Abstract
Tissue injury is associated with sensitization of nociceptors and subsequent changes in the excitability of central (spinal) neurons, termed central sensitization. Nociceptor sensitization and central sensitization are considered to underlie, respectively, development of primary hyperalgesia and secondary hyperalgesia. Because central sensitization is considered to reflect plasticity at spinal synapses, the spinal cord has been the principal focus of studies of mechanisms of hyperalgesia. Not surprisingly, glutamate, acting at a spinal N-methyl-d-aspartate (NMDA) receptor, has been implicated in development of secondary hyperalgesia associated with somatic, neural, and visceral structures. Downstream of NMDA receptor activation, spinal nitric oxide (NO⋅), protein kinase C, and other mediators have been implicated in maintaining such hyperalgesia. Accumulating evidence, however, reveals a significant contribution of supraspinal influences to development and maintenance of hyperalgesia. Spinal cord transection prevents development of secondary, but not primary, mechanical and/or thermal hyperalgesia after topical mustard oil application, carrageenan inflammation, or nerve-root ligation. Similarly, inactivation of the rostral ventromedial medulla (RVM) attenuates hyperalgesia and central sensitization in several models of persistent pain. Inhibition of medullary NMDA receptors or NO⋅ generation attenuates somatic and visceral hyperalgesia. In support, topical mustard oil application or colonic inflammation increases expression of NO⋅ synthase in the RVM. These data suggest a prominent role for the RVM in mediating the sensitization of spinal neurons and development of secondary hyperalgesia. Results to date suggest that peripheral injury and persistent input engage spinobulbospinal mechanisms that may be the prepotent contributors to central sensitization and development of secondary hyperalgesia.
Hardy et al. (1) investigated two types of experimentally produced cutaneous hyperalgesia, primary and secondary. Primary hyperalgesia occurs at the site of injury; secondary hyperalgesia is associated with the injury, but occurs in “undamaged tissues adjacent to and at some distance from the site of an injury.” They proposed a “new formulation” to explain the spread of hyperalgesia away from the site of injury, namely that a central (spinal) excitatory state, and not a peripheral mechanism as advanced by Lewis (2), was responsible for secondary hyperalgesia. Subsequent intensive study of the altered sensations that arise from and adjacent to injured tissues has supported this “formulation” and it is now widely accepted that mechanisms of primary and secondary hyperalgesia are, respectively, peripheral and central (e.g., see refs. 3, 4).
The increase in excitability of spinal neurons after peripheral injury, termed central sensitization, has been extensively studied by Woolf and colleagues (see ref. 5 for overview). They documented that the enhanced reflex excitability after peripheral tissue damage did not require ongoing peripheral input, and that spinal dorsal horn neuron receptive fields expanded, responsiveness to suprathreshold stimuli increased, response thresholds decreased, and sensitivity to novel stimuli was acquired after peripheral injury. The focus of investigation has remained the spinal cord, and many investigators have since documented the importance of the spinal N-methyl-d-aspartate (NMDA) receptor to the induction and maintenance of central sensitization (see ref. 6 for recent overview). A growing body of evidence, however, reveals a significant contribution of descending influences from supraspinal sites in the development and maintenance of central sensitization/secondary hyperalgesia. We review here and discuss evidence that peripheral tissue injury engages spinobulbospinal circuitry that may be important to the development and maintenance of central sensitization and secondary hyperalgesia.
Descending Facilitation.
Although the potency of descending inhibitory influences has long been appreciated, the study and characterization of descending facilitatory influences have been more recent developments. Interestingly, inhibitory and facilitatory influences can be produced at many of the same sites in the brainstem, particularly in the rostral ventromedial medulla (RVM). Generally, low intensities of electrical stimulation or low concentrations of chemical (e.g., glutamate, neurotensin) facilitate spinal nociception, whereas greater intensities of stimulation or concentrations of chemical at the same sites typically inhibit spinal nociception (7–10). These dual influences appear to involve anatomically distinct independent spinal pathways and are mediated by different lumbar spinal receptors. For example, high-intensity electrical stimulation or high-dose glutamate or neurotensin injection into the RVM inhibits spinal nociceptive transmission via descending projections in the dorsolateral funiculi and activation of spinal cholinergic and monoaminergic receptors. In contrast, facilitatory influences from the RVM produced by electrical stimulation, glutamate injection, or neurotensin injection involve descending projections in the ventrolateral funiculi and are mediated by spinal serotonin and cholecystokinin receptors. (7, 9, 11–13).
In addition to the RVM, adjacent medullary sites also have been implicated in descending facilitation of spinal nociceptive transmission. Electrical and/or selective chemical stimulation in these areas have been shown to enhance spinal behavioral and dorsal horn neuron responses to noxious stimulation (14).
Fields et al. (15) have characterized cells in the RVM that may constitute the physiological basis for generation of bidirectional modulation of spinal nociceptive transmission. They have operationally defined three classes of neurons in the RVM: on-cells, off-cells, and neutral cells, which are intermixed in the RVM and not anatomically separable. Off-cells display an abrupt pause in ongoing activity immediately before nociceptive reflexes and are proposed to contribute to inhibitory influences that descend from the RVM. On-cells display a burst of activity immediately before nociceptive reflexes and are proposed to contribute to facilitatory influences that descend from the RVM. Neutral cells show no nociception-related change in activity. Off-cells, on-cells, and neutral cells all project to the spinal dorsal horn (16), placing on-cell and off-cell terminals in appropriate laminae (I, II, and V) to modulate nociceptive transmission. That on- and off-cells mediate descending facilitatory and inhibitory influences from the RVM is supported by several reports demonstrating enhanced on- or off-cell activity during facilitation or inhibition of spinal nociceptive transmission, respectively (17–19).
We hypothesize that there exists a spinobulbospinal circuit that contributes significantly to central sensitization and secondary hyperalgesia. Anatomically, this circuit is in place. Both the RVM and adjacent areas receive direct afferent input from the superficial spinal dorsal horn and in turn send descending projections through spinal funiculi that terminate in the superficial dorsal horn, completing a spinobulbospinal loop (20–23). We review below recent studies that document that spinal transection, or inactivation of supraspinal sites, prevents the expression of secondary hyperalgesia in a variety of animal models of persistent inflammatory, neurogenic, or neuropathic pain, thus providing the functional context in support of the anatomy (see Table 1).
Table 1.
Summary of supraspinal contributions to hyperalgesia
| Model of hyperalgesia | Nociceptive response | Manipulation | Effect | Ref. |
|---|---|---|---|---|
| Inflammation/neurogenic | ||||
| Mustard oil | Tactile allodynia, foot | Spinal transection | Block | 26 |
| (ankle) | Intra-RVM lidocaine | |||
| Mustard oil | Enhanced excitability of | Spinal transection | Block | 27 |
| (foot, outside receptive field) | WDR dorsal horn neurons | Intra-RVM lidocaine | ||
| Mustard oil | Facilitation of the thermal | Spinal transection | Block | 28, 29 |
| (leg) | tail-flick reflex | Electrolytic RVM lesion | ||
| Ibotenic acid RVM lesion | ||||
| Carrageenan | Enhanced C-fiber-mediated | Spinal transection | Block | 37 |
| (knee joint) | flexor motoneuron wind-up | |||
| Carrageenan | Facilitation of the thermal | Intra-RVM lidocaine | Block | 29 |
| (knee joint) | paw-withdrawal response | Ibotenic acid RVM lesion | ||
| Carrageenan | Facilitation of the thermal | Intra-RVM lidocaine | No effect | 29 |
| (plantar foot) | paw-withdrawal response | Ibotenic acid RVM lesion | ||
| Formalin | Facilitation of the thermal | Spinal transection | block | 41, 42 |
| (foot) | tail-flick reflex | Electrolytic RVM lesion | ||
| Neuropathic | ||||
| Spinal nerve ligation | Tactile allodynia, foot | Intra-RVM lidocaine | Block | 44 |
| Spinal nerve ligation | Tactile allodynia, foot | Spinal transection | Block | 45 |
| Facilitation of the thermal | ||||
| paw-withdrawal response | ||||
| Spinal nerve cut | Tactile allodynia, foot | Spinal transection | Block | 46 |
| Illness | ||||
| LPS | Facilitation of the thermal | Electrolytic RVM lesion | Block | 42, 50 |
| (intraperitoneal) | tail-flick reflex | Electrolytic NTS lesion |
Inflammatory/Neurogenic Models of Hyperalgesia.
Mustard oil. Mustard oil (allyl isothiocyanate) is a chemical irritant that produces a neurogenic inflammation and excites chemosensitive C-fibers, resulting in behavioral hyperalgesia and central sensitization (24, 25). An involvement of supraspinal sites in mustard oil-induced sensitization was reported by Mansikka and Pertovaara (26), who found that tactile allodynia of the glabrous skin of the foot after topical application of mustard oil to the ankle was prevented in animals that had received spinal transection. Additionally, in spinally intact rats, the tactile allodynia was blocked after inactivation of the medial RVM by local lidocaine microinjection. The authors concluded that persistent nociceptor stimulation by topical mustard oil activates a positive feedback loop involving descending facilitatory influences from the RVM. In an electrophysiological study of spinal cord neurons, Pertovaara (27) subsequently reported that midthoracic spinal transection or lidocaine inactivation of the RVM blocked mustard oil-induced enhanced excitability of wide dynamic range neurons to mechanical stimulation. In these experiments, mustard oil was applied 1–2 cm outside the border of the receptive field of the spinal neuron. Thus, in both studies, the allodynia/hyperalgesia was tested at a site distant from the site of application of mustard oil (i.e., it was secondary in nature).
In related studies, we documented a significant contribution of descending facilitatory influences in a model of thermal hyperalgesia involving topical application of mustard oil to the hind leg and measurement of the spinal nociceptive tail-flick reflex (28). It was found that midthoracic spinal transection or electrolytic lesion of the RVM prevented facilitation of the tail-flick reflex produced by mustard oil. To confirm an involvement of cells in the RVM in modulating this secondary thermal hyperalgesia, we found that RVM lesion using the soma-selective neurotoxin ibotenic acid resulted in a similar block of mustard oil-induced hyperalgesia (29).
Active participation of descending facilitatory influences from the RVM in modulating mustard oil-induced hyperalgesia is supported further by evidence that NMDA and neurotensin receptors in the RVM modulate this secondary thermal hyperalgesia. As indicated above, neurotensin receptors (7, 8) and NMDA receptors (30, 31) in the RVM have been implicated in descending facilitation of spinal nociception. Selective blockage of these receptors should then modulate hyperalgesia. Indeed, intra-RVM injection of a selective neurotensin receptor antagonist (SR48692) or NMDA receptor antagonist [2-amino-5-phosphonovaleric acid (APV)] fully and dose dependently prevented mustard oil-induced facilitation of the tail-flick reflex (28, 30). It is known that generation of nitric oxide (NO⋅) is one downstream consequence of NMDA receptor activation (32). In complementary studies, we showed that intra-RVM administration of the NO⋅-synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME), like the NMDA receptor antagonist APV, attenuated mustard oil-induced hyperalgesia (30). Conversely, microinjection of the NO⋅ donor GEA 5024 (or of NMDA itself) dose dependently facilitated the tail-flick reflex in naïve rats. The involvement of NO⋅ in the RVM was further supported by a significant increase in the number of NADPH–diaphorase-labeled cells at the time of maximal mustard oil-induced hyperalgesia. Finally, in a model of visceral hyperalgesia in which the inflammogen zymosan is instilled into the colon, both APV and l-NAME given into the RVM 3 hr after colonic inflammation reversed the hyperalgesia for the duration of drug action, suggesting that the RVM plays a role in maintenance of the hyperalgesia (31). Similar to what was seen in the model of mustard oil hyperalgesia, both NADPH–diaphorase-labeled cell numbers and the number of cells immunostained for the neuronal isoform of NO⋅-synthase were significantly increased in the RVM 3 hr after colonic inflammation. These results support a role for descending facilitatory influences in the maintenance of mustard oil-induced and visceral hyperalgesia involving activation of NMDA and neurotensin receptors in the RVM.
Carrageenan.
Several models of hyperalgesia involving subcutaneous injection of carrageenan have been characterized. Carrageenan is a water-extractable polysaccharide obtained from various seaweeds. Injection of lambda carrageenan (a hydrocolloid that does not form a gel) into the plantar foot, or intraarticular injection into the knee joint, results in a localized inflammation, decreased weight bearing, guarding of the affected limb, and hyperalgesia (e.g., refs. 33 and 34). Carrageenan-induced hyperalgesia is believed to occur as a consequence of sensitization of primary afferent nociceptors and neuron plasticity intrinsic to the spinal cord (35, 36).
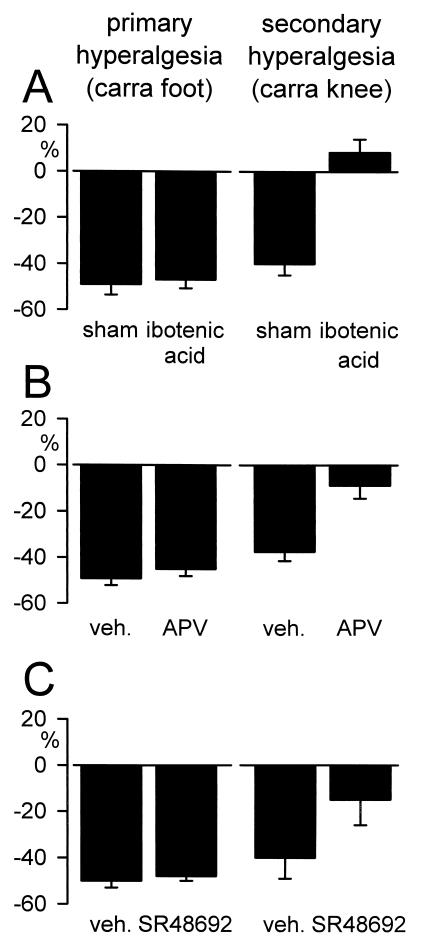
Herrero and Cervero (37) first reported that the A- and C-fiber mediated wind-up of flexor motoneurons after intraarticular (knee) carrageenan injection was prevented by spinal transection. They concluded that supraspinal modulatory systems, either direct excitatory influences on spinal neurons or release of local inhibitory controls, are essential for wind-up. We examined a potential contribution of descending facilitatory influences from the RVM to enhanced behavioral nociceptive responses after intraplantar or intraarticular (knee) injection of carrageenan (29). Intraplantar injection of carrageenan and subsequent thermal stimulation of the plantar surface of the hindpaw is a model of primary hyperalgesia; intraarticular injection of carrageenan and subsequent thermal stimulation of the plantar surface of the hindpaw is a model of secondary hyperalgesia. Inactivation of the RVM by lidocaine microinjection reversed, and prior permanent inactivation of the RVM by ibotenic acid lesion completely blocked, facilitation of the thermal paw-withdrawal response after intraarticular carrageenan injection. RVM inactivation by either lidocaine or ibotenic acid was ineffective, however, in preventing thermal hyperalgesia after intraplantar carrageenan injection (i.e., model of primary hyperalgesia). These results suggest that these two models of carrageenan-induced thermal hyperalgesia are differentially modulated in the central nervous system. Additionally, similar to mustard oil-induced secondary hyperalgesia, intra-RVM injection of a selective neurotensin receptor antagonist (SR48692) or NMDA receptor antagonist (APV) was found to block facilitation of the thermal paw-withdrawal response after intraarticular, but not intraplantar, carrageenan injection (Fig. 1). These results further support a contribution of descending facilitatory influences to secondary hyperalgesia that is mediated by neurotensin and NMDA receptors in the RVM.
Figure 1.
Involvement of descending facilitatory influences from the RVM in models of secondary, but not primary, thermal hyperalgesia after peripheral inflammation. (A) RVM lesion produced by ibotenic acid prevented facilitation of the thermal paw-withdrawal response after intraarticular carrageenan/kaolin injection into the knee (t test, P < 0.05), but was ineffective in preventing facilitation of the thermal paw-withdrawal response after intraplantar carrageenan injection into the foot (model of primary hyperalgesia). (B) Intra-RVM microinjection of the NMDA receptor antagonist APV (1 pmol/1 μl), or (C) intra-RVM microinjection of the neurotensin receptor antagonist SR48692 (3 nmol/1 μl) attenuated secondary, but not primary, hyperalgesia (t test, P < 0.05). All data are represented as mean ± SEM of the percent change in thermal paw-withdrawal latency (%) from the control response for the ipsilateral (inflamed) hindlimb. In experiments involving ibotenic acid RVM lesion, responses are represented at the time of maximal hyperalgesia (3 hr after carrageenan injection). Intra-RVM microinjection of APV or SR48692 was performed at the time of maximal hyperalgesia (3 hr), and responses are represented at the time of maximal drug effect after intra-RVM injection (10 min).
Formalin.
Subcutaneous injection of formalin into the dorsum of the rodent hindpaw is a well characterized model in which animals exhibit spontaneous pain behaviors (shaking, licking of the injected hindpaw) as well as hyperalgesia (38, 39). Additionally, formalin has been shown to produce secondary hyperalgesia after subcutaneous injection into either the hindpaw or tail (40, 41). A significant contribution of supraspinal sites to formalin-produced secondary hyperalgesia was reported by Wiertelak et al. (41), who found that spinal transection prevented facilitation of the tail-flick reflex after formalin injection into the hindpaw. That activation of descending facilitatory influences from the RVM modulates this hyperalgesia was subsequently supported by the finding that electrolytic lesion of the RVM prevented facilitation of the tail-flick reflex after formalin injection (42).
Neuropathic Models of Hyperalgesia.
Animal models of neuropathic pain generally involve loose ligation of peripheral nerves, which results in spontaneous pain behaviors, enhanced responses of spinal-dorsal horn nociceptive neurons, and hyperalgesia (for review, see ref. 43). A contribution of supraspinal sites to neuropathic pain after spinal nerve ligation was initially reported by Pertovaara et al. (44). In that study, the tactile allodynia that develops after unilateral ligation of the L5 and L6 spinal nerves was found to be attenuated by inactivation of the RVM by lidocaine injection. The lidocaine effect was determined to be localized within the RVM and independent of an opioid mechanism, suggesting an inactivation of a descending facilitatory influence from the RVM. These results were supported in a subsequent study (45), in which spinal transection was found to abolish the tactile allodynia as well as thermal hyperalgesia produced by ligation of the L5 and L6 spinal nerves. Additionally, Kauppila (46) found spinal transection to block mechanical hyperalgesia observed after a chronic sciatic nerve cut. Thus, neuropathic pain after peripheral nerve injury appears to involve, at least in part, activation of descending facilitatory influences from supraspinal sites, including the RVM. ÷ropcapli9
Illness-Induced Models of Hyperalgesia.
The systemic administration of lipopolysaccharide (LPS) has been shown to produce a number of symptoms associated with illness, such as fever, lethargy, decreased food and water intake, and increased sleep (for review, see ref. 47). Additionally, administration of LPS produces hyperalgesia through the release of peripheral cytokines (e.g., IL-1β) from immune cells (48, 49). In a series of experiments, Watkins et al. (49, 50) determined that facilitation of the tail-flick reflex after intraperitoneal injection of LPS does not involve primary afferent nociceptor input to the spinal dorsal horn. Instead, a novel circuit was proposed involving IL-1β activation of hepatic vagal afferent fibers that terminate in the nucleus tractus solitarius (NTS). Consistent with this proposal, electrolytic lesion of the NTS or RVM was found to block facilitation of the tail-flick reflex produced by intraperitoneal LPS. Because the NTS and RVM are reciprocally connected, direct afferent input to the RVM may mediate this effect, although Watkins et al. (50) implicated an unidentified site rostral to the midmesencephalon as an important relay. This interpretation is consistent with earlier studies of biphasic effects of electrical stimulation of vagal afferent fibers (see ref. 51 for review). In those experiments, low-intensity stimulation of vagal afferent fibers was documented to facilitate spinal nociceptive reflexes (tail-flick reflex) and spinal dorsal horn neuron responses to noxious stimuli. The facilitatory effect of vagal stimulation was abolished after midcollicular decerebration, implicating an NTS–forebrain circuit in descending influences that ultimately exit the brainstem via the RVM. Although the tail-flick reflex is a spinally organized response, facilitation of this reflex after intraperitoneal LPS similarly appears to involve activation of descending facilitatory influences from the RVM.
Primary vs. Secondary Hyperalgesia.
We and others have studied the effects of spinal cord transection and of reversible (lidocaine) or permanent (ibotenic acid) inactivation of the RVM in models of primary and secondary hyperalgesia after peripheral tissue insult. The results reviewed above uniformly support the hypothesis that facilitatory influences from the brainstem significantly contribute to secondary, but not primary, hyperalgesia. What has not yet been addressed specifically is whether the RVM is necessary and sufficient for development or for maintenance of secondary hyperalgesia. Intra-RVM injection of lidocaine reverses, in a time-limited fashion, already established secondary hyperalgesia, suggesting a clear role for the RVM in maintenance of secondary hyperalgesia. Other studies reveal that spinal-cord transection or soma-selective lesion of the RVM prevents development of secondary, but not primary, hyperalgesia. Accordingly, available evidence suggests that the RVM is important to both the development and maintenance of secondary hyperalgesia. The studies reviewed here all have examined behavioral consequences of peripheral tissue insult, and there are limited data available yet with respect to the direct influence of the RVM on spinal neuron plasticity (central sensitization).
Two studies have examined changes in spinal neuron behavior associated with peripheral tissue insult. Schaible et al. (52) examined, in the cat, the effect of acute inflammation of the knee joint with a mixture of kaolin and carrageenan on spinal dorsal horn neurons. They documented that spontaneous activity and responses to both innocuous and noxious stimulation of the joint were increased progressively as the inflammation progressed. Neuron activity and responses to stimulation were increased further when spinal cord transmission was interrupted temporarily by cold block of the lower thoracic spinal cord. They concluded that spinal neuron hyperexcitability associated with a peripheral inflammation was counteracted by enhancement of descending inhibitory influences. Ren and Dubner (53) studied, in the rat, the effect of lidocaine injection into the midline RVM on spinal neuron responses to stimulation of a hindpaw inflamed with complete Freund’s adjuvant. During the action of lidocaine, neuron spontaneous activity and responses to mechanical and thermal stimulation applied to the hindpaw were significantly increased, which was interpreted to indicate that peripheral inflammation leads to an enhanced descending inhibition. Both of these studies used models of primary hyperalgesia (stimuli were applied to the injured tissue). Both also noted, however, an increase in the size of neuron receptive fields, usually taken as an indication of secondary hyperalgesia. Although neither report directly addresses the hypothesis advanced here, both contribute relevant information. Both document an active modulation by the brainstem of spinal neuron excitability in the presence of tissue injury, confirming activation by peripheral noxious inputs of descending inhibition that can modulate further spinal nociceptive transmission.
The generality of the present hypothesis remains to be established. Most of the studies done to date have examined secondary thermal hyperalgesia. Thermal hyperalgesia is widely used in studies with nonhuman animals, but secondary thermal hyperalgesia is not of significant consequence in most instances of tissue injury in humans. The extent to which secondary mechanical hyperalgesia is modulated by the RVM is unclear. The limited data available to date relate to tactile allodynia and mechanical hyperalgesia in models of neuropathic pain. Additional studies that use other models of hyperalgesia are necessary. Models of chemically produced hyperalgesia, which may involve more selective actions on different types of nociceptors, have not been studied extensively. Secondary thermal hyperalgesia produced by topical application of the C-fiber excitant mustard oil has been documented by several investigators to be influenced by the RVM. Whether secondary hyperalgesia produced by intradermal injection of capsaicin, which acts at the vanilloid-1 receptor, is similarly modulated by the RVM has not been reported.
It is also unknown how blockage of central sensitization at the level of the spinal cord (by antagonism of the NMDA receptor, for example) influences the RVM. It may be that the spinal cord and RVM are both necessary and sufficient to development and maintenance of secondary hyperalgesia. Results reviewed here clearly indicate that central sensitization at the level of the spinal cord can be modulated by the RVM, even if the spinal cord is the portal of first entry of the relevant input. Temporally, input to the spinal cord likely precedes receipt of similar input in the brain stem, but it may be that other avenues of input (e.g., via the vagus) provide an important (more important?) trigger for the RVM.
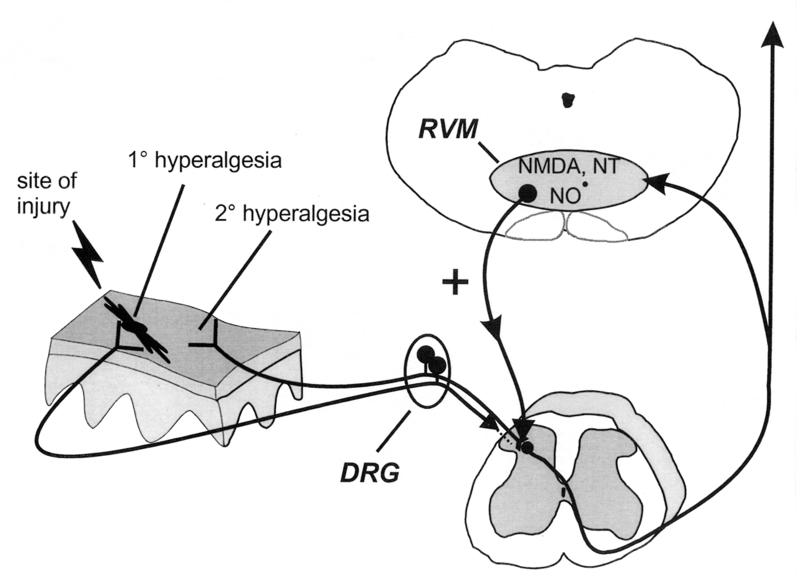
Returning to the formulation advanced almost 50 years ago by Hardy et al. (1), we believe that a dominant active influence from the brainstem is necessary for the expression of secondary hyperalgesia (see Fig. 2). We acknowledge that there are likely multiple supraspinal sites involved in responding to peripheral tissue insult. Indeed, the limited data available suggest that forebrain sites can play an important role, even if the RVM is the final common pathway of facilitatory influences that mediate spinal neuron excitability.
Figure 2.
Summary diagram illustrating a significant supraspinal contribution to secondary, but not primary, thermal hyperalgesia after peripheral inflammation. Peripheral injury results in activation and sensitization of peripheral nociceptors and subsequent enhanced excitability of dorsal horn nociceptive neurons (central sensitization) that contributes to primary hyperalgesia (at site of injury) and secondary hyperalgesia (adjacent/distant from site of injury). Additionally, it is proposed that stimulation of nociceptors activates a spinobulbospinal loop, engaging a centrifugal descending nociceptive facilitatory influence from the RVM. Facilitatory influences are activated by NMDA receptors and NO⋅, and neurotensin (NT) receptors in the RVM and descend to multiple spinal segments to contribute significantly to secondary hyperalgesia. In contrast, primary hyperalgesia does not involve descending facilitatory influences from supraspinal sites and is likely the direct result of peripheral nociceptor sensitization and neuroplasticity intrinsic to the spinal cord. For clarity, the afferent input to the spinal dorsal horn from the site of injury is illustrated as not entering the spinal cord (which it certainly does).
Acknowledgments
This work was supported by National Institutes of Health awards DA11431 (M.O.U.), NS19912 (G.F.G.), and DA02879 (G.F.G.).
ABBREVIATIONS
- NMDA
N-methyl-d-aspartate
- RVM
rostral ventromedial medulla
- NO⋅
nitric oxide
- LPS
lipopolysaccharide
- NTS
nucleus tractus solitarius
- APV
2-amino-5-phosphonovaleric acid
References
- 1.Hardy J D, Wolff H G, Goodell H. J Clin Invest. 1950;29:115–140. doi: 10.1172/JCI102227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis T. Clin Sci. 1936;2:373–421. [Google Scholar]
- 3.Woolf C J. Nature (London) 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 4.LaMotte R H, Shain C N, Simone D A, Tsai E F P. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 5.Woolf C J. In: Hyperalgesia and Allodynia. Willis W, editor. New York: Raven; 1992. pp. 221–243. [Google Scholar]
- 6.Urban M O, Gebhart G F. Prog Brain Res. 1998;116:407–420. doi: 10.1016/s0079-6123(08)60452-5. [DOI] [PubMed] [Google Scholar]
- 7.Urban M O, Gebhart G F. J Neurophysiol. 1997;78:1550–1562. doi: 10.1152/jn.1997.78.3.1550. [DOI] [PubMed] [Google Scholar]
- 8.Urban M O, Smith D J. J Pharmacol Exp Ther. 1993;265:580–586. [PubMed] [Google Scholar]
- 9.Zhuo M, Gebhart G F. J Neurophysiol. 1992;67:1599–1614. doi: 10.1152/jn.1992.67.6.1599. [DOI] [PubMed] [Google Scholar]
- 10.Zhuo M, Gebhart G F. J Neurophysiol. 1997;78:746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]
- 11.Urban M O, Smith D J, Gebhart G F. J Pharmacol Exp Ther. 1996;278:90–96. [PubMed] [Google Scholar]
- 12.Zhuo M, Gebhart G F. Brain Res. 1990;535:67–78. doi: 10.1016/0006-8993(90)91825-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhuo M, Gebhart G F. Brain Res. 1991;550:35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]
- 14.Almeida A, Tjolsen A, Lima D, Coimbra A, Hole K. Brain Res Bull. 1996;39:7–15. doi: 10.1016/0361-9230(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 15.Fields H L, Bry J, Hentall I, Zorman G. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields H L, Malick A, Burstein R. J Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- 17.Fields H L, Vanegas H, Hentall I D, Zorman G. Nature (London) 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- 18.Bederson J B, Fields H L, Barbaro N M. Somatosens Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- 19.Morgan M M, Fields H L. J Neurophysiol. 1994;72:1161–1170. doi: 10.1152/jn.1994.72.3.1161. [DOI] [PubMed] [Google Scholar]
- 20.Almeida A, Tavares I, Lima D, Coimbra A. Neuroscience. 1993;55:1093–1106. doi: 10.1016/0306-4522(93)90323-8. [DOI] [PubMed] [Google Scholar]
- 21.Basbaum A I, Clanton C H, Fields H L. J Comp Neurol. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- 22.Craig A D. J Comp Neurol. 1995;361:225–248. doi: 10.1002/cne.903610204. [DOI] [PubMed] [Google Scholar]
- 23.Martin G F, Vertes R P, Waltzer R. Exp Brain Res. 1985;58:154–162. doi: 10.1007/BF00238963. [DOI] [PubMed] [Google Scholar]
- 24.Woolf C J, Wall P D. J Neurosci. 1986;6:1433–1442. doi: 10.1523/JNEUROSCI.06-05-01433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolf C J, Shortland P, Sivilotti L G. Pain. 1994;58:141–155. doi: 10.1016/0304-3959(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 26.Mansikka H, Pertovaara A. Brain Res Bull. 1997;42:359–365. doi: 10.1016/s0361-9230(96)00313-9. [DOI] [PubMed] [Google Scholar]
- 27.Pertovaara A. Exp Neurol. 1998;149:193–202. doi: 10.1006/exnr.1997.6688. [DOI] [PubMed] [Google Scholar]
- 28.Urban M O, Jiang M C, Gebhart G F. Brain Res. 1996;737:83–91. doi: 10.1016/0006-8993(96)00631-2. [DOI] [PubMed] [Google Scholar]
- 29.Urban M O, Zahn P K, Gebhart G F. Neuroscience. 1999;90:349–352. doi: 10.1016/s0306-4522(99)00002-0. [DOI] [PubMed] [Google Scholar]
- 30.Urban M O, Coutinho S V, Gebhart G F. Pain. 1999;81:45–55. doi: 10.1016/s0304-3959(98)00265-6. [DOI] [PubMed] [Google Scholar]
- 31.Coutinho S V, Urban M O, Gebhart G F. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- 32.Meller S T, Gebhart G F. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 33.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. Pain. 1998;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 34.Sluka K A, Westlund K N. Pain. 1993;55:367–377. doi: 10.1016/0304-3959(93)90013-F. [DOI] [PubMed] [Google Scholar]
- 35.Schaible H-G, Schmidt R F. J Neurophysiol. 1985;54:1109–1122. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 36.Schaible H-G, Schmidt R F, Willis W D. Exp Brain Res. 1987;66:466–489. doi: 10.1007/BF00270681. [DOI] [PubMed] [Google Scholar]
- 37.Herrero J F, Cervero F. Neurosci Lett. 1996;209:21–24. doi: 10.1016/0304-3940(96)12588-x. [DOI] [PubMed] [Google Scholar]
- 38.Coderre T J, Vaccarino A L, Melzack R. Brain Res. 1990;535:155–158. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- 39.Dubuisson D, Dennis S G. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 40.Bianchi M, Panerai A E. Neurosci Lett. 1997;237:89–92. doi: 10.1016/s0304-3940(97)00819-7. [DOI] [PubMed] [Google Scholar]
- 41.Wiertelak E P, Furness L E, Horan R, Martinez J, Maier S F, Watkins L R. Brain Res. 1994;649:19–26. doi: 10.1016/0006-8993(94)91044-8. [DOI] [PubMed] [Google Scholar]
- 42.Wiertelak E P, Roemer B, Maier S F, Watkins L R. Brain Res. 1997;748:143–150. doi: 10.1016/s0006-8993(96)01289-9. [DOI] [PubMed] [Google Scholar]
- 43.Bennett G J. Muscle Nerve. 1993;16:1040–1048. doi: 10.1002/mus.880161007. [DOI] [PubMed] [Google Scholar]
- 44.Pertovaara A, Wei H, Hamalainen M M. Neurosci Lett. 1996;218:127–130. doi: 10.1016/s0304-3940(96)13136-0. [DOI] [PubMed] [Google Scholar]
- 45.Bian D, Ossipov M H, Zhong C M, Malan T P, Porreca F. Neurosci Lett. 1998;241:79–82. doi: 10.1016/s0304-3940(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 46.Kauppila T. Brain Res. 1997;770:310–312. doi: 10.1016/s0006-8993(97)00904-9. [DOI] [PubMed] [Google Scholar]
- 47.Watkins L R, Maier S F, Goehler L E. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 48.Maier S F, Wiertelak E P, Martin D, Watkins L R. Brain Res. 1993;623:321–324. doi: 10.1016/0006-8993(93)91446-y. [DOI] [PubMed] [Google Scholar]
- 49.Watkins L R, Wiertelak E P, Goehler L E, Smith K P, Martin D, Maier S F. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 50.Watkins L R, Wiertelak E P, Goehler L E, Mooney-Heiberger K, Martinez J, Furness L, Smith K P, Maier S F. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- 51.Randich A, Gebhart G F. Brain Res Rev. 1992;17:77–99. doi: 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- 52.Schaible H-G, Neugebauer V, Cervero F, Schmidt R F. J Neurophysiol. 1991;66:1021–1032. doi: 10.1152/jn.1991.66.3.1021. [DOI] [PubMed] [Google Scholar]
- 53.Ren K, Dubner R. J Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]