Abstract
Hyperplastic polyps have traditionally been considered not to have malignant potential. New pathological classification of serrated polyps and recent discoveries about the serrated pathway of carcinogenesis have revolutionized the concepts and revitalized the research in this area. Until recently, it has been thought that most colorectal cancers arise from conventional adenomas via the traditional tumor suppressor pathway initiated by a mutation of the APC gene, but it has been found that this pathway accounts for only approximately 70%-80% of colorectal cancer (CRC) cases. The majority of the remaining colorectal cancer cases follow an alternative pathway leading to CpG island methylator phenotype carcinoma with BRAF mutation and with or without microsatellite instability. The mechanism of carcinomas arising from this alternative pathway seems to begin with an activating mutation of the BRAF oncogene. Serrated polyposis syndrome is a relatively rare condition characterized by multiple and/or large serrated polyps of the colon. Clinical characteristics, etiology and relationship of serrated polyposis syndrome to CRC have not been clarified yet. Patients with this syndrome show a high risk of CRC and both sporadic and hereditary cases have been described. Clinical criteria have been used for diagnosis and frequent colonoscopy surveillance should be performed in order to prevent colorectal cancer. In this review, we try to gather new insights into the molecular pathogenesis of serrated polyps in order to understand their possible clinical implications and to make an approach to the management of this syndrome.
Keywords: Colorectal cancer, Hyperplastic polyps, CpG island methylator phenotype, Serrated polyposis, Serrated pathway
INTRODUCTION
Colorectal cancer (CRC) is a common and lethal disease. It is a major health issue in western countries where it represents the second most common fatal malignancy after lung cancer[1]. Until recently, it has been thought that most CRCs arise from conventional adenomas via the traditional tumor suppressor pathway initiated with a mutation of the APC gene, but it has been found that this pathway accounts for only approximately 70%-80% of CRC cases[2-4]. The majority of the remaining CRC cases follow an alternative pathway leading to CpG island methylator phenotype (CIMP+) carcinoma with BRAF mutation and with or without microsatellite instability. This pathway is called the serrated pathway of colorectal carcinogenesis[3]. The mechanism of carcinomas arising from this alternative pathway seems to begin with an activating mutation of the BRAF oncogene. This BRAF mutation provokes the development of serrated lesions that are mainly microvesicular hyperplastic polyps or sessile serrated polyps[5]. These lesions are prone to methylation of CpG islands in the promoter regions of genes resulting in their epigenetic silencing. The best characterized gene silenced by this mechanism is MLH1. This gene is one of the mismatch repair genes and its epigenetic silencing results in sporadic tumors with microsatellite instability (MSI). However, other genes such as P16, MGMT, or IGFBP7 may also be epigenetically inactivated. The serrated polyposis syndrome (SPS) is a relatively rare condition characterized by multiple and/or large serrated polyps of the colon. Diagnosis of this disease is made by the fulfillment of any of the World Health Organization’s (WHO) clinical criteria[6] (Table 1). SPS exhibits an increased risk of CRC[7], which occurs on average in subjects aged between 50 to 60 years. There is a high incidence of synchronous cancers[8] and CRC shows a trend to be located in the proximal colon[9]. These patients and their relatives should receive strict surveillance strategies because of the high risk of CRC. This review focuses on the SPS, its genetics and management.
Table 1.
The World Health Organization's clinical criteria for the identification of serrated polyposis
| Criterion A | At least five serrated polyps proximal to the sigmoid colon, two of which are greater than 10 mm in diameter |
| Criterion B | Any number of serrated polyps occurring proximal to the sigmoid colon in an individual who has a first-degree relative with serrated polyposis |
| Criterion C | More than 20 serrated polyps of any size distributed throughout the colon |
A diagnosis of serrated polyposis syndrome can be made if a patient fulfils any of these criteria.
SERRATED POLYPOSIS SYNDROME
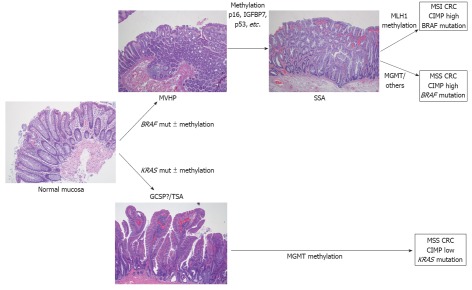
Serrated polyposis syndrome is the paradigm of the serrated pathway of carcinogenesis and an excellent and interesting human model for the study of the features that drive progression from hyperplastic polyps (HP) to serrated carcinoma (Figure 1). These patients show clinical, pathological and molecular features that are very useful for expanding the knowledge of this particular and alternative carcinogenetic pathway.
Figure 1.
Model of serrated pathway of colorectal carcinogenesis. MVHP: Microvesicular hyperplastic polyp; SSA: Sessile serrated adenoma; MGMT: Methylguanine methyltransferase; MSI: Microsatellite instability; MSS: Microsatellite stable; CRC: Colorectal cancer; CIMP: CpG island methylator phenotype; GCSP: Goblet cell serrated polyp; TSA: Traditional sessile adenomas.
Diagnostic criteria
Diagnostic criteria of SPS were first defined by Burt and Jass in 2000 for the WHO. These criteria have been recently redefined and this entity is now called Serrated Polyposis[6]. A patient is diagnosed with SPS if at least one of the following criteria is met: (1) At least five serrated polyps proximal to the sigmoid colon, two of which are greater than 10 mm in diameter; (2) Any number of serrated polyps occurring proximal to the sigmoid colon in an individual who has a first-degree relative with serrated polyposis; and (3) More than 20 serrated polyps of any size distributed throughout the colon (Table 1). This arbitrary definition has been considered over the years somewhat restrictive. Moreover, SPS probably comprises a heterogeneous group of patients that includes several phenotypes of serrated polyposis. However, until the molecular basis of this syndrome is better understood, this clinical definition is applicable.
Clinical characteristics
Characteristics of patients with SPS have been defined mainly based on the publication of series of cases[7,9-13] (Table 2). There is no sex predominance and the mean age at diagnosis is around 55 years. SPS has largely been considered a genetic disease, but the pattern of inheritance remains unknown: both autosomal recessive and autosomal dominant patterns have been suggested. Published case series report that between 10%-50% of patients meeting SPS criteria have a family history of CRC[9,11-13]. In this way, Boparai et al[14] have recently described an increased risk of CRC [relative risk (RR) = 5.4] and SPS (RR = 39) in first-degree relatives of probands diagnosed with SPS compared to the general population.
Table 2.
Summary findings from publications including patients that fulfil World Health Organization criteria of serrated polyposis syndrome
| Author | Patients (n) | Age at diagnosis (median, yr) | CRC (%) | CRC family history (%) |
| Lage et al[12] | 14 | 54 | 43 | 36 |
| Ferrández et al[10] | 15 | 52 | 7 | 0 |
| Rubio et al[13] | 10 | 61 | 70 | 10 |
| Chow et al[9] | 38 | 44 | 26 | 50 |
| Boparai et al[7] | 77 | 56 | 35 | NR |
CRC: Colorectal cancer; NR: Not reported.
It is important to point out that conventional adenomas may coexist with serrated polyps in patients with SPS[7,9-13]. Some authors have suggested the existence of various phenotypes within the SPS definition. Kalady et al[11] described three phenotypic patterns in a series of 115 patients with multiple serrated polyps: (1) The patients presented a right-sided phenotype with large sessile serrated adenomas (SSAs) and with a CRC onset in younger individuals (48%); (2) Left-sided phenotype with a greater amount of small polyps (16%); and (3) Mixed phenotype with shared features of the previous phenotypes (37%). These different patterns should be revised in future studies.
Environmental factors could be partially responsible for the phenotypic differences and model the unknown pattern of inheritance. Smoking, being overweight and some drugs have been postulated as potential risk factors of HPs. Samowitz et al[15] described a statistically significant dose-response association between CIMP+ CRC and smoking. Moreover, Walker et al[16] found a strong association between cigarette smoking and SPS (odds ratio = 8.3; 95% CI: 3.0-22.9) in a case-control study comparing SPS patients with a population-based registry. Wallace et al[17], using the data of multicenter chemoprevention trials, came upon the association of some environmental factors with an increased risk of colonic serrated polyps (not necessary SPS criteria). On the one hand, in the left colon, obesity, smoking, increased dietary fat and red meat intake were linked with serrated polyps. On the other hand, in the right colon, the risk factors were folate intake and family history of polyps, whereas aspirin treatment was shown as a protective factor. These results should be confirmed by targeted studies.
Somatic molecular characteristics of polyps in patients with serrated polyposis syndrome
Molecular heterogeneity among polyps from patients with SPS has been described[18]. In fact, although a mixed phenotype has been identified[11], SPS patients can be molecularly classified into two defined groups[19]. The first group is characterized by the presence of relatively few large right-sided polyps which show BRAF mutation while the other group presents with many small left-sided polyps associated with KRAS mutation[11,19]. Mutations in KRAS and BRAF are more common in HPs from SPS patients as well as in younger cases. The frequency of BRAF mutations in SPS patients is higher than KRAS mutations[19].
The combined incidence of BRAF and KRAS mutations in serrated polyps ranges from 64% to 75%[19,20]. The presence of epithelial dysplasia is associated with higher rates (90%) of mutation in either BRAF or KRAS, indicating the importance of the activation of the RAS-RAF-MAP kinase pathway in the pathogenesis of the serrated lesions[20]. Furthermore, nearly 90% of all CIMP+ CRCs have either BRAF or KRAS mutations[21]. Serrated polyps from patients with SPS have different frequencies of BRAF mutation and it is higher in those lesions that show typical features of SSA[21-23]. However, there are differences between studies due to the methodology used for the detection of BRAF mutation[22] or because of the lack of consensus about the diagnostic terms for serrated lesions[24,25].
Carvajal-Carmona et al[19] proposed molecular criteria that could complement the clinical WHO criteria for SPS. They recommended that SPS should be diagnosed if BRAF or KRAS mutations are present at a significantly higher frequency in a patient’s polyps than in sporadic HPs. In addition, SPS could be excluded if both BRAF and KRAS mutations are present in less than 10% of HPs from one patient, or if less than 5% of HPs are MSI.
Genetic predisposition in patients with serrated polyposis syndrome
SPS is a very heterogeneous condition[26] and it has been suggested that each phenotype may result from different underlying genetic causes[11]. Familial cases of SPS have been reported[19,26]. Although the genetic basis of SPS remains unknown, both recessive and dominant transmission patterns have been proposed[9,23,26]. Young et al[27] provided evidence for a syndrome of familial CRC distinct from hereditary nonpolyposis colorectal cancer by describing 11 families, of which 6 met the Amsterdam I criteria, with multiple members across several generations with CRC with variable MSI phenotype, BRAF mutation in 70% and hypermethylation of MINT31 in 80%. Moreover CRCs showed early age at diagnosis and were more likely to show a serrated architecture. Frazier et al[28] observed that patients whose CRC show methylation in p16, MINT1, MINT31 and MLH1 are 14 times more likely to have a family history of cancer than patients with methylation at none of the four loci. Taking into account these studies and the fact that extensive DNA methylation in normal colorectal mucosa has been described in patients with SPS[19,29,30], it has been postulated that the hypermethylation of gene promoters is due to genetic predisposition[23].
On the other hand, patients meeting criteria for hereditary nonpolyposis colorectal cancer may also fulfil criteria of SPS[31]. Occasional HPs have also been described in MYH-associated polyposis (MAP) patients and some of them met the criteria for SPS. Moreover HPs and SSAs can also be considered a phenotypic expression of MAP[32] and pathogenic biallelic MYH mutations were detected in 1 patient with SPS[9]. For that reason MYH mutations should be studied in SPS patients, especially when adenomas occur simultaneously with HPs in the same patient[9]. PTEN mutations have also been identified in patients with a combination of hyperplastic and adenomatous polyps[33].
A recent study from Roberts et al[34] has showed linkage to 2q32.2-q33.3 in approximately half of the SPS families studied. Sequencing of coding regions and exon-intron boundaries of five potential candidate genes in this region did not reveal any variants segregating with disease.
Together these data support the existence of more than one genetic cause of SPS. Identification of the underlying genetic defect of SPS will help to improve management of these patients and may identify therapeutic targets for the treatment of CRC associated with this disease.
Risk of cancer in serrated polyposis syndrome
Serrated polyposis syndrome has been associated with an increased incidence of CRC. In the published series[7,9,10,12,13], about 25%-70% of patients with SPS had CRC at time of diagnosis or during follow-up. In the largest series with patients meeting WHO criteria for SPS[7], 35% of patients had CRC (28.5% at initial endoscopy and 6.5% during the mean follow-up of 5.6 years). In this study, increased number of polyps and the presence of serrated adenomas were associated with CRC. The results of the larger published series are summarized in Table 2. In addition, first degree relatives of SPS patients have an increased risk for both CRC and SPS compared to the general population[14].
Recommendations for treatment and surveillance
The management of patients with SPS should be based on regular screening colonoscopies in order to remove potential premalignant lesions. It is important to point out that it could be difficult to detect these serrated polyps and colonoscopy should be done under high quality conditions (Figure 2). Serrated polyps are less likely than adenomas to bleed, so fecal occult blood test could be less suitable for an early diagnosis. Surveillance recommendations can be done as follows[4]: (1) Colonoscopy with pancolonic chromoendoscopy every 1-2 years with removal of all polyps. It is recommended that this resection be performed at a tertiary centre, if possible; (2) If colonoscopy does not allow the total control of colonic polyps because of their size or number or the patient does not wish to have such frequent colonoscopies or cancer is detected, colectomy with ileorectal anastomosis should be indicated; and (3) First-degree relatives should be offered 1-2 years screening colonoscopy from 10 years younger than the index case and if it is possible by pancolonic chromoendoscopy.
Figure 2.
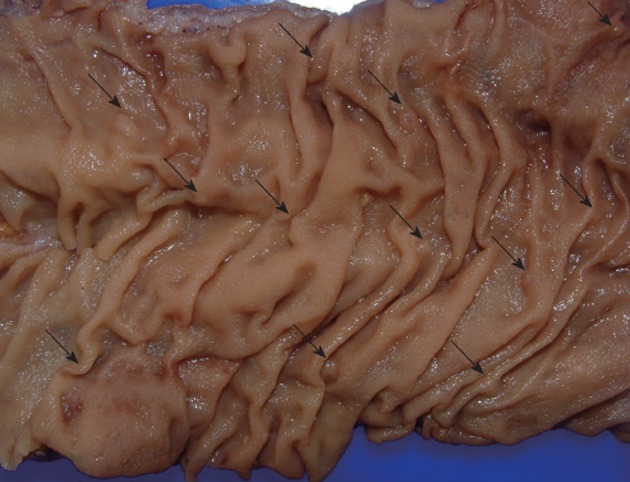
Segment of colectomy in a case of serrated polyposis. Polyps are frequently small (arrows) and flat, making their endoscopic detection difficult.
Pedunculated polyps can be removed by conventional electrocautery snare polypectomy. The technique of choice for removal of flat and large HPs is endoscopic mucosal resection. Besides, it may be advisable to apply argon plasma coagulation in the lesion borders in order to reduce the risk of recurrence[35].
SERRATED PATHWAY OF CARCINOGENESIS
In 1999, Iino et al[36] suggested that a proportion of hyperplastic polyps may serve as precursors of some CRC cases. Now, there is increasing evidence showing that, in some conditions, hyperplastic polyps can be the initial premalignant lesion in the serrated pathway of carcinogenesis. Some studies have reported the existence of BRAF mutations in sporadic MSI CRCs which show CIMP[21,23,27,37-41] suggesting the existence of this alternative pathway. BRAF mutations and DNA methylation would be early events in this pathway with serrated polyps as precursor lesions[4,21,27,41]. The lack of adenoma-specific mutations such as APC, KRAS and TP53 in sporadic MSI CRCs, and the fact that BRAF mutation and methylation of CpG islands are exceptional in classic adenomas[42] supports the existence of this pathway[43]. Tumors following this pathway show some specific characteristics, being more frequent in females and located in the right colon[4]. Moreover, some preliminary studies suggest that these tumors could be unresponsive to 5-fluorouracil chemotherapy[44].
Molecular characteristics of serrated polyps
As mentioned above, BRAF mutations and DNA methylation would be early events in this pathway. In fact, epigenetic changes in normal mucosa in patients with SPS have been described[29]. The first role of BRAF in the serrated pathway is probably to allow the apoptosis evasion[21,45]. Then, under normal conditions, these cells are eliminated by regular senescence. However, the silencing of key cell cycle regulatory genes such as p16, IGFBP7 or p53 through promoter methylation allows the cell to escape from senescence[4], facilitating its proliferation (Figure 1). When cells acquire other mutations, activated BRAF itself could also drive proliferation[21] and facilitate the maintenance of an invasive phenotype[45]. BRAF mutation has even been observed in serrated hyperplastic aberrant crypt foci, suggesting that these lesions are probably the earliest histological evident lesions in the serrated pathway[4,46].
There are several lines of evidence suggesting the existence of two parallel serrated pathways depending on the oncogene involved: BRAF or KRAS (Figure 1). The serrated pathway that involves BRAF mutations usually leads to CIMP tumors[4,29,43,47] and tumors are located in the proximal colon[38,47,48]. These tumors will be MSI or microsatellite stable (MSS) depending on the involvement of MLH1. As has been already stated, SSA seems to be the precursor lesion in the BRAF serrated pathway. In contrast, serrated tumors with KRAS mutations are more frequently MSI-low or MSS and are frequently associated with MGMT silencing[47,49]. These tumors are predominantly located in the left colorectum[4,29,47,48]. Differently, traditional sessile adenomas (TSA) would be the intermediate lesion in the KRAS serrated pathway.
CpG island methylator phenotype in colorectal cancer
CpG islands are 0.5- to 2-kb regions rich in cytosine guanine dinucleotides and are present in the 5’ region of approximately 50% of human genes[21,29]. CIMP is characterized by methylation of CpG islands within the promoter regions of multiple genes resulting in the silencing of gene expression[18,21,29,45]. It might be assumed that methylation of CpG islands in most cancers arises stochastically[18,23,50]. This phenomenon can alter the expression of genes which are known to be important in neoplastic development, such as p16, MGMT, and the mismatch repair gene MLH1. However, the role of some genes affected by hypermethylation is not associated with colorectal carcinogenesis suggesting that not all de novo events are subject to growth selection. Taking into account that particular sequence motifs are significantly overrepresented among promoters vulnerable to CIMP, it is not surprising that some CpG islands are more likely to undergo hypermethylation than others[23]. The balance between DNA methyltransferases and the transcriptional machinery will determine the extent of methylation. Moreover, an active transcription may provide protection from de novo methylation[50]. The CIMP pathway is heterogeneous with respect to MSI status[27] and appears to be responsible for approximately 30% of all sporadic CRC[27,39].
There are different studies showing that a high proportion of polyps in SPS are CIMP+[18,29]. Chan et al[29] also observed that 75% of serrated polyps from patients with SPS showed CIMP frequently and had methylation of the p16 gene. Moreover, extensive DNA methylation in normal colorectal mucosa has been described in patients with SPS[19,29,30], suggesting a field defect in epigenetic regulation and, consequently, a possible underlying genetic predisposition to extensive and early onset of DNA methylation. Furthermore, this phenomenon would be associated with a predisposition to CRC that would arise through the serrated pathway[51].
Endoscopic characteristics of serrated polyps
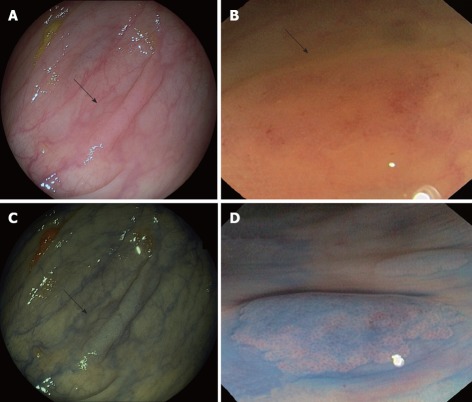
Serrated and hyperplastic polyps present endoscopic features that could help to differentiate them from adenomatous polyps. HPs appear pale, glistening, and very similar to the surrounding mucosa and usually covered by mucus. The vascular network is weak, in contrast to that of hypervascular adenomas. In addition, serrated polyps, mainly SSAs, are typically sessile or flat, making their detection even more difficult[48] (Figures 2 and 3, panels A and B). Since the malignant potential of these lesions, particularly in the context of SPS, has been shown, early endoscopic detection becomes more important. In this regard, the new advanced endoscopic techniques such as chromoendoscopy and narrow-band imaging (NBI) (Figure 3, panels C and D) become significant.
Figure 3.
Endoscopic appearance of serrated polyps. A and B: Sessile serrated adenoma (SSA) (arrows) as flat polyp on conventional optical colonoscopy; C: Narrow-band imaging appearance of polyp (arrow) seen in panel A; D: Chromoendoscopy image of SSA revealing Kudo II pattern (Images courtesy of Dr. Adolfo Parra, Hospital Central de Asturias, Oviedo, Spain).
Chromoendoscopy in the SPS should be carried out by spraying contrast over the entire surface of the colon and using a magnification endoscope. The most widely used contrast is indigo carmine, which accumulates in pits and innominate grooves of the colonic mucosa, outlining the limits of flat lesions and drawing the described Kudo patterns[52]. Hyperplastic and serrated polyps typically show Kudo type I (normal) or type II (‘‘stellate’’ or ‘‘papillary’’) (Figure 3, panel D). Published randomized trials have shown that pancolonic chromoendoscopy almost doubles the rate of detection of sporadic serrated polyps compared to conventional endoscopy[53-56]. In these studies, HPs were found in 20% of patients using chromoendoscopy vs 10% of patients with conventional endoscopy, and this difference was statistically significant. New endoscopic technologies, such as NBI and confocal laser endomicroscopy (CLE) should also be taken into consideration (Figure 4).
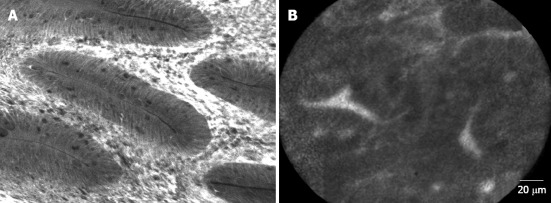
Figure 4.
Confocal endomicroscopy of (A) tubular adenoma, low-grade dysplasia and (B) serrated polyp. Both types of polyps show different shape as well as differences in the cellular structures (Images courtesy of Dr. Maria Pellisé, Hospital Clinic, Barcelona, Spain).
It is accepted that the vascular pattern evaluation by chromoendoscopy or NBI could be an appropriate method to differentiate adenomas from HPs[57,58], but it has not been specifically studied in the SPS. In this way, Boparai et al[59] ran a prospective series including 7 patients with SPS who underwent a colonoscopy with trimodal imaging (high resolution, AFI and NBI): they obtained an unsatisfactory diagnostic accuracy for differentiate between HPs and SSAs but distinguishing adenomas from HPs was possible with NBI (accuracy 94%). Highest accuracy (76%) was achieved by the combination of a size of 3 mm or larger and a proximal location. Comparing CLE with virtual chromoendoscopy, it was shown that CLE demonstrated higher sensitivity (91% vs 77%; P = 0.010) with similar specificity in histological classification of colorectal polyps. However, further studies are needed to implement the CLE in clinical practice. The limited field of view and the horizontal sections of CLE hinder the detection of architectural distortion of sessile polyps (Figure 4).
Pathological characteristics of serrated polyps
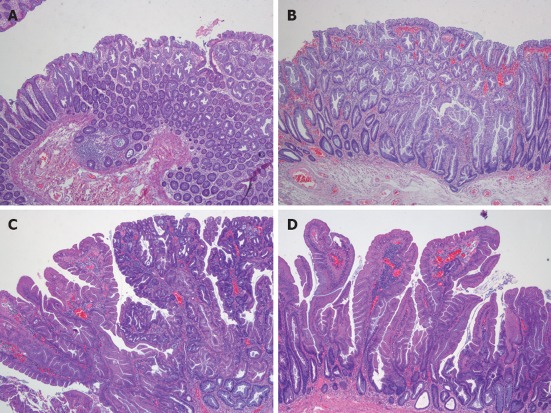
Confirmation of the serrated character of polyps can only be made by pathological study. Serrated polyps are defined as epithelial lesions that show serrated appearance on histological section due to infolding of crypt epithelium. There are different types of serrated polyps (Table 3). HPs, considered for a long time as a benign and non-premalignant colorectal lesion, SSA, mixed polyps (MP), and TSA are included in this group[4,48,60,61] (Figure 5).
Table 3.
Characteristics of serrated polyps
| Polyp name | Alternative terminology | Morphology and significance | Predominant location | Molecular features |
| Hyperplastic polyp, goblet type | Type 1 hyperplastic polyp | Subtype of hyperplastic polyp with conspicuous goblet cells and showing the least morphologic deviation from normal; Described as goblet-cell rich type | Distal colon: Sigmoid and rectum | Frequent KRAS mutation (54%) |
| Hyperplastic polyp, microvesicular type | Type 2 hyperplastic polyp | Variant of hyperplastic polyp in which columnar cells have mucin-filled vesicles within the apical cytoplasm and goblet cells are relatively inconspicuous | Right colon and distal colon | Frequent BRAF mutation (76%) and CIMP (68%) |
| Sesile serrated adenoma | Sessile serrated polyp; Serrated polyp with atypical proliferation | Advanced type of serrated polyp with abnormalities of architecture and proliferation but lacking the classic features of epithelial dysplasia (intraepithelial neoplasia) | Right colon | Frequent BRAF mutation (75%-82%) and CIMP (92%) |
| Sessile serrated adenoma with cytological dysplasia | Mixed polyp | Rare serrated polyp that includes two separate components: Nondysplastic (usually SSA) and either traditional adenoma or serrated adenoma | Right and left colon | Frequent BRAF mutation, (89%) |
| Serrated adenoma | Mixed hyperplastic adenomatous polyp; Atypical hyperplastic polyp; TSA | Relatively rare neoplastic polyp having a serrated architecture reminiscent of hyperplastic polyp but with unequivocal traditional adenomatous dysplasia; Comprises < 5% of serrated polyps | Left colon | Marked molecular heterogeneity; May have either KRAS or BRAF mutation |
SSA: Sessile serrated adenoma; TSA: Traditional serrated adenoma; CIMP: CpG island methylator phenotype.
Figure 5.
Pathological types of serrated polyps. A: Microvesicular serrated polyp; B: Sessile serrated adenomas; C: Traditional serrated adenoma; D: Mixed polyp.
HPs are the most common colorectal polyps. Sporadic HPs are usually small (2-5 mm)[10], multiple and mainly distributed in the rectum and sigmoid colon[10,47]. HPs have been divided into two main histological subtypes: microvesicular serrated polyps (MVSPs) (Figure 5, panel A), in which columnar cells have mucin-filled vesicles within atypical cytoplasm, and goblet cell serrated polyps (GCSPs) with conspicuous goblet cells that are predominantly found in the distal colon[4,23,47]. MVSPs seem to be the precursor lesion of SSA, especially when located in the right colon. In fact, both have the same molecular genetic abnormalities such as mutations in BRAF and CIMP. MVSPs show large and regular stellate pit openings. However, the large GCSPs are likely to have KRAS mutation, which is infrequently found in SSA. There is some evidence that large GCSPs are potential precursors of dysplastic serrated polyps which show KRAS mutations[47,61,62] . A third type of HP has been added, mucin poor type, but its frequency and importance is lower than the two main HP types[6].
SSA is an atypical HP variant described by Torlakovic and Snover in 1996[63]. SSAs are larger than (usually greater than 1 cm) HPs and more frequently located in the right colon[10]. Histologically, SSAs are distinguished from typical HPs by the presence of inverted T- or L-shaped crypt bases that reflect disordered proliferation (Figure 5, panel B). Other features include dilated crypts and serration extending into the lower third of the crypt. Focal nuclear stratification, mild nuclear atypia, or dystrophic goblet cells may be seen in the crypt bases[47,60,61]. Moreover, SSAs show increased mucin production, absence of enteroendocrine cells, and absence of a thickened basement membrane under the surface[43]. Other less common features include small foci of pseudostratification and eosinophilic change (identical to that seen in TSAs) of the surface epithelium. Small prominent nucleoli, open chromatin, and irregular nuclear contours also might be present, along with mitoses in the upper third of the crypts or on the surface itself[61]. SSAs are thought to represent approximately 2% of all colonoscopically removed polyps, over 8% of all polyps that were previously diagnosed as HPs and around 18% of all serrated polyps[60].
MP, also called SSA with cytological dysplasia include two separate hyperplastic and adenomatous components (Figure 5, panel C)[21,23]. One component is usually SSA (nondysplastic) whereas the second dysplastic component is either adenoma or TSA.
TSAs, usually present on distal location, are dysplastic serrated polyps which lack SSA patterns and more closely resemble conventional adenoma with tubulovillous architecture (Figure 5, panel D)[4,24,47,60]. Ectopic crypt formation, defined by the presence of crypts with bases not seated adjacent to the muscularis mucosae, is a feature that makes it possible to distinguish between TSAs and SSAs[4]. Columnar cells from the epithelium show eosinophilic cytoplasm, centrally placed elongated nuclei that are hyperchromatic and display pseudostratification[46].
There is no strong morphological evidence suggesting that SSAs are the precursor of TSAs, otherwise there are some histological and epidemiologic differences for keeping these lesions apart in different categories[4,61]. SSAs have been associated with proximal CRCs, high level of CIMP, BRAF mutations and MSI-high[47,48]. TSAs have been associated with distal location and MSS, CIMP-low CRCs with KRAS mutations[48]. SSAs, TSAs and MPs are described as “advanced serrated polyps” and represent approximately 5%-15% of all serrated polyps found in colonoscopy patients[23].
FUTURE DIRECTIONS
Advances in the knowledge about the serrated pathway of carcinogenesis are making it possible to differentiate a new type of CRC with different natural history, prognosis and response to chemotherapy treatment. For this reason it is important to be able to easily identify this kind of colorectal tumor and its precursors. Identification of molecular markers in both polyps and cancers that follow this pathway will provide the opportunity of a better understanding of how these tumours grow and how we could explain differences in clinical presentation, evolution and symptoms in different types of CRC. These molecular markers will also allow improvement in the identification of patients with serrated polyposis, moving forward the currently used clinical criteria, and will give us better rationale for appropriate management and surveillance intervals for patients and their relatives.
Footnotes
Supported by Grants from Instituto de Salud Carlos III, INT09/208 and PI08/0726, to Jover R; Fundación de la CV para la Investigación en el Hospital General Universitario de Alicante, to Alenda C, Payá A and Jover R; a predoctoral grant from Conselleria d’Educació de la Generalitat Valenciana, VALi+d. EXP ACIF/2010/018, to Guarinos C; a grant from Fundación de la CV para la Investigación en el Hospital General Universitario de Alicante, to Rodríguez-Soler M
Peer reviewers: Michael Linnebacher, PhD, Molecular Oncology and Immunotherapy, University of Rostock, Schillingallee 35, 18055 Rostock, Germany; Lodewijk AA Brosens, MD, PhD, Department of Pathology, University Medical Center Utrecht, Postbus 85500, 3508 GA Utrecht, The Netherlands
S- Editor Gou SX L- Editor O’Neill M E- Editor Li JY
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 3.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50:113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 4.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 5.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated (“hyperplastic”) polyposis. In: Bosman ST, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of tumours of the digestive system. Berlin: Springer-Verlag; 2010. [Google Scholar]
- 7.Boparai KS, Mathus-Vliegen EM, Koornstra JJ, Nagengast FM, van Leerdam M, van Noesel CJ, Houben M, Cats A, van Hest LP, Fockens P, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: a multicentre cohort study. Gut. 2010;59:1094–1100. doi: 10.1136/gut.2009.185884. [DOI] [PubMed] [Google Scholar]
- 8.Yeoman A, Young J, Arnold J, Jass J, Parry S. Hyperplastic polyposis in the New Zealand population: a condition associated with increased colorectal cancer risk and European ancestry. N Z Med J. 2007;120:U2827. [PubMed] [Google Scholar]
- 9.Chow E, Lipton L, Lynch E, D’Souza R, Aragona C, Hodgkin L, Brown G, Winship I, Barker M, Buchanan D, et al. Hyperplastic polyposis syndrome: phenotypic presentations and the role of MBD4 and MYH. Gastroenterology. 2006;131:30–39. doi: 10.1053/j.gastro.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Ferrández A, Samowitz W, DiSario JA, Burt RW. Phenotypic characteristics and risk of cancer development in hyperplastic polyposis: case series and literature review. Am J Gastroenterol. 2004;99:2012–2018. doi: 10.1111/j.1572-0241.2004.30021.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalady MF, Jarrar A, Leach B, LaGuardia L, O’Malley M, Eng C, Church JM. Defining phenotypes and cancer risk in hyperplastic polyposis syndrome. Dis Colon Rectum. 2011;54:164–170. doi: 10.1007/DCR.0b013e3181fd4c15. [DOI] [PubMed] [Google Scholar]
- 12.Lage P, Cravo M, Sousa R, Chaves P, Salazar M, Fonseca R, Claro I, Suspiro A, Rodrigues P, Raposo H, et al. Management of Portuguese patients with hyperplastic polyposis and screening of at-risk first-degree relatives: a contribution for future guidelines based on a clinical study. Am J Gastroenterol. 2004;99:1779–1784. doi: 10.1111/j.1572-0241.2004.30178.x. [DOI] [PubMed] [Google Scholar]
- 13.Rubio CA, Stemme S, Jaramillo E, Lindblom A. Hyperplastic polyposis coli syndrome and colorectal carcinoma. Endoscopy. 2006;38:266–270. doi: 10.1055/s-2006-925026. [DOI] [PubMed] [Google Scholar]
- 14.Boparai KS, Reitsma JB, Lemmens V, van Os TA, Mathus-Vliegen EM, Koornstra JJ, Nagengast FM, van Hest LP, Keller JJ, Dekker E. Increased colorectal cancer risk in first-degree relatives of patients with hyperplastic polyposis syndrome. Gut. 2010;59:1222–1225. doi: 10.1136/gut.2009.200741. [DOI] [PubMed] [Google Scholar]
- 15.Samowitz WS, Albertsen H, Sweeney C, Herrick J, Caan BJ, Anderson KE, Wolff RK, Slattery ML. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 16.Walker RG, Landmann JK, Hewett DG, Worthley DL, Buttenshaw RL, Knight N, Webb PM, Whiteman DC, Whitehall VL, Leggett BA. Hyperplastic polyposis syndrome is associated with cigarette smoking, which may be a modifiable risk factor. Am J Gastroenterol. 2010;105:1642–1647. doi: 10.1038/ajg.2009.757. [DOI] [PubMed] [Google Scholar]
- 17.Wallace K, Grau MV, Ahnen D, Snover DC, Robertson DJ, Mahnke D, Gui J, Barry EL, Summers RW, McKeown-Eyssen G, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev. 2009;18:2310–2317. doi: 10.1158/1055-9965.EPI-09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynter CV, Walsh MD, Higuchi T, Leggett BA, Young J, Jass JR. Methylation patterns define two types of hyperplastic polyp associated with colorectal cancer. Gut. 2004;53:573–580. doi: 10.1136/gut.2003.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvajal-Carmona LG, Howarth KM, Lockett M, Polanco-Echeverry GM, Volikos E, Gorman M, Barclay E, Martin L, Jones AM, Saunders B, et al. Molecular classification and genetic pathways in hyperplastic polyposis syndrome. J Pathol. 2007;212:378–385. doi: 10.1002/path.2187. [DOI] [PubMed] [Google Scholar]
- 20.Chan TL, Zhao W, Leung SY, Yuen ST. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63:4878–4881. [PubMed] [Google Scholar]
- 21.Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beach R, Chan AO, Wu TT, White JA, Morris JS, Lunagomez S, Broaddus RR, Issa JP, Hamilton SR, Rashid A. BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol. 2005;166:1069–1075. doi: 10.1016/S0002-9440(10)62327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young J, Jass JR. The case for a genetic predisposition to serrated neoplasia in the colorectum: hypothesis and review of the literature. Cancer Epidemiol Biomarkers Prev. 2006;15:1778–1784. doi: 10.1158/1055-9965.EPI-06-0164. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus R, Junttila OE, Karttunen TJ, Mäkinen MJ. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol. 2005;123:349–359. doi: 10.1309/VBAG-V3BR-96N2-EQTR. [DOI] [PubMed] [Google Scholar]
- 25.Lindor NM. Hereditary colorectal cancer: MYH-associated polyposis and other newly identified disorders. Best Pract Res Clin Gastroenterol. 2009;23:75–87. doi: 10.1016/j.bpg.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young J, Barker MA, Simms LA, Walsh MD, Biden KG, Buchanan D, Buttenshaw R, Whitehall VL, Arnold S, Jackson L, et al. Evidence for BRAF mutation and variable levels of microsatellite instability in a syndrome of familial colorectal cancer. Clin Gastroenterol Hepatol. 2005;3:254–263. doi: 10.1016/s1542-3565(04)00673-1. [DOI] [PubMed] [Google Scholar]
- 28.Frazier ML, Xi L, Zong J, Viscofsky N, Rashid A, Wu EF, Lynch PM, Amos CI, Issa JP. Association of the CpG island methylator phenotype with family history of cancer in patients with colorectal cancer. Cancer Res. 2003;63:4805–4808. [PubMed] [Google Scholar]
- 29.Chan AO, Issa JP, Morris JS, Hamilton SR, Rashid A. Concordant CpG island methylation in hyperplastic polyposis. Am J Pathol. 2002;160:529–536. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minoo P, Baker K, Goswami R, Chong G, Foulkes WD, Ruszkiewicz AR, Barker M, Buchanan D, Young J, Jass JR. Extensive DNA methylation in normal colorectal mucosa in hyperplastic polyposis. Gut. 2006;55:1467–1474. doi: 10.1136/gut.2005.082859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jarrar AM, Church JM, Fay S, Kalady MF. Is the phenotype mixed or mistaken? Hereditary nonpolyposis colorectal cancer and hyperplastic polyposis syndrome. Dis Colon Rectum. 2009;52:1949–1955. doi: 10.1007/DCR.0b013e3181b5450c. [DOI] [PubMed] [Google Scholar]
- 32.Boparai KS, Dekker E, Van Eeden S, Polak MM, Bartelsman JF, Mathus-Vliegen EM, Keller JJ, van Noesel CJ. Hyperplastic polyps and sessile serrated adenomas as a phenotypic expression of MYH-associated polyposis. Gastroenterology. 2008;135:2014–2018. doi: 10.1053/j.gastro.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Sweet K, Willis J, Zhou XP, Gallione C, Sawada T, Alhopuro P, Khoo SK, Patocs A, Martin C, Bridgeman S, et al. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. JAMA. 2005;294:2465–2473. doi: 10.1001/jama.294.19.2465. [DOI] [PubMed] [Google Scholar]
- 34.Roberts A, Nancarrow D, Clendenning M, Buchanan DD, Jenkins MA, Duggan D, Taverna D, McKeone D, Walters R, Walsh MD, et al. Linkage to chromosome 2q32.2-q33.3 in familial serrated neoplasia (Jass syndrome) Fam Cancer. 2011;10:245–254. doi: 10.1007/s10689-010-9408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooker JC, Saunders BP, Shah SG, Thapar CJ, Suzuki N, Williams CB. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc. 2002;55:371–375. doi: 10.1067/mge.2002.121597. [DOI] [PubMed] [Google Scholar]
- 36.Iino H, Jass JR, Simms LA, Young J, Leggett B, Ajioka Y, Watanabe H. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52:5–9. doi: 10.1136/jcp.52.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goel A, Nagasaka T, Arnold CN, Inoue T, Hamilton C, Niedzwiecki D, Compton C, Mayer RJ, Goldberg R, Bertagnolli MM, et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007;132:127–138. doi: 10.1053/j.gastro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst. 2001;93:1307–1313. doi: 10.1093/jnci/93.17.1307. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Cho NY, Choi M, Yoo EJ, Kim JH, Kang GH. Clinicopathological features of CpG island methylator phenotype-positive colorectal cancer and its adverse prognosis in relation to KRAS/BRAF mutation. Pathol Int. 2008;58:104–113. doi: 10.1111/j.1440-1827.2007.02197.x. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka H, Deng G, Matsuzaki K, Kakar S, Kim GE, Miura S, Sleisenger MH, Kim YS. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer. 2006;118:2765–2771. doi: 10.1002/ijc.21701. [DOI] [PubMed] [Google Scholar]
- 41.Velho S, Moutinho C, Cirnes L, Albuquerque C, Hamelin R, Schmitt F, Carneiro F, Oliveira C, Seruca R. BRAF, KRAS and PIK3CA mutations in colorectal serrated polyps and cancer: primary or secondary genetic events in colorectal carcinogenesis? BMC Cancer. 2008;8:255. doi: 10.1186/1471-2407-8-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jass JR. Colorectal polyposes: from phenotype to diagnosis. Pathol Res Pract. 2008;204:431–447. doi: 10.1016/j.prp.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Jass JR. Hyperplastic polyps and colorectal cancer: is there a link? Clin Gastroenterol Hepatol. 2004;2:1–8. doi: 10.1016/s1542-3565(03)00284-2. [DOI] [PubMed] [Google Scholar]
- 44.Jover R, Nguyen TP, Pérez-Carbonell L, Zapater P, Payá A, Alenda C, Rojas E, Cubiella J, Balaguer F, Morillas JD, et al. 5-Fluorouracil adjuvant chemotherapy does not increase survival in patients with CpG island methylator phenotype colorectal cancer. Gastroenterology. 2011;140:1174–1181. doi: 10.1053/j.gastro.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minoo P, Moyer MP, Jass JR. Role of BRAF-V600E in the serrated pathway of colorectal tumourigenesis. J Pathol. 2007;212:124–133. doi: 10.1002/path.2160. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg DW, Yang S, Pleau DC, Greenspan EJ, Stevens RG, Rajan TV, Heinen CD, Levine J, Zhou Y, O’Brien MJ. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res. 2007;67:3551–3554. doi: 10.1158/0008-5472.CAN-07-0343. [DOI] [PubMed] [Google Scholar]
- 47.Huang CS, Farraye FA, Yang S, O’Brien MJ. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106:229–240; quiz 241. doi: 10.1038/ajg.2010.429. [DOI] [PubMed] [Google Scholar]
- 48.East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am. 2008;37:25–46, v. doi: 10.1016/j.gtc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Whitehall VL, Walsh MD, Young J, Leggett BA, Jass JR. Methylation of O-6-methylguanine DNA methyltransferase characterizes a subset of colorectal cancer with low-level DNA microsatellite instability. Cancer Res. 2001;61:827–830. [PubMed] [Google Scholar]
- 50.Hesson LB, Hitchins MP, Ward RL. Epimutations and cancer predisposition: importance and mechanisms. Curr Opin Genet Dev. 2010;20:290–298. doi: 10.1016/j.gde.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Worthley DL, Whitehall VL, Buttenshaw RL, Irahara N, Greco SA, Ramsnes I, Mallitt KA, Le Leu RK, Winter J, Hu Y, et al. DNA methylation within the normal colorectal mucosa is associated with pathway-specific predisposition to cancer. Oncogene. 2010;29:1653–1662. doi: 10.1038/onc.2009.449. [DOI] [PubMed] [Google Scholar]
- 52.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 53.Brooker JC, Saunders BP, Shah SG, Thapar CJ, Thomas HJ, Atkin WS, Cardwell CR, Williams CB. Total colonic dye-spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2002;56:333–338. doi: 10.1016/s0016-5107(02)70034-5. [DOI] [PubMed] [Google Scholar]
- 54.Hurlstone DP, Cross SS, Slater R, Sanders DS, Brown S. Detecting diminutive colorectal lesions at colonoscopy: a randomised controlled trial of pan-colonic versus targeted chromoscopy. Gut. 2004;53:376–380. doi: 10.1136/gut.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lapalus MG, Helbert T, Napoleon B, Rey JF, Houcke P, Ponchon T. Does chromoendoscopy with structure enhancement improve the colonoscopic adenoma detection rate? Endoscopy. 2006;38:444–448. doi: 10.1055/s-2006-925265. [DOI] [PubMed] [Google Scholar]
- 56.Le Rhun M, Coron E, Parlier D, Nguyen JM, Canard JM, Alamdari A, Sautereau D, Chaussade S, Galmiche JP. High resolution colonoscopy with chromoscopy versus standard colonoscopy for the detection of colonic neoplasia: a randomized study. Clin Gastroenterol Hepatol. 2006;4:349–354. doi: 10.1016/j.cgh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 57.Rastogi A, Bansal A, Wani S, Callahan P, McGregor DH, Cherian R, Sharma P. Narrow-band imaging colonoscopy--a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc. 2008;67:280–286. doi: 10.1016/j.gie.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 58.Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092–1096. doi: 10.1055/s-2007-966781. [DOI] [PubMed] [Google Scholar]
- 59.Boparai KS, van den Broek FJ, van Eeden S, Fockens P, Dekker E. Hyperplastic polyposis syndrome: a pilot study for the differentiation of polyps by using high-resolution endoscopy, autofluorescence imaging, and narrow-band imaging. Gastrointest Endosc. 2009;70:947–955. doi: 10.1016/j.gie.2009.03.1172. [DOI] [PubMed] [Google Scholar]
- 60.Liang JJ, Alrawi S, Tan D. Nomenclature, molecular genetics and clinical significance of the precursor lesions in the serrated polyp pathway of colorectal carcinoma. Int J Clin Exp Pathol. 2008;1:317–324. [PMC free article] [PubMed] [Google Scholar]
- 61.Sandmeier D, Benhattar J, Martin P, Bouzourene H. Serrated polyps of the large intestine: a molecular study comparing sessile serrated adenomas and hyperplastic polyps. Histopathology. 2009;55:206–213. doi: 10.1111/j.1365-2559.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 62.Hiraoka S, Kato J, Fujiki S, Kaji E, Morikawa T, Murakami T, Nawa T, Kuriyama M, Uraoka T, Ohara N, et al. The presence of large serrated polyps increases risk for colorectal cancer. Gastroenterology. 2010;139:1503–1510. doi: 10.1053/j.gastro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]