasis or aggravation of the original insult.
Keywords: endothelial cells, hormesis, oxidative stress, nitrosative stress, acute kidney injury
Nowadays laymen need not be told that “Cry havoc!” attracts more attention than the night watchman’s reassuring “All’s well, all’s well”.
Peter Medawar “The threat and the glory” Oxford Univ Press, 1991
The endothelium is one of the largest organs in the human body, weighing about 1 kg and covering up to 7000 m2 of the surface area. The endothelium lines the entire circulatory system. Despite the common anatomical location inside the vessel wall, the physiologic functions of endothelium are quite diverse, as has been comprehensively reviewed1.
The kidney has unique vascular system. The main renal arteries originate from the aorta and the amount of blood passing through the renal arteries every minute approximates 25% of the cardiac output. The main renal artery gives rise to several branches, which divide into segmental arteries and later into interlobar arteries, arcuate arteries, interlobular arteries and afferent arterioles. Afferent arterioles give rise to glomerular capillaries, which later merge into efferent arterioles. The efferent arterioles form peritubular capillaries in the cortex and vasa recta in the renal medulla. Endothelial cells in glomerular capillaries participate in the ultrafiltration of plasma, the first step in urine formation, have a unique phenotype, for instance they are fenestrated. Fenestrations in the glomerular endothelial cells are varying in the diameter from 70 to 100 nm. Endothelium in some of the the peritubular capillaries is also fenestrated. Vasa recta have a smooth muscle cells layer, which becomes attenuated as the vasa recta descends into the medulla and completely disappears in the ascending vasa recta. Endothelial cells in the venous part of the renal vasculature are similar to their peers in other veins2.
Endothelial cells as a part of vascular integrated system
Endothelial cells (ECs) are involved in regulation of many vascular functions, including production of vasoconstrictive and vasodilating biologically active substances, and hence the control of blood pressure; blood coagulation and fibrinolysis; formation of new blood vessels; regulation of inflammatory processes and the transit of white blood cells into and out of the vascular lumen; formation of a selective barrier between the blood and interstitium and controlling the transcapillary passage of different molecules (Figure 1).
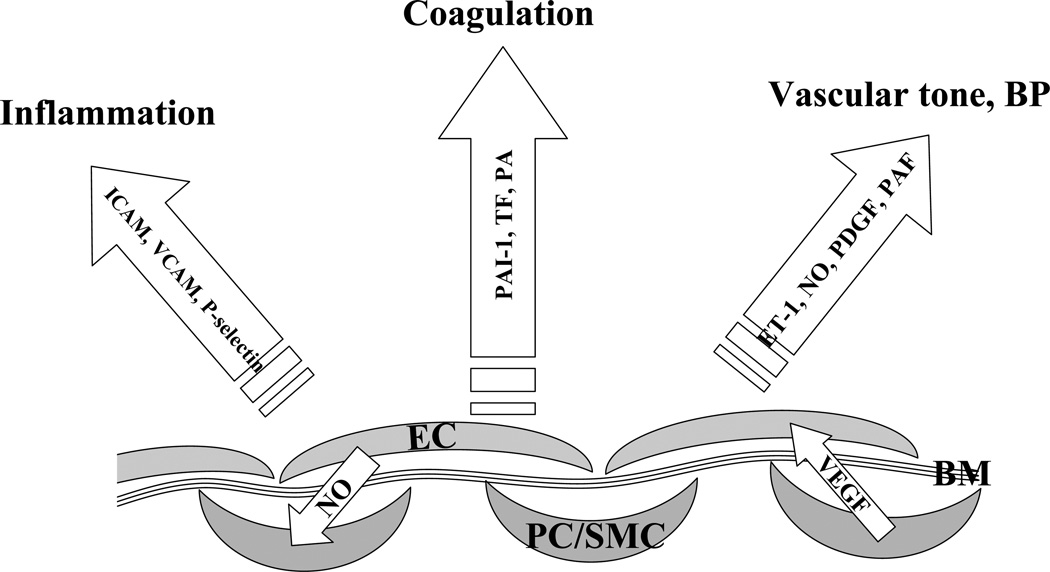
Figure 1. Endothelial and perivascular cells as parts of the vascular integrated system with mutual maintenance and benefits for different cell types.
Endothelial cells (EC) are located in the proximity to other cell types, such as pericytes (PC) and smooth muscle cells (SMC). The endothelium is separated from PC/SMC by a thin basement membrane (BM). Endothelial cells constitutively produce nitric oxide (NO), which regulates tonicity and serves as an anti-proliferative factor for SMC. On the other hand, perivascular cells produce VEGF, a survival factor for EC. Endothelial cells are involved in the regulation of many vascular functions through production of endothelin-1 (ET-1), platelet-derived growth factor (PDGF), platelet-activating factor (PAF), tissue factor (TF), plasminogen activators (PA) and plasminogen activator inhibitors (PAI-1), expression of intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), P-selectin, among others.
As any other organ, the endothelium may become dysfunctional. This was recently recognized as a syndrome of endothelial cell dysfunction (ECD). It has been widely appreciated that altered function of endothelial nitric oxide synthase (eNOS), its uncoupling and/or decreased availability of nitric oxide (NO) are a common denominator for a wide range of clinical manifestations of ECD. In this brief overview of the subject “Endothelium under Stress” we shall focus on some recent data describing local and systemic signaling by the endothelium. For detailed information on the broader subject, a reader is referred to several outstanding reviews3–5.
Endothelial cells are located in close proximity to other cell types. The endothelium in the kidney is in a constant dialog with adjacent smooth muscle cells, pericytes, podocytes and tubular epithelial cells. Endothelial cells constitutively produce NO, platelet-derived growth factor (PDGF), endothelin-1 (ET-1) and other regulatory substances. In contrast, their neighbors synthesize vascular endothelial growth factor (VEGF) and angiopoietin-1, which are pro-angiogenic, pro-survival and vessel-stabilizing factors that can act on neighbouring endothelial cells. This cross-talk signalling is necessary for the mutual maintenance of endothelial cells and their neighbors6. Hence, perturbations in EC functions have far-reaching repercussions, as do primary perturbations in the neighboring cells, which affect the endothelium as well. For instance, endothelial generation of PDGF-B is required for investment of pericytes and mesangial cells to the vascular wall and glomerular tufts, respectively, proliferation of vascular smooth muscle cells and differentiation of mesenchymal cells7. A myriad of endothelial gene products participate in cellular cross-talk. Moreover, new ones continued to be described. For instance, a recently described SCUBE-1, a late stress-response endothelial product secreted after ischemic injury, is involved in epithelial proliferation and kidney repair8.
In the kidney, different stressors affect differently endothelial cells in various vascular beds. Thus, the endothelium lining of large and medium-size arteries may be subjected to turbulent blood flow leading to morphologic changes similar to those found in large vessels in other organs, namely atherosclerotic lesions and intimal thickening9. Moreover, shear stress regulates NO production and calcium influx in endothelial cells lining vessels of all calibers10. Endothelial cells in small arteries and arterioles are the target for immune diseases, such as lupus erythematosus or thrombotic microangiopathy, cryoglobulinemic vasculopathy, Henoch-Schonlein purpura or targets of ANCA-related pauci-immune diseases11. Glomerular and peritubular capillary endothelial cells are affected by antibody-mediated complement injury, as seen in kidney transplantation12. All endothelial cells are affected by ischemia-reperfusion injury, a common model that is utilized in experimental models. Usually ischemia-reperfusion injury, similar to experimental animal models, is seen in patients with aortic cross-clamping, transplanted organs, after prolonged resuscitation, severe hemorrhage or other types of hypovolemic shock13. Other stressors, affecting the endothelium in all vascular beds, may include endotoxemia, hyperglycemia, and drugs, such as calcineurin inhibitors and cytotoxic agents.
Endothelial cell response to stress: local signals
Endothelial responses to the stressors depend on their intensity. Therefore responses to the same stressor can range from pro-regenerative to pro-lethal. Such a biphasic behavior is known as hormesis. “Stress-response hormesis” refers to the induction of stress-protective mechanisms14. The toxicological axiom states that “the dose determines the poison”; accordingly, sublethal exposure to stressors induces a response that results in stress resistance, whereas lethal level of stressor accelerates cell demise. This response has been conceptualized as a “fight-or-flight” cellular reaction and has been previously detailed15.
Damage to endothelial cells results in the influx of extracellular calcium and rapid upregulation of immediate early genes, like c-fos message and protein levels that orchestrate an adaptive response to stress16. Another early response to structural damage involves induction of intermediary filamental network that “provides a structural scaffold” 17. Stressed endothelial cells release stored basic fibroblast growth factor (FGF) into their microenvironment; this potent endothelial mitogen stimulates wound repair by uninjured cells18. A similar function is played by ATP released from the damaged or stressed cells and acting via P2-purinergic receptors19.
Non-lethal stress may stimulate mitochondrial biogenesis via activation of leucine zipper transcription factors, nuclear factor-E2-related factor (Nrf2) and ATF4, which regulate the expression of antioxidant response element-containing genes such as glutathione-S-transferase, glutathione peroxidase, glutathione reductase, and heme oxygenase-1, among others20. This process is mediated, at least in part, by the mitochondrial ROS stimulating Nrf2 binding to the promoter region (rich in antioxidant response element motifs) of the nuclear respiratory factor-1 (NRF-1) in the Akt-dependent de-repression of Nrf2 nuclear translocation21. Activation of NRF-1 is required for transcriptional activation of mitochondrial transcription factor A (Tfam) and induction of mitochondrial DNA replication/transcription and mitochondrial biogenesis22,23.
Another recently discovered mechanism stimulating mitochondrial biogenesis is adenosine monophosphate-activated protein kinase (AMPK) activation. Induction of the ataxia telangiectasia mutated (ATM)-dependent pathway is attributed to adenosine monophosphate-activated protein kinase (AMPK) activation24.AMPK is a key sensor of cellular energy state that, when activated by increased AMP:ATP ratio, switches on catabolic pathways capable of generating ATP and turns off energy-requiring processes that are not essential for cell survival25. Non-metabolic activation of AMPK may also occur and it involves elevation in cytosolic calcium concentration and activation of Ca-calmodulin- dependent protein kinase. Targets of AMPK are many and include NRF-1, HIF-1α, eNOS, FoxO1, FoxO3, sirtuin-1, among others. Activation of AMPK also affords anti-inflammatory action via downregulation of NF-kB, suppression of VCAM-1, E-selectin and JNK expression and anti-oxidant action via induction of several defense enzymes, such as Mg-SOD, catalase and thioredoxin. In addition, activation of AMPK results in the pro-survival stimuli like induction of Bcl-2 and survivin expression.
All the above programs are engaged in rendering endothelial cells resistant to non-lethal stress. However, in the cases of severe stress, the balance is shifted: ROS and reactive nitrogen species (such as peroxynitrite) are generated in excess of defense capacity, caspases and calpains are activated, mitochondrial, endoplasmic reticulum and lysosomal stress ensue to result in endothelial cell death26. Although substantial literature exists on the contribution of individual mechanisms to cell demise, there is little understanding of the fine orchestration of these processes and cell sensors that may be involved in decision making as to fight or flight or succumb to death.
One of the mechanisms that emerged during the past few years and has a potential to turn on cell-destructive programs is ascribed to mitochondrial oxidative stress. One of the primary targets of oxidative stress is represented by an anionic phospholipid of the inner mitochondrial membrane (IMM), namely cardiolipin. Cardiolipin is well known to the anti-phospholipid literature. Under normal conditions, cardiolipin restricts cytochrome c (cyt-c) to the IMM. Oxidation of the highly unsaturated acyl chains of cardiolipin reduces its binding affinity for cyt-c and liberates it from the IMM. This event, in conjunction with permeabilization of the outer mitochondrial membrane by pro-apoptotic members of Bcl-2 family of proteins, results in the release of cytochrome c into the cytosol. This results in the formation of the apoptosome and activation of the caspase cascade culminating in apoptosis27.
Endothelial cell response to stress: systemic signals
Similar to the local signals generated by stressed endothelial cells, systemic signals may also be conducive of repair or promoting further inflammatory reaction. Notably, the distinction between the two is blurred and context-dependent, as will be seen below. Reparative signals by stressed endothelial cells most often lead to well-orchestrated mobilization and recruitment of endothelial progenitor cells (EPC) that tend to restore vascular integrity. Examples of this signaling pathway are numerous. Hypoxia induces endothelial secretion of macrophage migration inhibitory factor (MIF): it initially peaks at 60 min and originated from preformed MIF stores, and by 8 h MIF secretion peaks again due to the de novo synthesis28. One of the functions of MIF is to recruit EPC to the site(s) of injury; this chemotactic response is CXCR4- dependent. Importantly, increased MIF secretion cooperates with the injury- or hypoxia-induced secretion of VEGF, erythropoietin, and SDF-1, all potent mobilizers of EPC29–31. Some pro-inflammatory cytokines produced by stressed endothelial cells, like IL-8, are potent stimulators of EPC mobilization, thus linking this response to stressors with pro-inflammatory conditions32. An additional mechanism for EPC recruitment and differentiation has been attributed to apoptotic endothelial microparticles generated upon injury to mature endothelial cells33, a mechanism that creates a feedback loop that enhances the number and initiates the differentiation of EPC engrafting the vessel wall when injury to mature endothelial cells occurs. Yet another mechanism of communication between injured and intact cells has been recently described – microvesicular transfer of genetic information from EPC to mature endothelial cells which is guided by the α4β1 integrin34.
Exocytosis of Weibel-Palade bodies, unique endothelial organelles containing von Willebrand factor, interleukin-8, angiopoietin-2, endothelin-1, among other biologically active molecules, releases to the bloodstream all these previously sequestered substances. While traditionally linked to inflammation and coagulation cascades, released constituents of Weibel-Palade bodies are able to mobilize endothelial progenitors and hematopoietic stem cells and, by this mechanism, promote regeneration32.
Some pro-regenerative signals, on the one hand, have a pro-inflammatory component, but, on the other, are opposed by several default responses that may aggravate the insult to the endothelium. Production of endothelin-1 has been documented in the ischemic kidney or after nephrotoxic or endotoxic insults to the kidney35–37. In combination with the uncoupling of eNOS and reduced endothelium-dependent NO production, this surge in endothelin-1 may lead to a prolonged and profound renal vasoconstriction38. It has recently been discovered, however, that under these conditions inflamed endothelium becomes the source of a metabolic product of tryptophan, kynurenine, which is a powerful vasorelaxing factor39, emphasizing again the remarkable functional vascular diversity that is elicited following cellular stress.
Another example of a default response that may have lasting effects on the vasculature is the induction of collagen XVIII synthesis and formation of a C-terminal cleavage product, endostatin, a powerful anti-angiogenic substance40–43 that may account for the eventual pruning of blood vessels. One of the mechanisms of anti-angiogenic effect of endostatin consists in its ability to induce endothelial-to-mesenchymal transdifferentiation43.
The role of the endothelium in the pathogenesis of AKI
It is almost 40 years ago that Flores et al. demonstrated that ischemia-stressed endothelial cells in the renal vasculature undergo an early swelling, leading to the narrowing of the lumen, an observation that lead to the concept of "no-reflow". This hypothesis has been supported by the observation that ischemic renal vasculature is characterized by a profound loss of acetylcholine-induced vasorelaxation. In addition, NO production in response to bradykinin was found to be suppressed in ischemic kidneys44. Overexpression of intracellular adhesion molecule-1 (ICAM-1) by the vascular endothelium of the ischemic kidney has been demonstrated to play a pathophysiological role in the development of renal dysfunction, and neutralizing anti-ICAM-1 antibodies significantly improved the outcome of renal ischemia45. Indeed, mice lacking ICAM-1 are protected against acute ischemic kidney injury45. These and other observations together with the recognition that epithelial cell injury is much less prominent in humans than in experimental animals46 lead to the re-evaluation of the pathophysiology of AKI and relative contribution of endothelial and epithelial damage.
The response of the endothelium of kidney to various forms of AKI is characterized by an inflammatory pattern. Thus, ischemia-reperfusion injury results in upregulation of different adhesion molecules promoting endothelial-leukocyte interactions. These adhesion molecules include integrins, selectins, and members of the immunoglobulin superfamily, including ICAM-1, vascular cell adhesion molecule (VCAM), and P-selectin47,48. The endothelium of renal microvessels in ischemic kidneys shows a loss of polarity in the expression of arginine-glycine-aspartic acid-binding (RGD) integrins, similar to that seen in the tubular epithelium49. Indeed, treatment of cultured endothelial cells in vitro with hydrogen peroxide or peroxynitrite resulted in a rapid detachment of endothelial cells, leading to the gradual loss of integrity of endothelial cell monolayers. This was confirmed using scanning electron microscopy, which showed frequent gaps in monolayer integrity. In vivo, ischemia-reperfusion resulted in disorganization of endothelial integrity with areas of denudation, partial disappearance of cell-cell borders, or characteristic distortion of cell-cell contacts in a rat model. These defects in the integrity of endothelial cell lining were most prominent in the renal microvasculature50. Ischemia-reperfusion injury compromises the barrier function of the endothelium in renal microvasculature, as has been shown using fluorescent dextrans and two-photon intravital imaging51.
Treatment with a cyclic RGD peptide resulted in significant amelioration of AKI52, in part by inhibiting α4β1-VCAM-1-mediated monocyte-endothelial adhesion53 and potentially reducing macrophage infiltration of the kidney54, suggesting an important role of the renal endothelium in the inflammatory response to acute ischemia-reperfusion and other stressors.
In addition, the renal tubular epithelium in response to ischemia-reperfusion injury generates a number of mediators that may affect the endothelium directly or by potentiation of inflammatory response. This is a further example of cellular cross-talk. These mediators include proinflammatory cytokines such as TNF-α, IL-6, IL-1β, and transforming growth factor-β (TGF-β); chemokines, including monocyte chemoattractant protein-1 (MCP-1), IL-8, epithelial neutrophil-activating protein 78 (ENA-78), CCL5 (formerly called regulated upon activation, normal T-cell expressed and secreted (RANTES)), and CX3CL1 (also known as fractalkine in humans and neurotactin in mice)47,55. One of the consequences of cytokine and chemokine release involves the induction of inducible NOS (iNOS) in epithelial and endothelial cells. Selective inhibition of iNOS protected renal function against ischemia52,56. Furthermore, it has been demonstrated that iNOS knockout mice have lesser degree of renal injury after ischemia-reperfusion57.
Collectively, these observations are indicative of endothelial cell activation and dysfunction in AKI. Indeed, using minimally invasive intravital microscopy of peritubular capillary blood flow in control and postischemic kidneys, we confirmed the existence of the “no-reflow” phenomenon. Renal microvascular hemodynamics was characterized by a sudden cessation of the peritubular capillary flow within 1–3 min after the removal of the clamp, followed by a gradual and partial recovery of the microcirculatory blood flow. During the recovery process, restoration of blood flow was non-uniform with sporadic capillaries showing oscillating stagnation or cessation of flow58. Injection of endothelial cells or surrogate cells expressing endothelial NO synthase into rats subjected to renal artery clamping resulted in the implantation of these cells in the renal microvasculature and a dramatic functional protection of ischemic kidneys50. Furthermore, we have demonstrated that skeletal muscle-derived stem cells differentiated along the endothelial lineage, but not non-differentiated cells, also ameliorate acute kidney injury in mice59. These beneficial effects of adoptive transfer of endothelial cells or their surrogates expressing eNOS may be related to the restoration of the integrity of the renal endothelium. In addition, it has been shown in human post-transplant ischemic kidneys that allografts with less damaged endothelium of peritubular capillaries had better post-transplant recovery of function as compared to those that had more severe damage of the endothelium60.
Oxidative and nitrosative stress in the pathogenesis of AKI
One of the important pathophysiologic mechanisms of the ischemia-reperfusion injury in kidneys is oxidative and nitrosative stress. Reactive oxygen species (ROS) are derived from the one-electron reduction of molecular oxygen. They include O2.−, which is a shor-tlived and poorly diffusible across biological membranes molecule, as well as a long-lived, uncharged and easily diffusible H2O2. ROS are produced by plasma membrane-encored enzymes, such as NADPH oxidase and uncoupled eNOS, cytosolic enzymes like xanthine oxidoreductase, iNOS, cytochrome P450 systems, and mitochondrial enzymes. Protection from cytotoxic effects of ROS is accomplished by antioxidant systems, including superoxide dismutases (SOD) (extracellular, cytosolic and mitochondrial isoforms), peroxisomal catalase, glutathione peroxidase, peroxiredoxin, thioredoxin, and glutaredoxin. Oxidative stress occurs when the net production of ROS outweighs the capacity of antioxidant systems to neutralize them. By the same token, when NO production overcomes the reductive capacity of antinitrosative systems, nitrosative stress ensues.
Generation of ROS and excess of NO produced by iNOS in ischemia/reperfusion injury results in formation of a cytotoxic metabolite, peroxynitrite, which is capable of causing lipid peroxidation, DNA damage and 3-nitrotyrosine modification of proteins (Figure 2). Treatment with either iNOS inhibitor or SOD resulted in a significant amelioration of ischemia-reperfusion injury in rats61. Similar results were reported by using treatment with Tempol, a membrane-permeable radical scavenger62. Direct scavenging of peroxynitrite by Ebselen also resulted in a significant improvement of renal function after acute ischemia-reperfusion injury61.
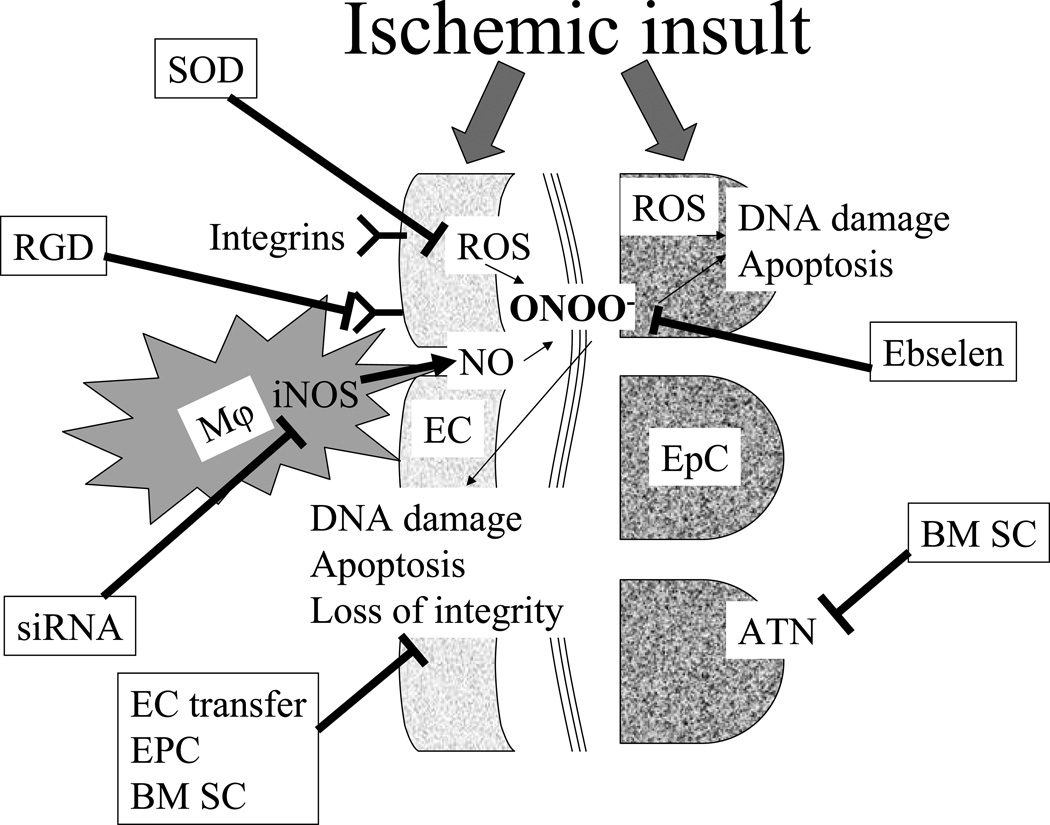
Figure 2. Pathophysiologic mechanisms of ischemia-reperfusion injury in the kidney.
Ischemic insult affects both endothelial (EC) and epithelial (EpC) cells in the kidney. In EpC, formation of reactive oxygen species (ROS) results in DNA damage, apoptosis and can culminate in acute tubular necrosis (ATN). In EC, ischemic insult induces expression of adhesion molecules which increase rolling, tight adhesion and migration of circulating pro-inflammatory cells. These inflammatory cells produce excessive amount of nitric oxide (NO) by inducible nitric oxide synthase (iNOS). On the other hand, ischemic insult results in ROS formation by EC as well. NO and ROS form peroxynitrite (ONOO−), which is directly responsible for DNA damage and apoptosis in both EC and EpC. Loss of EC integrity plays an important role in the pathogenesis of acute kidney injury, as it reduces further the already compromised microcirculation and exacerbates ischemia. Experimental strategies to reduce kidney injury include scavenging of ROS [by superoxide dismutases (SOD)] or peroxynitrite (by Ebselen); decreasing inflammatory cells adhesion to EC [by arginine-glycine-aspartic acid (RGD)-peptide]; decreasing NO production by iNOS [by small interfering RNA (siRNA)]; restoration of the EC integrity [by EC, endothelial progenitor cells (PEC) or bone marrow stem cells (BM SC) transfer].
It has been demonstrated that in renal ischemia reperfusion injury the polyunsaturated fatty acyl group of membrane phospholipids are highly susceptible to O∸2 and a self-propagating chain of reaction produces a wide variety of aldehydes, alkenals, and hydroxyalenals, such as malondialdehyde, 4-hydroxy hexenol (HHE), and 4-hydroxy-2-nonenal (HNE)61, 63, 64. Membrane-permeable HNE is noxious and one of the best investigated mediators of free-radical damage. Mitochondrial proteins are targets of HNE (see cardiolipin above) and HNE inactivates the 2-oxoglutarate dehydrogenase, pyruvate dehydrogenase complex, cytochrome-c oxidase, and NADH-linked respiration in isolated mitochondria.
Mitochondria are richly endowed with Mn-SOD, Gpx1, Prx3, Trx2 and Grx2. All of these antioxidant systems protect mitochondria from uncontrolled formation of ROS and accumulation of ROS-induced injury to proteins, lipids and mitochondrial DNA. Excessive ROS in quiescent mitochondria poses the risk to this organelle and to the viability of the cell because it may result in the opening of mitochondrial membrane channels including the mitochondrial permeability transition pore (MPTP) and the inner membrane anion channel (IMAC)65 leading to the collapse of mitochondrial membrane potential and further transient increase in ROS generation by the electron transport chain66. These events may result in autophagy, apoptosis or necrosis65.
Indeed, apoptosis via the mitochondrial pathway contributes to ischemic acute kidney injury at least in tubular epithelial cell67. AKI-associated fragmentation of mitochondria involves the activation of mitochondrial fission via dynamin-related protein 1 (Drp1). Evidence has been provided that suppression of Drp1 and mitochondrial fragmentation abrogates mitochondrial damage, cytochrome-c release, apoptosis, and renal/cellular injury both in vitro and in vivo. The mitochondrial fragmentation, clearly induced by azide- or cisplatin-induced cell apoptosis, was reduced with Bcl-2 but not by pan-caspase inhibition. A fission protein Drp1 was translocated to mitochondria under these stimuli but the fragmentation was inhibited in cells with dominant-negative Drp1 in association with decrease in apoptosis. They further demonstrated the efficacy of Drp1 pharmacological inhibitor, mdivi-1, in acute kidney injury67. Another prominent mechanism for development and perpetuation of mitochondrial dysfunction is related to the induction of a mitochondrial anion carrier protein, uncoupling protein-2 (UCP2)68. Oxidative stress results in the accumulation of UCP2 in mitochondria leading to the inward proton leak which competes with the function of ATP synthase and results in reduction of ATP synthesis from ADP. Collectively, these ROS-induced events may result in autophagy, apoptosis or necrosis.
In conclusion, the intent of this brief overview summarizing responses of endothelial cells to stressors is to emphasize three in our view important points. Firstly, the same stressor, depending on its intensity, may elicit either resistance to further stimulation or precipitate cell death. Secondly, diverse stressors turn on several default programs within the cells, some programs tend to induce anti-oxidative, anti-inflammatory and pro-survival systems, whereas others tend to switch on pro-apoptotic systems; these all are a part of local signaling. Thirdly, stressed endothelial cells broadcast distress signals systemically; these signals can be directed to restoration of homeostasis or toward aggravation of the original insult. Sorting out whether any particular signal belongs to one or another category may pose significant problems. Hence, despite the sarcastic pronouncement by Sir Peter Medawar on the attractiveness of “cry havoc” message, there are intriguing and as yet poorly explored gradations that may bring help or exacerbate destruction.
Acknowledgements
Studies in the authors’ laboratories were supported in part by NIH grants DK54602, DK052783 and DK45462 (MSG), Westchester Artificial Kidney Foundation (MSG) and the start-up found from the Department of Pathology, The Ohio State University (SVB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LIST OF REFERENCES
- 1.Aird WC. Endothelial cell heterogeneity. Crit Care Med. 2003;31(4 Suppl):S221–S230. doi: 10.1097/01.CCM.0000057847.32590.C1. [DOI] [PubMed] [Google Scholar]
- 2.Bonsib SM. Heptinstall' Pathology of the Kidney by J. Charles Jennette, Jean L Olson, Melvin M Schwartz, and Fred G Silva. Sixth Edition Edition. Lippincott Williams & Wilkins; 2006. Renal Anatomy and histology; pp. 1–70. [Google Scholar]
- 3.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 4.Peters K, Unger R, Brunner J, Kirkpatrick J. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003;60:49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- 5.Thomas S, Witting P, Drummond G. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxidants Redox Signaling. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 6.Kim BS, Chen J, Weinstein T, Noiri E, Goligorsky MS. VEGF expression in hypoxia and hyperglycemia: reciprocal effect on branching angiogenesis in epithelial-endothelial co-cultures. J Am Soc Nephrol. 2002;13(8):2027–2036. doi: 10.1097/01.asn.0000024436.00520.d8. [DOI] [PubMed] [Google Scholar]
- 7.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fass C, Betscholtz C. Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 8.Zhuang J, Deane J, Yang R, Li J, Ricardo S. SCUBE-1, a novel developmental gene involved in renal regeneration and repair. Nephrol Dial Transplant. 2010;25:1421–1428. doi: 10.1093/ndt/gfp637. [DOI] [PubMed] [Google Scholar]
- 9.Nishi T, Bond C, Jr, Brown G, Solez K, Heptinstall RH. A morphometric study of arterial intimal thickening in kidneys of dialyzed patients. Am J Pathol. 1979;95(3):597–610. [PMC free article] [PubMed] [Google Scholar]
- 10.Pittner J, Wolgast M, Casellas D, Persson AE. Increased shear stress-released NO and decreased endothelial calcium in rat isolated perfused juxtamedullary nephrons. Kidney Int. 2005;67(1):227–236. doi: 10.1111/j.1523-1755.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 11.Feldman JD, Mardiney MR, Unanue ER, Cutting H. The vascular pathology of thrombotic thrombocytopenic purpura. An immunohistochemical and ultrastructural study. Lab Invest. 1966;15(6):927–946. [PubMed] [Google Scholar]
- 12.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10(10):2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 13.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010 Jan;77(1):9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 14.Gems D, Partridge L. Stress-response hormesis and aging: "that which does not kill us makes us stronger". Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Goligorsky MS. The concept of cellular “fight-or-flight” reaction to stress. Am J Physiol: Renal. 2001;280:F551–F561. doi: 10.1152/ajprenal.2001.280.4.F551. [DOI] [PubMed] [Google Scholar]
- 16.Grembowicz K, Sprague D, McNeil P. Temporary disruption of the plasma membrane is required for c-fos expression in response to mechanical stress. Mol Biol Cell. 1999;10:1247–1257. doi: 10.1091/mbc.10.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wooley K, Martin P. Conserved mechanisms of repair: from damaged single cells to wounds in multicellular tissues. BioEssays. 2000;22:911–919. doi: 10.1002/1521-1878(200010)22:10<911::AID-BIES6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 18.McNeil P, Muthukrishnan L, Warder E, D’Amore P. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989;109:811–822. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubyak G, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 20.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 23.Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X, Wan S, Lyu YL, Liu LF, Qi H. Etoposide induces ATM-dependent mitochondrial biogenesis through AMPK activation. PLoS One. 2008;3:e2009. doi: 10.1371/journal.pone.0002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisslthaller B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 26.Dickhout J, Krepinsky J. Endoplasmic reticulum stress and renal disease. Antioxidants Redox Signaling. 2009;11:2341–2352. doi: 10.1089/ars.2009.2705. [DOI] [PubMed] [Google Scholar]
- 27.Ott M, Zhivotovsky B, Orrenius S. Role of cardiolipin in cytochrome c release from mitochondria. Cell Death Differ. 2007;14:1243–1247. doi: 10.1038/sj.cdd.4402135. [DOI] [PubMed] [Google Scholar]
- 28.Simons D, Grieb G, Hristov M, Pallua N, Weber C, Bernhagen J, Steffens G. Hypoxia-induced endothelial secretion of macrophage migration inhibitory factor and role in endothelial progenitor cell recruitment. J Cell Mol Med. 2010 Feb 22; doi: 10.1111/j.1582-4934.2010.01041.x. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill M, Dias S, Hattori K, Rivera M, Hicklin D, Witte L, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+) AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 30.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 31.Ceradini D, Kulkarni A, Callaghan M, Tepper O, Bastidas N, Kleinman M, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 32.Kuo MC, Patschan D, Patschan S, Cohen-Gould L, Park HC, Ni J, Addabbo F, Goligorsky MS. Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. J Am Soc Nephrol. 2008;19(12):2321–2330. doi: 10.1681/ASN.2007111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hristov M, Erl W, Linder S, Weber P. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104:2761–2766. doi: 10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- 34.Deregibus M, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell-derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 35.Kon V, Yoshioka T, Fogo A, Ichikawa I. Glomerular actions of endothelin in vivo. J Clin Invest. 1989;83(5):1762–1767. doi: 10.1172/JCI114079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsden PA, Brenner BM. Nitric oxide and endothelins - novel autocrine paracrine regulators of the circulation. Seminars in Nephrology. 1991;11(2):169–185. [PubMed] [Google Scholar]
- 37.Kon V, Sugiura M, Inagami T, Harvie BR, Ichikawa I, Hoover RL. Role of endothelin in cyclosporine-induced glomerular dysfunction. Kidney Int. 1990;37(6):1487–1491. doi: 10.1038/ki.1990.139. [DOI] [PubMed] [Google Scholar]
- 38.Iijima K, Lin L, Nasjletti A, Goligorsky MS. Intracellular ramification of the endothelin signal. Am.J.Physiol, Cell. 1991;260:C982–C992. doi: 10.1152/ajpcell.1991.260.5.C982. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Liu H, McKenzie G, Witting P, Stasch J, Hahn M, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16:279–287. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 41.Hanai J, Dhanabal M, Karumanchi A, Albanese C, Waterman M, Chan B, et al. Endostatin causes G1 arrest of endothelial cells through inhibition of cyclin D1. J Biol Chem. 2002;277:16464–16469. doi: 10.1074/jbc.M112274200. [DOI] [PubMed] [Google Scholar]
- 42.Paddenberg R, Faulhammer P, Goldenberg A, Kummer W. Hypoxia-induced increase of endostatin in murine aorta and lung. Histochem Cell Biol. 2006;125:497–508. doi: 10.1007/s00418-006-0158-5. [DOI] [PubMed] [Google Scholar]
- 43.O’Riordan E, Mendelev N, Patschan S, Chander P, Goligorsky MS. Chronic NOS inhibition actuates endothelial-mesenchymal transformation. Am J Physiol. 2007;292:H285–H294. doi: 10.1152/ajpheart.00560.2006. [DOI] [PubMed] [Google Scholar]
- 44.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281(5):F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 45.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97(4):1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racusen LC, Fivush BA, Li YL, Slatnik I, Solez K. Dissociation of tubular cell detachment and tubular cell death in clinical and experimental "acute tubular necrosis". Lab Invest. 1991;64(4):546–556. [PubMed] [Google Scholar]
- 47.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66(2):480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 48.Goligorsky MS, Patschan D, Kuo C, Park H-C, Hochegger K, Rosenkranz A, Brady H, Mayadas T. Cell Adhesion Molecules in Renal Injury. In: Schnellmann R, editor. Comprehensive Toxicology. vol 7. Elsevier Ltd.; 2010. pp. 813–845. [Google Scholar]
- 49.Romanov V, Noiri E, Czerwinski G, Finsinger D, Kessler H, Goligorsky MS. Two novel probes reveal tubular and vascular Arg-Gly-Asp (RGD) binding sites in the ischemic rat kidney. Kidney Int. 1997;52(1):93–102. doi: 10.1038/ki.1997.308. [DOI] [PubMed] [Google Scholar]
- 50.Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol Renal Physiol. 2002;282(6):F1140–F1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- 51.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285(2):F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 52.Noiri E, Peresleni T, Miller F, Goligorsky MS. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest. 1996;97(10):2377–2383. doi: 10.1172/JCI118681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Chen A, De Leon D, Li H, Noiri E, Moy VT, Goligorsky MS. Atomic force microscopy measurement of leukocyte-endothelial interaction. Am J Physiol Heart Circ Physiol. 2004;286(1):H359–H367. doi: 10.1152/ajpheart.00491.2003. [DOI] [PubMed] [Google Scholar]
- 54.Elitok S, Brodsky SV, Patschan D, Orlova T, Lerea KM, Chander P, Goligorsky MS. Cyclic arginine-glycine-aspartic acid peptide inhibits macrophage infiltration of the kidney and carotid artery lesions in apo-E-deficient mice. Am J Physiol Renal Physiol. 2006;290(1):F159–F166. doi: 10.1152/ajprenal.00227.2005. [DOI] [PubMed] [Google Scholar]
- 55.Bonventre JV. Pathophysiology of AKI: injury and normal and abnormal repair. Contrib Nephrol. 2010;165:9–17. doi: 10.1159/000313738. [DOI] [PubMed] [Google Scholar]
- 56.Noiri E, Nakao EA, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Scavenging of peroxynitrite ameliorates lipid peroxidation and DNA damage in experimental ischemic acute renal failure. Am J Physiol: Renal. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 57.Ling H, Edelstein C, Gengaro P, Meng X, Lucia S, Knotek M, Wangsiripaisan A, Shi Y, Schrier R. Attenuation of renal ischemia-reperfusion injury in inducible nitric oxide synthase knockout mice. Am J Physiol. 1999;277:F383–F390. doi: 10.1152/ajprenal.1999.277.3.F383. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T, Tada T, Brodsky SV, Tanaka H, Noiri E, Kajiya F, Goligorsky MS. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am J Physiol Renal Physiol. 2002;282(6):F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- 59.Arriero M, Brodsky SV, Gealekman O, Lucas PA, Goligorsky MS. Adult skeletal muscle stem cells differentiate into endothelial lineage and ameliorate renal dysfunction after acute ischemia. Am J Physiol Renal Physiol. 2004 Oct;287(4):F621–F627. doi: 10.1152/ajprenal.00126.2004. [DOI] [PubMed] [Google Scholar]
- 60.Kwon O, Hong SM, Sutton TA, Temm CJ. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol. 2008;295(2):F351–F359. doi: 10.1152/ajprenal.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281(5):F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 62.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58(2):658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 63.Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;65:1714–1723. doi: 10.1111/j.1523-1755.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 64.Sano M, Fukuda K. Activation of mitochondrial biogenesis by hormesis. Circ Res. 2008;103:1191–1193. doi: 10.1161/CIRCRESAHA.108.189092. [DOI] [PubMed] [Google Scholar]
- 65.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, et al. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]