Abstract
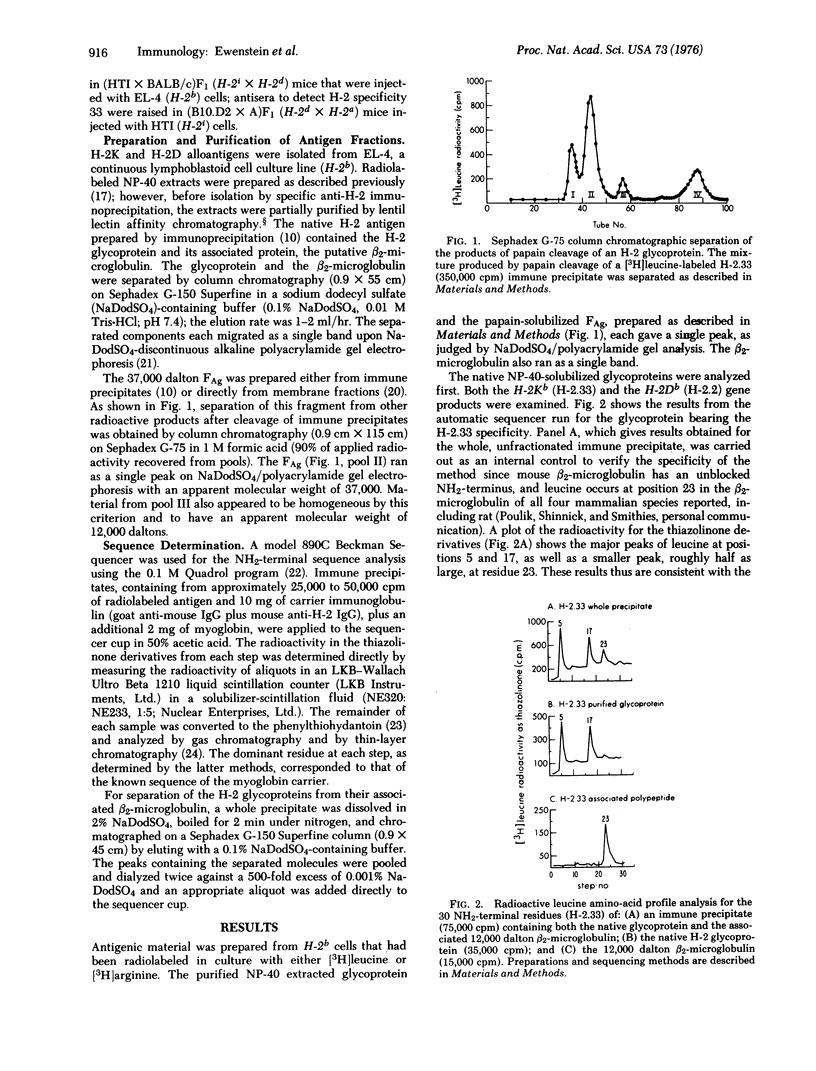
The antigenic products of the murine H-2K and H-2D genes are glycoproteins of about 45,000 molecular weight which are tightly integrated within the cell surface membrane. A glycoprotein fragment (FAg, antigenic fragment) of 37,000 daltons carrying the carbohydrate, antigenic sites, and the associated putative beta2-microglobulin of 12,000 daltons can be generated by papain cleavage either of the native molecules in the cell membrane or of immune precipitates made from the antigen solubilized by nonionic detergent. Partial NH2-terminal sequence analyses of the native H-2 glycoprotein and of the papain-cleaved glycoprotein fragment establish that the fragment is, in fact, the NH2-terminal portion of the native molecule. Thus, the cleavage by papain proteolysis is near the COOH-terminus, and removal of the COOH-terminal portion (Fm, membrane fragment) converts the glycoprotein to a water-soluble form. This observation suggests that the NH2-terminus of the native glycoprotein extends out of the hydrophobic bilayer of the cell membrane, and that the COOH-terminus contains the membrane binding region and is buried within the bilayer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Kato K., Silver J., Nathenson S. G. Notable diversity in peptide composition of murine H-2K and H-2D alloantigens. Biochemistry. 1974 Jul 16;13(15):3174–3178. doi: 10.1021/bi00712a027. [DOI] [PubMed] [Google Scholar]
- Démant P. H-2 gene complex and its role in alloimmune reactions. Transplant Rev. 1973;15:162–200. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Hinzová E., Démant P., Iványi P. Genetic control of haemolytic complement in mice: association with H-2. Folia Biol (Praha) 1972;18(4):237–243. [PubMed] [Google Scholar]
- Iványi P., Hámpl R., Stárka L., Micková M. Genetic association between H-2 gene and testosterone metabolism in mice. Nat New Biol. 1972 Aug 30;238(87):280–281. doi: 10.1038/newbio238280a0. [DOI] [PubMed] [Google Scholar]
- McKean D. J., Peters E. H., Waldby J. I., Smithies O. Amino acid sequence determination with radioactive proteins. Biochemistry. 1974 Jul 16;13(15):3048–3051. doi: 10.1021/bi00712a008. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Edidin M. Association of mouse liver adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels with histocompatibility-2 genotype. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2644–2648. doi: 10.1073/pnas.72.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathenson S. G., Cullen S. E. Biochemical properties and immunochemical-genetic relationships of mouse H-2 alloantigens. Biochim Biophys Acta. 1974 Apr 8;344(1):1–25. doi: 10.1016/0304-4157(74)90006-9. [DOI] [PubMed] [Google Scholar]
- Rask L., Lindblom J. B., Peterson P. A. Subunit structure of H-2 alloantigens. Nature. 1974 Jun 28;249(460):833–834. doi: 10.1038/249833a0. [DOI] [PubMed] [Google Scholar]
- Schechter I. Partial amino acid sequence of the precursor of immunoglobulin light chain programmed by messenger RNA in vitro. Science. 1975 Apr 11;188(4184):160–162. doi: 10.1126/science.803715. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Kato K., Cullen S. E., Nathenson S. G. H-2 histocompatibility alloantigens. Some biochemical properties of the molecules solubilized by NP-40 detergent. Biochemistry. 1973 May 22;12(11):2157–2164. doi: 10.1021/bi00735a023. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Isolation of H-2 alloantigens solubilized by the detergent NP-40. J Immunol. 1971 Nov;107(5):1363–1367. [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Regeneration of transplantation antigens on mouse cells. Transplant Proc. 1971 Mar;3(1):180–182. [PubMed] [Google Scholar]
- Shimada A., Nathenson S. G. Murine histocompatibility-2 (H-2) alloantigens. Purification and some chemical properties of soluble products from H-2b and H-2d genotypes released by papain digestion of membrane fractions. Biochemistry. 1969 Oct;8(10):4048–4062. doi: 10.1021/bi00838a023. [DOI] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Silver J., Hood L. An approach to amino acid sequence analysis of transplantation antigens. Nature. 1975 Jul 3;256(5512):63–64. doi: 10.1038/256063a0. [DOI] [PubMed] [Google Scholar]
- Silver J., Hood L. Detergent-solubilised H-2 alloantigen is associated with a small molecular weight polypeptide. Nature. 1974 Jun 21;249(459):764–765. doi: 10.1038/249764a0. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W., Boyse E. A. Association of a beta2-microglobulin-like subunit with H-2 and TL alloantigens on murine thymocytes. J Immunol. 1975 Jan;114(1 Pt 1):252–254. [PubMed] [Google Scholar]
- Yamane K., Shimada A., Nathenson S. G. Peptide comparison of two histocompatibility-2 (H-2b and H-2d) alloantigens. Biochemistry. 1972 Jun 20;11(13):2398–2402. doi: 10.1021/bi00763a002. [DOI] [PubMed] [Google Scholar]


