Abstract
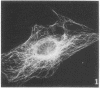
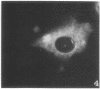
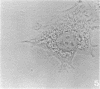
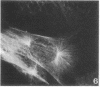
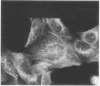
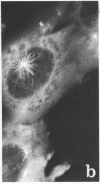
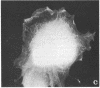
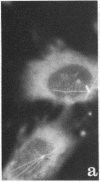
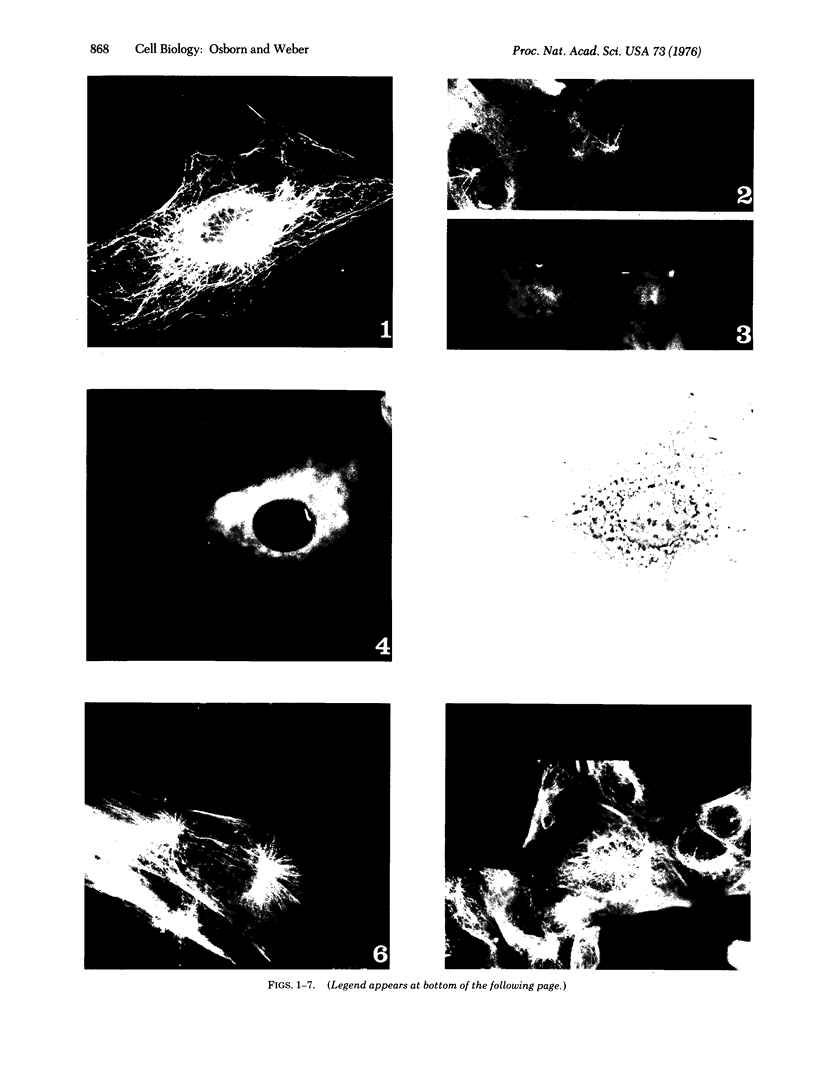
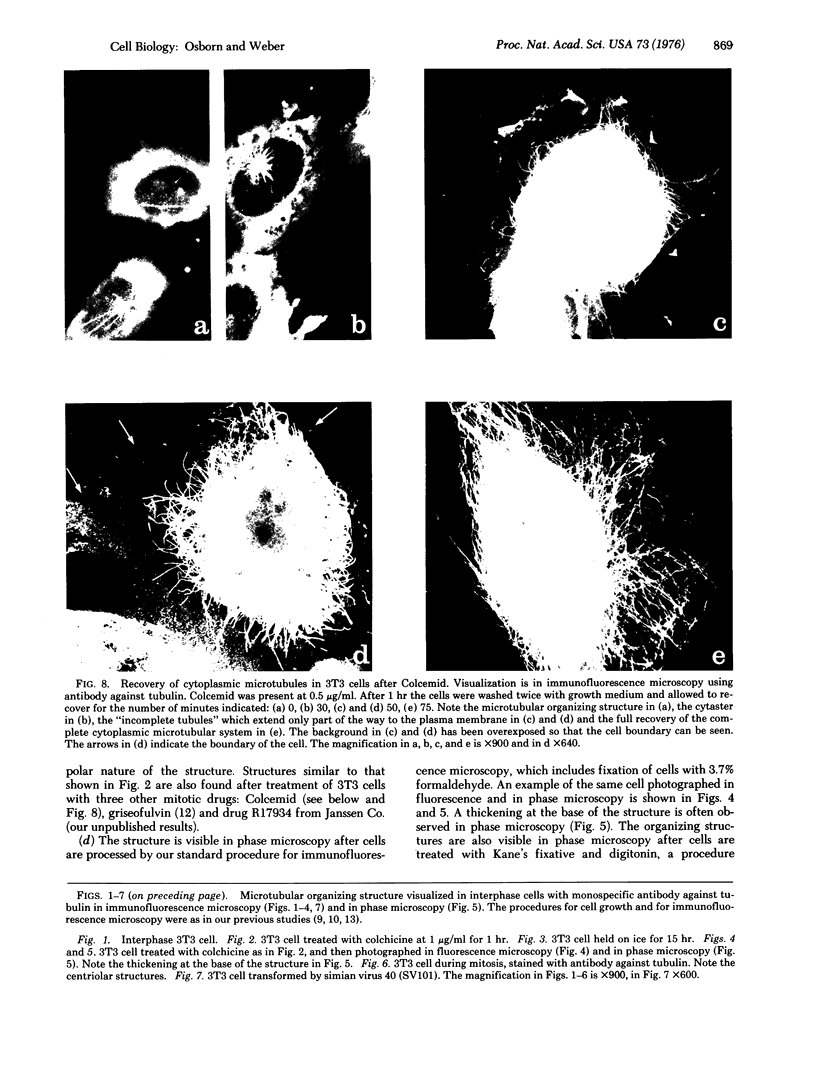
A structure which appears to organize cytoplasmic microtubules in interphase mouse 3T3 cells can be visualized by immunofluorescence microscopy. Purified monospecific antibody against homogeneous tubulin from brain visualizes, in addition to cytoplasmic microtubules, a cytoplasmic polar structure as the focal point from which the microtubules seem to radiate. The structure is preserved after treatments that depolymerize cytoplasmic microtubules, i.e., exposure of cells to mitotic drugs or to low temperature. When cells recover from these treatments one end of each microtubule organizing structure acts as a nucleating center from which cytoplasmic microtubules grow toward the plasma membrane. Thus cytoplasmic microtubules assemble in vivo in an ordered unidirectional manner, and therefore the cell must be able to avoid the assembly of unwanted, unoriented, and disconnected microtubules. These results suggest that the assembly of tubulin into microtubules is regulated in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Stubblefield E., Hsu T. C. The effects of colcemid inhibition and reversal on the fine structure of the mitotic apparatus of Chinese hamster cells in vitro. J Ultrastruct Res. 1967 Jul;19(1):1–18. doi: 10.1016/s0022-5320(67)80057-1. [DOI] [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- McGill M., Brinkley B. R. Human chromosomes and centrioles as nucleating sites for the in vitro assembly of microtubules from bovine brain tubulin. J Cell Biol. 1975 Oct;67(1):189–199. doi: 10.1083/jcb.67.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Jentzsch G., Micali A. The centriole cycle in synchronized HeLa cells. J Cell Biol. 1968 Feb;36(2):329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield E., Brinkley B. R. Cilia formation in Chinese hamster fibroblasts in vitro as a response to colcemid treatment. J Cell Biol. 1966 Sep;30(3):645–652. doi: 10.1083/jcb.30.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEG D. E. CYTOPLASMIC MICROTUBULES IN DIFFERENT ANIMAL CELLS. J Cell Biol. 1964 Nov;23:265–275. doi: 10.1083/jcb.23.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Pollack R., Bibring T. Antibody against tuberlin: the specific visualization of cytoplasmic microtubules in tissue culture cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):459–463. doi: 10.1073/pnas.72.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley D. N. Cilia in cell-cultured fibroblasts. I. On their occurrence and relative frequencies in primary cultures and established cell lines. J Anat. 1969 Sep;105(Pt 2):351–362. [PMC free article] [PubMed] [Google Scholar]
- Wheatley D. N. Cilia in cell-cultured fibroblasts. IV. Variation within the mouse 3T6 fibroblastic cell line. J Anat. 1972 Oct;113(Pt 1):83–93. [PMC free article] [PubMed] [Google Scholar]