Abstract
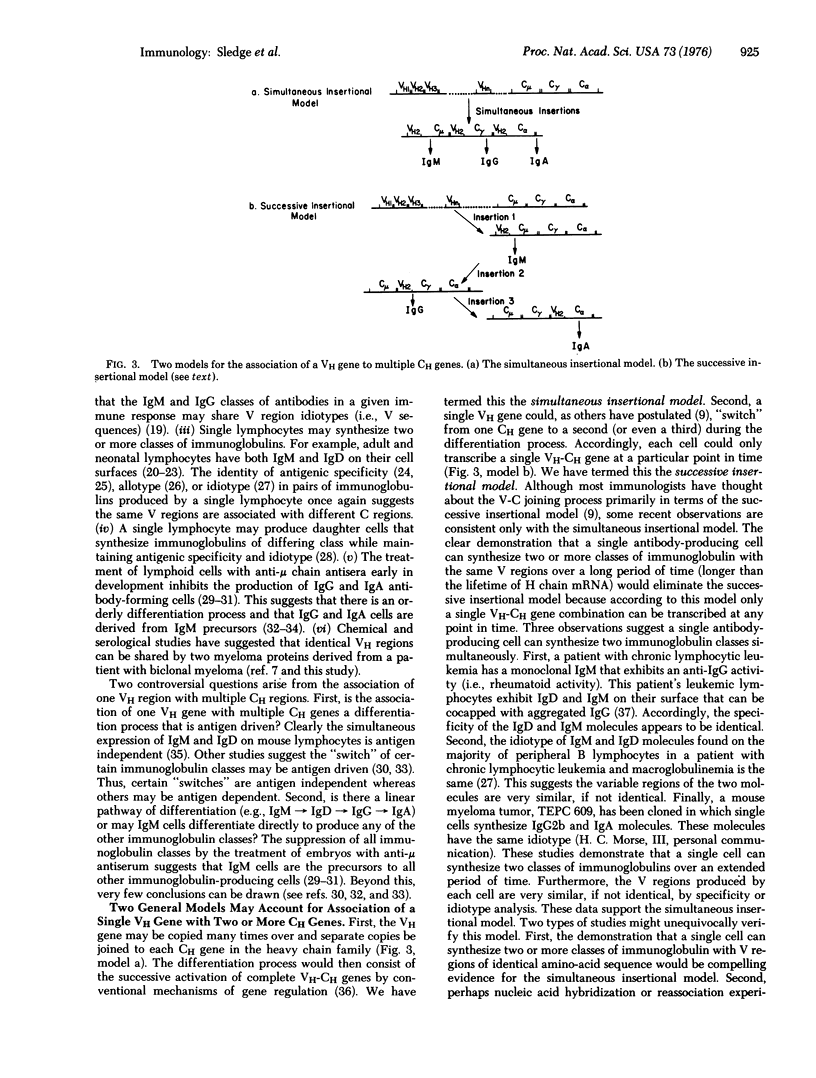
We have analyzed a pair of human myeloma immunoglobulins (biclonal proteins) of the IgG and IgA classes from a single patient, GR. The light chains are identical in amino-acid sequence over 40 residues at their NH2-terminus, hwereas the heavy chains are identical throughout 45 residues of their NH2-terminus. Additional chemical and serological studies suggest the light chains and variable regions of the heavy chains (VH) are very similar, if not identical. The implications of these and of other published studies are discussed with regard to (i) the association of one VH region with multiple constant regions of the heavy chain (CH regions), (ii) two alternative types of V-C joining mechanisms, (iii) the differentiation of antibody-producing cells, and (iv) three categories of biclonal immunoglobulins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J., Parkhouse R. M., Williamson A. R., Askonas B. A. Biosynthesis of immunoglobulins. Prog Biophys Mol Biol. 1972;25:133–162. doi: 10.1016/0079-6107(72)90018-1. [DOI] [PubMed] [Google Scholar]
- Bihrer R., Flury R., Morell A. Biklonale Paraproteinämie. Schweiz Med Wochenschr. 1974 Jan 12;104(2):39–45. [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M. Variable region sequences of five human immunoglobulin heavy chains of the VH3 subgroup: definitive identification of four heavy chain hypervariable regions. Proc Natl Acad Sci U S A. 1974 Mar;71(3):845–848. doi: 10.1073/pnas.71.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., 3rd The development of the immune system. Sci Am. 1974 Nov;231(5):58–72. doi: 10.1038/scientificamerican1174-58. [DOI] [PubMed] [Google Scholar]
- Fair D. S., Krueger R. G., Gleich G. J., Kyle R. A. Studies on IgA and IgG monoclonal proteins derived from a single patient. I. Evidence for shared individually specific antigenic determinants. J Immunol. 1974 Jan;112(1):201–209. [PubMed] [Google Scholar]
- Fair D. S., Sledge C., Krueger R. G., Mann K. G., Hood L. E. Studies on IgA and IgA monoclonal proteins derived from a single patient. Evidence for identical light chains and variable regions of the heavy chain. Biochemistry. 1975 Dec 30;14(26):5561–5568. doi: 10.1021/bi00697a004. [DOI] [PubMed] [Google Scholar]
- Fröland S. S., Natvig J. B. Lymphocytes with membrane-bound immunoglobulin (B-lymphocytes) in new-born babies. Clin Exp Immunol. 1972 Aug;11(4):495–505. [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Winchester R. J., Kunkel H. G. Occurrence of surface IgM, IgD, and free light chains of human lymphocytes. J Exp Med. 1974 Feb 1;139(2):451–456. doi: 10.1084/jem.139.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S. M., Winchester R. J., Kunkel H. G. Similar idiotypic specificity for the membrane IgD and IgM of human B lymphocytes. J Immunol. 1975 Jan;114(1 Pt 1):250–252. [PubMed] [Google Scholar]
- Fudenberg H. H., Wang A. C., Pink J. R., Levin A. S. Studies of an unusual biclonal gammopathy: implications with regard to genetic control of normal immunoglobulin synthesis. Ann N Y Acad Sci. 1971 Dec 31;190:501–506. doi: 10.1111/j.1749-6632.1971.tb13559.x. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. Somatic translocation of antibody genes. Nature. 1970 Jul 25;227(5256):341–348. doi: 10.1038/227341a0. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. The genetic control of immunoglobulin synthesis. Annu Rev Genet. 1972;6:1–46. doi: 10.1146/annurev.ge.06.120172.000245. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Sigal N. H., Klinman N. R. Production of antibodies of identical idiotype but diverse immunoglobulin classes by cells derived from a single stimulated B cell. Proc Natl Acad Sci U S A. 1975 May;72(5):1707–1711. doi: 10.1073/pnas.72.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad K., Sletten K. Multiple M-components in a single individual. 3. Heterogeneity of M-components in two macroglobulinemia sera with anti-polysaccharide activity. J Biol Chem. 1971 Nov 25;246(22):6982–6990. [PubMed] [Google Scholar]
- Harboe M., Hannestad K., Sletten K. Oligoclonal macroglobulinaemia. Scand J Immunol. 1972;1(1):13–26. doi: 10.1111/j.1365-3083.1972.tb03731.x. [DOI] [PubMed] [Google Scholar]
- Herrod H. G., Warner N. L. Inhibition by anti- chain sera of the cellular transfer of antibody and immunoglobulin synthesis in mice. J Immunol. 1972 Jun;108(6):1712–1717. [PubMed] [Google Scholar]
- Hood L. E. Two genes, one polypeptide chain--fact or fiction? Fed Proc. 1972 Jan-Feb;31(1):177–187. [PubMed] [Google Scholar]
- Hood L., McKean D., Farnsworth V., Potter M. Mouse immunoglobulin chains. A survey of the amino-terminal sequences of kappa chains. Biochemistry. 1973 Feb;12(4):741–749. doi: 10.1021/bi00728a026. [DOI] [PubMed] [Google Scholar]
- Kabat D. Gene selection in hemoglobin and in antibody-synthesizing cells. Science. 1972 Jan 14;175(4018):134–140. doi: 10.1126/science.175.4018.134. [DOI] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A. S., Fudenberg H. H., Hopper J. E., Wilson S. K., Nisonoff A. Immunofluorescent evidence for cellular control of synthesis of variable regions of light and heavy chains of immunoglobulins G and M by the same gene. Proc Natl Acad Sci U S A. 1971 Jan;68(1):169–171. doi: 10.1073/pnas.68.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning D. D. Recovery from anti-IG induced immunosuppression: implications for a model of Ig-secreting cell development. J Immunol. 1974 Aug;113(2):455–463. [PubMed] [Google Scholar]
- Martin L. N., Leslie G. A. IgM-forming cells as the immediate precursor of IgA-producing cells during ontogeny of the immunoglobulin-producing system of the chicken. J Immunol. 1974 Jul;113(1):120–126. [PubMed] [Google Scholar]
- NOSSAL G. J., SZENBERG A., ADA G. L., AUSTIN C. M. SINGLE CELL STUDIES ON 19S ANTIBODY PRODUCTION. J Exp Med. 1964 Mar 1;119:485–502. doi: 10.1084/jem.119.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H. Incidence of cells simultaneously secreting IgM and IgG antibody to sheep erythrocytes. Cell Immunol. 1971 Feb;2(1):41–53. doi: 10.1016/0008-8749(71)90024-4. [DOI] [PubMed] [Google Scholar]
- Oudin J., Michel M. Idiotypy of rabbit antibodies. II. Comparison of idiotypy of various kinds of antibodies formed in the same rabbits against Salmonella typhi. J Exp Med. 1969 Sep 1;130(3):619–642. doi: 10.1084/jem.130.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Brouet J. C., Seligmann M. IgD and IgM on the membrane of lymphoid cells in macroglobulinemia. Evidence for identity of membrane IgD and IgM antibody activity in a case with anti-IgG receptors. Eur J Immunol. 1974 Nov;4(11):776–778. doi: 10.1002/eji.1830041114. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulins as cell receptors. Ann N Y Acad Sci. 1971 Dec 31;190:420–431. doi: 10.1111/j.1749-6632.1971.tb13552.x. [DOI] [PubMed] [Google Scholar]
- Pierce C. W., Solliday S. M., Asofsky R. Immune responses in vitro. IV. Suppression of primary M, G, and A plaque-forming cell responses in mouse spleen cell cultures by class-specific antibody to mouse immunoglobulins. J Exp Med. 1972 Mar 1;135(3):675–697. doi: 10.1084/jem.135.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Faulk W. P., McCormick J. N., Gerber H. IgD on the surface of peripheral blood lymphocytes of the human newborn. Nat New Biol. 1973 Apr 4;242(118):155–157. doi: 10.1038/newbio242155a0. [DOI] [PubMed] [Google Scholar]
- Rowe D. S., Hug K., Forni L., Pernis B. Immunoglobulin D as a lymphocyte receptor. J Exp Med. 1973 Oct 1;138(4):965–972. doi: 10.1084/jem.138.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudders R. A., Yakulis V., Heller P. Double myeloma. Production of both IgG type lambda and IgA type lambda myeloma proteins by a single plasma cell line. Am J Med. 1973 Aug;55(2):215–221. doi: 10.1016/0002-9343(73)90171-x. [DOI] [PubMed] [Google Scholar]
- Seon B. K., Yagi Y., Pressman D. Comparative chemical study of alpha-and mu-chains from a single patient (SC). J Immunol. 1973 Oct;111(4):1285–1287. [PubMed] [Google Scholar]
- Smithies O. Pathways through networks of branched DNA. Science. 1970 Aug 28;169(3948):882–883. doi: 10.1126/science.169.3948.882. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Melcher U., McWilliams M., Lamm M. E., Phillips-Quagliata J. M., Uhr J. W. Cell surface immunoglobulin. XI. The appearance of an IgD-like molecule on murine lymphoid cells during ontogeny. J Exp Med. 1975 Jan 1;141(1):206–215. doi: 10.1084/jem.141.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Immunoglobulin-receptors revisited. Science. 1975 Sep 19;189(4207):964–969. doi: 10.1126/science.1083069. [DOI] [PubMed] [Google Scholar]
- Wang A. C., Gergely J., Fudenberg H. H. Amino acid sequences at constant and variable regions of heavy chains of monotypic immunoglubulins G and M of a single patient. Biochemistry. 1973 Jan 30;12(3):528–534. doi: 10.1021/bi00727a027. [DOI] [PubMed] [Google Scholar]
- Wang A. C., Wilson K. S., Hopper J. E., Fudenberg H. H., Nisonoff A. Evidence for control of synthesis of the varible regions of the heavy chains of immunoglobulins G and M by the same gene. Proc Natl Acad Sci U S A. 1970 Jun;66(2):337–343. doi: 10.1073/pnas.66.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenstein-Todel C., Franklin E. C., Rudders R. A. Similarities of the light chains and the variable regions of the heavy chains of the IgG2 lambda and IgA1 lambda myeloma proteins from a single patient. J Immunol. 1974 Mar;112(3):871–876. [PubMed] [Google Scholar]



