Abstract
Hyaluronan (HA) is an abundant matrix molecule whose functions in the skin remain to be fully defined. To explore the roles of HA in cutaneous injury responses, double-knockout mice (abbreviated as Has1/3 null) that lack two HA synthase enzymes (Has1 and Has3) but still express functional Has2, were used in two types of experiments: (i) application of 12-O-tetradecanoylphorbol-13-acetate (TPA), and (ii) full-thickness wounding of the skin. Uninjured Has1/3 null mice were phenotypically normal. However, after TPA, the accumulation of HA that normally occurs in wildtype epidermis was blunted in Has1/3 null epidermis. In excisional wound healing experiments, wound closure was significantly faster in Has1/3 null than in wildtype mice. Coincident with this abnormal wound healing, a marked decrease in epidermal and dermal HA and a marked increase in neutrophil efflux from cutaneous blood vessels were observed in Has1/3 null skin relative to wildtype skin. Has1/3 null wounds displayed an earlier onset of myofibroblast differentiation. In summary, selective loss of Has1 and Has3 leads to a pro-inflammatory milieu that favors recruitment of neutrophils and other inflammation-related changes in the dermis.
INTRODUCTION
Hyaluronan (HA) is an abundant extracellular matrix molecule in the dermis and epidermis of skin whose functions are only now beginning to be elucidated. It is a linear, non-sulfated glycosaminoglycan comprised of long chains of disaccharides (N-acetyl-glucosamine and glucuronic acid) repeated thousands of times, thereby reaching >106 Da in molecular weight (Itano, 2008; Itano et al., 1999). In the epidermis, HA is present in the extracellular matrix around keratinocytes (Tammi et al., 1988); its rapid turnover rate (t1/2 of 1-2 days) (Tammi et al., 1991) is determined by a balance between HA degradation (with the exact hyaluronidases responsible still undetermined), and synthesis by three hyaluronan synthases (Has1, Has2, and Has3) that when active reside in the plasma membrane (Rilla et al., 2005). The Has enzymes differ in kinetic properties and length of the HA chain produced (Itano et al., 1999). HA is normally extruded directly into the extracellular space (Wang and Hascall, 2004). The externalized HA interacts with an HA membrane receptor, CD44, which affects intracellular signaling and thereby regulates cellular proliferation and differentiation (Toole, 2004).
The three Has isoforms and their biological activities have been investigated in gene targeting studies. Functional deletion of the Has2 gene results in embryonic lethality with severe cardiac and vascular malformations (Camenisch et al., 2000). During early embryogenesis, Has2 is responsible for HA production, and loss of Has2 is not compensated for by Has1 or Has3. Has3 null (knockout) mice on the other hand are viable and fertile, with normal lifespans (Bai et al., 2005). Has1 null mice also appear perfectly normal under non-stressed conditions (Kobayashi et al., 2010).
In the skin, all three HAS enzymes are expressed throughout the epidermis and dermis (Sugiyama et al., 1998). Current studies suggest that Has3 may be more highly expressed in keratinocytes (Sayo et al., 2002), Has1 in fibroblasts (Yamada et al., 2004), and Has2 in both cell types. In vitro models have been used to evaluate the role of Has enzymes during various perturbations of the epidermis. Treatment of 3-D organotypic keratinocyte cultures with EGF or KGF results in increased proliferation, increased Has2 and Has3 expression, and elevated HA levels (Karvinen et al., 2003; Pasonen-Seppanen et al., 2003). Physical injury (needle stick) also triggers HA accumulation in the 3-D organotypic keratinocyte cultures, further supporting a causal relationship between inducible Has2 and Has3 expression and increased epidermal HA levels after injury (Monslow et al., 2009). Elevated HA levels are often observed in hyperproliferative epidermis in the setting of acute inflammation, so-called inflammatory hyperplasia. Tape-stripping of the skin, a form of physical injury, induces epidermal hyperplasia, inflammatory cell infiltration, transient induction of HA, and elevations in Has2 and Has3 mRNA levels (Tammi et al., 2005). Skin biopsies from patients with acute eczematous dermatitis (an inflammatory process) demonstrate increased levels of HA in areas of spongiosis, mediated by preferential increases in Has3 mRNA expression (Ohtani et al., 2009). Other evidence also suggests important, complex relationships between the production of HA, leukocyte recruitment, (e.g., gamma/delta T cells) and cytokine production within cutaneous wounds (Jameson et al., 2005). Therefore, strong evidence suggests links between the expression of Has enzymes, accumulation of HA, dermal inflammatory cell infiltrates, and epidermal hyperplasia after skin injury. How these events are connected mechanistically, however, remains to be determined.
We utilized two different injury models to elucidate the associations between HA, cutaneous inflammation, and epidermal hyperplasia. The phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA) is a well-established pro-inflammatory agent that stimulates inflammatory cytokine release, tissue edema, epidermal thickening, and cornified envelope formation when topically applied (Wang and Smart, 1999). TPA-driven inflammatory hyperplasia involves an imbalance between cell cycle stimulation and growth arrest (Marks and Furstenberger, 1993), and protein kinase C (PKC) isoforms are known to be major regulators (Cataisson et al., 2005). In studies with PKCα-overexpressing mice harboring an epidermally targeted transgene, TPA application was shown to cause the release of pro-proliferative and chemotactic factors including TNFα, MIP-2, KC, VEGF, and GM-CSF (Cataisson et al., 2005). Another type of skin injury that involves cytokine release, inflammation, and epidermal hyperplasia is full-thickness incisional wounding (Singer and Clark, 1999). Wounding triggers recruitment of leukocytes (neutrophils first, followed by macrophages) that have roles in modulating epidermal responses and in regulating the differentiation of fibroblasts into myofibroblasts (Singer and Clark, 1999).
In this paper, we examined possible links between changes in HA accumulation and epidermal and dermal events after skin injury, using a new mouse model that expresses only one of the HA synthetic enzymes, Has2. These mice are nullizygous for Has1 and Has3 (abbreviated as Has1/3 null). In response to skin injury, HA fails to accumulate in Has1/3 null mice. Using this model, we show that Has1 and Has3 are dispensable for epidermal hyperplasia, but are in fact necessary to properly regulate acute inflammation and fibroblast behavior in the skin following injury.
RESULTS
Mice harboring deletions in the Has1 gene (Kobayashi et al., 2010), or the Has3 gene (Bai et al., 2005) were intercrossed to create Has1 -/- Has3 -/- animals (Has1/3 null). These mice appear grossly normal, with good fertility and normal life spans.
Epidermal induction of HA and CD44 following TPA application is blunted in Has1/3 null mice
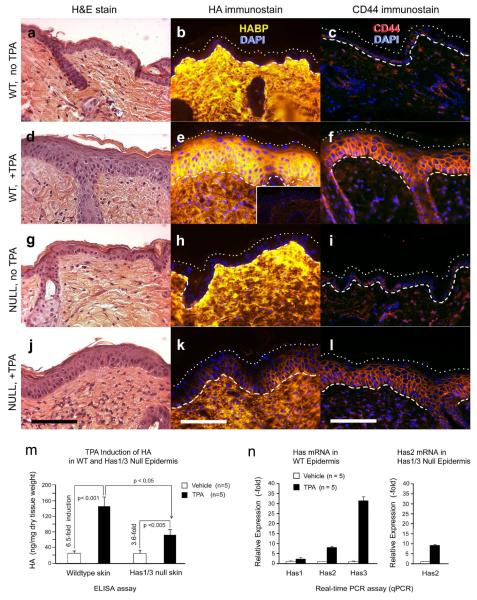
Dorsal skin was treated with TPA, and the skin was examined histologically for changes in morphology, HA, and CD44 (Fig. 1). In wildtype (WT) skin, TPA treatment caused epidermal hyperplasia (compare Fig. 1a and 1d) and a strong epidermal induction of HA, which rose from nearly undetectable levels (Tammi et al., 2005) to a very high amount (compare Fig. 1b and 1e). Immunohistochemical analysis of CD44 revealed strongly increased expression after TPA treatment (compare Figs. 1c and 1f). In contrast to WT mice, Has1/3 null mice showed a blunted response to TPA in terms of epidermal HA levels (compare Figs. 1h and 1k) and CD44 (compare Figs. 1i and 1l). Despite the loss of Has1 and Has3, hyperplastic epidermal thickening in response to TPA still occurred in Has1/3 null mice, to the same extent as in WT mice (compare Figs. 1g and 1j).
Figure. 1. TPA-stimulated accumulation of HA in the epidermis is blunted in Has1/3 null mice.
(a-l) Dorsal skin was treated with TPA or vehicle alone (x 3 days), biopsied, and evaluated histologically for morphologic changes (H&E stains), HA levels (bHABP and streptavidin-Cy3), and CD44 protein levels (anti-CD44/rhodamine). Specific HA staining was confirmed by pretreatment of specimens with hyaluronidase (panel e, Inset). (m, n) TPA-treated or control epidermis was separated using dispase, and analyzed for concentrations (ng/mg) of HA using an ELISA-like assay (m), or analyzed for relative expression of Has1, Has2, and Has3 mRNA using quantitative real-time PCR (qPCR) (n). In Has1/3 null skin, only Has2 expression was analyzed. The p values from two-sided Student t-test are indicated. Dashed/dotted lines: epidermal boundaries. Scale bars, 100 μm.
To confirm these findings by an independent method, an ELISA-like assay for HA was utilized (Fig. 1m). When compared to the 6.5-fold increase in HA in WT epidermis (Fig. 1m, left), the 3.6-fold HA induction in Has1/3 null epidermis was significantly less (Fig. 1m, right).
Epidermal induction of Has enzymes after TPA application in WT and Has1/3 null mice
To understand the basis for increased HA accumulation after TPA exposure, mRNA expression of HA-synthetic enzymes in the epidermis was examined. Epidermal sheets from vehicle- and TPA-treated WT mouse skin were separated and evaluated by quantitative real-time PCR (Fig. 1n, left). Has3 mRNA was preferentially increased by 31-fold, Has2 by 8-fold, Has1 by 2-fold. In Has1/3 null epidermis, Has2 expression after TPA treatment was examined (Fig. 1n, right), and found to be induced to a nearly identical extent (9-fold) as in WT epidermis.
Immunostaining revealed identical expression patterns for markers of keratinocyte proliferation (Ki67) and differentiation (keratins K10 and K14) in the WT and Has1/3 null epidermis (data not shown), indicating that Has1/3 mice mount a normal proliferation and differentiation response to TPA even in the absence of a full HA induction in the epidermis.
Wound healing in Has1/3 null mice is abnormal, with accelerated wound closure
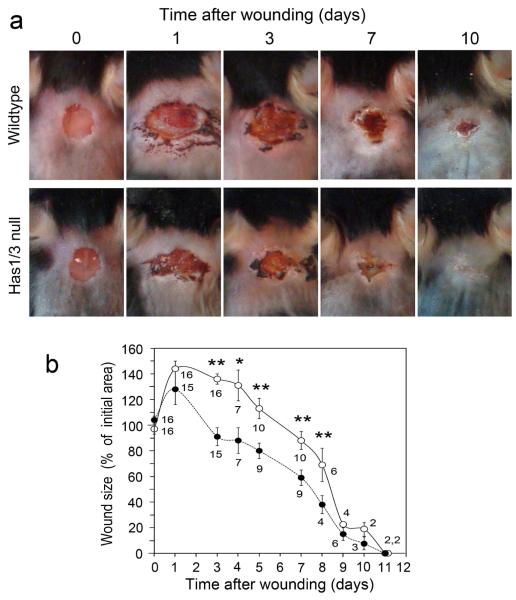
In a different model of cutaneous injury, full-thickness excisional wounds were generated in WT and Has1/3 null mice, and the course of wound healing in normal versus null mice was compared (Fig. 2). A striking difference was observed during the first 9 days post-wounding; namely, the rate of wound closure was higher in Has1/3 null mice than in WT controls (Fig. 2a). Whereas all excisional wounds in mice tended to stretch and expand during the first day after wound placement, Has1/3 null wounds had returned to the initial 5-mm wound diameter by day 3 and achieved 90% wound closure by day 9. WT wounds did not reach those benchmarks until days 6 and 10, respectively (Fig. 2b). No other major differences were discernable at a macroscopic level.
Figure 2. Wound closure is accelerated in Has1/3 null mice.
(a) Typical examples of 5-mm diameter full-thickness excisional wounds. Wounds were photographed daily until closure. (b) Graphical summary of changes in wound area, expressed relative to the initial size of the wound at day zero. Data are mean ± SEM; the number of mice analyzed at each time point is shown beneath each data symbol. Open circles, wildtype; Closed circles, Has1/3 null. (*), p < 0.01 ; (**), p < 0.005 by two-sided Student t-test.
Both epidermal and dermal levels of HA after skin injury are lower in Has1/3 null mice, relative to WT mice
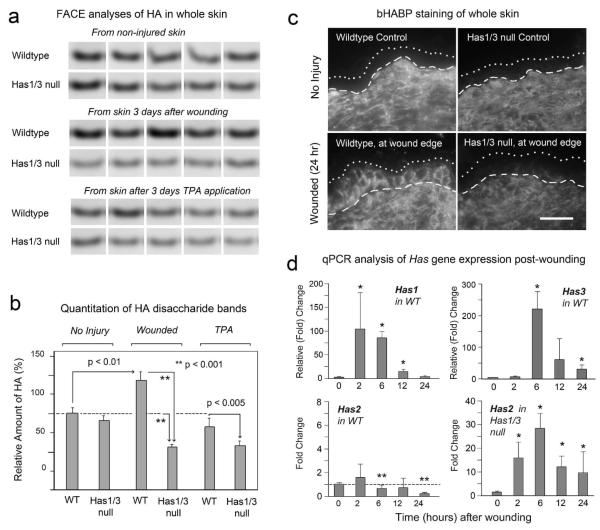
We were also interested in evaluating HA in the dermis, in response to TPA and wounding, because dermal HA constitutes the great majority of HA in the skin. To do this, we utilized a biochemical technique called FACE (Fluorophore-Assisted Carbohydrate Electrophoresis) which measures the total mass of HA in tissues (Calabro et al., 2000). We measured total HA in WT and Has1/3 null skin after full-thickness wounding and in TPA treated skin (Fig. 3a, b). In WT mice, full-thickness wounding significantly induces HA levels by 3 days, to ~145% of control levels. While a portion of this increase may reflect increased epidermal HA, as visualized by bHABP staining (Fig. 3c), the bulk of the measured HA accumulation comes from the dermis due to the overwhelming proportion of HA in the dermal compartment. In Has1/3 null mice, HA levels in control (uninjured) skin were similar to levels in WT controls (Fig. 3a, b). After full-thickness wounding, however, the dermal HA content of Has1/3 null wounds was significantly less than in WT wounds (Fig. 3a, b), only ~55% the level of uninjured controls. This relative decrease in dermal HA content of Has1/3 null skin after wounding can also be seen by bHABP staining (Fig. 3c, compare lower left and right panels). Note also that epidermal HA fails to accumulate in Has1/3 null epidermis (Fig. 3c), as was seen with TPA treatment (Fig. 1).
Figure 3. HA levels are altered in Has1/3 null mice after wounding.
(a) Fluorescence-Assisted Carbohydrate Electrophoresis analysis of HA levels in equivalent amounts (weight) of skin from WT or Has1/3 null mice; treatments as shown. Disaccharide (Δ di-HA) bands are displayed for individual wounds (5 mice/condition). (b) Integrated fluorescent intensity of Δ di-HA bands from 3-day wounds or TPA-treated skin, relative to WT non-injured controls (mean ± SD, n=5). (c) HA in skin adjacent to incisional wounds (<1 mm from wound edge) at 24 h post-injury, immunostained with bHABP/streptavidin-Cy3. Bar, 25 μm. (d) Time course of Has mRNA expression post-wounding in WT mice or Has1/3 null mice. Mean ± SD, duplicate mice, triplicate qPCR reactions per wound. (*) significant increase or (**) significant decrease, at p<0.05 level.
To determine how changes in Has enzyme gene expression might contribute to the changes in overall HA levels after full-thickness injury, a time course experiment using real-time PCR was done to examine Has gene expression after wounding in WT and Has1/3 null mice (Fig. 3d). In WT mice, strong inductions in Has1 (~100-fold) and Has3 (~200-fold) were observed at 2-6 h post-wounding. These inductions were transient, declining toward baseline by ~24 h post-wounding. In contrast, Has2 expression was not induced in WT skin, and in fact declined after 2 h post-wounding. In Has1/3 null wounds, on the other hand, Has2 was strongly induced at all times after wounding. However, as shown in Fig. 3a and 3b, this upregulation of Has2 was not sufficient to compensate for loss of Has1 and Has3, as far as HA induction is concerned.
Increases in TPA-induced inflammation and wound-induced inflammation and fibrosis in Has1/3 null skin are associated with reduced HA levels
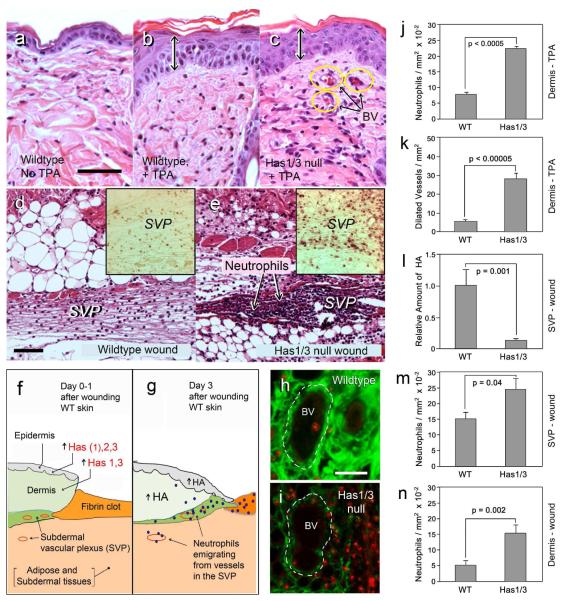
Because we observed a decrease in dermal HA in Has1/3 null injured skin, and knowing that HA influences inflammation in other systems (Wang et al., 2011; Wang and Hascall, 2004), we examined inflammatory responses in our two injury models. After TPA treatment, leukocyte infiltration into the dermis was greater in Has1/3 null mice than in control mice (Fig. 4a-c), despite the equivalent amounts of epidermal hyperplasia (Fig. 4b and 4c, double arrows). This was a mixed inflammatory infiltrate consisting of ~35% neutrophils (determined by comparing H&E stains and neutrophil-specific immunostains using the RB6/85c antibody; data not shown). Neutrophils displayed a 3-fold relative increase after TPA in Has1/3 null mice (Fig. 4j), along with a 5-fold increase in the number of dilated blood vessels in the upper dermis (Fig. 4c, k) suggesting an increased release of vasodilatory cytokines. In full-thickness wounds, a preferential increase in neutrophil recruitment was also seen in the Has1/3 mice (Fig. 4d, e). During normal wound healing, an influx of neutrophils begins within a few hours after injury and reaches a maximum between days 1 and 3 (Dechert et al., 2006). In WT mice at 24 h post-wounding, neutrophils were observed transmigrating from vessels of the subdermal vascular plexus (SVP), a region located immediately beneath the dermis and easily visualized in the skin adjacent to the wound (Fig. 4d; also see the drawings in Figs. 4f, g). In Has1/3 null mice at 24 h, the number of neutrophils effluxing from the SVP or present in the dermis was markedly increased (Fig. 4e, m, n). This increase in neutrophil recruitment was reflected in the number of intravascular neutrophil aggregates in the SVP that were detected in immunostained sections (Supplem Fig. 1c, c’).
Figure 4. Preferentially enhanced inflammation in Has1/3 null skin following TPA application (3 days) or full-thickness wounding (24 hours).
(a-c) H&E stains of non-injured wildtype, TPA-treated wildtype, or TPA-treated Has1/3 null skin. BV, dilated blood vessels. (d, e) Masson-Trichrome stains of subdermal vascular plexus (SVP) immediately adjacent to the wound, in wildtype or Has1/3 null mice. Insets, neutrophil-specific immunostains of the SVP. (f, g): Cartoon of events in wildtype skin after wounding. (h, i) Tissues in the SVP region, co-stained for HA (green) and neutrophils (red). GRAPHS: Data (mean ± SEM) for neutrophils (j) and dilated vessels (k) after TPA treatment; or HA staining intensity (l), neutrophils near blood vessels in the subdermis (m), and neutrophils in the dermis (n) of wounded skin. Scale bars, 50 μm.
Mechanistically, the SVP is of particular interest because this zone appears to contain the highest HA levels of the entire skin (Supplem Fig. 1a) and is a major site of neutrophil recruitment to vessels (Supplem Fig. 1b, b’). Levels of HA in the SVP region are reduced in Has1/3 null skin, both in the unwounded situation (Supplem Fig. 1a’) and at 24 h post-wounding (illustrated in Fig. 4h and 4i, and quantified in Fig. 4L). Notably, double-staining for HA and myeloperoxidase reveals that a robust neutrophil migration from blood vessels in the SVP appears to be significantly associated with low HA in the perivascular matrix (Fig. 4i, m).
A time course experiment provided further evidence that neutrophil recruitment is accelerated in the Has1/3 null wounds. Whereas more neutrophils were seen at Day 1, fewer were seen at Day 5 (relative to WT wounds), an observation consistent with faster resolution of the neutrophilic infiltrate (Supplem Table 1), although more detailed experiments will be needed to confirm this point. Macrophages, on the other hand, showed similar behavior in the Has1/3 null and WT wounds, first appearing at Day 3 and becoming equally abundant at Days 5 and 10 (Supplem Table 1).
We also examined the dermal and subcutaneous regions for evidence of relative changes in fibroblast proliferation and differentiation. The results suggest an earlier onset of myofibroblast differentiation in the Has1/3 null wounds (Supplem Table 1). Beginning at Day 5 after wounding, spindle-shaped cells (many of which expressed alpha-smooth muscle actin; Supplem Fig. 1d, d’) appeared in Has1/3 null skin at the wound edge and beneath the wound bed, but were much less evident in the WT wounds.
DISCUSSION
In this study we examined the epidermal and dermal injury responses in mice lacking Has1 and Has3, as compared to WT mice. Levels of HA, expression of Has enzymes, inflammation, and fibroblast behavior were evaluated. Two different kinds of injury, TPA and wounding, were utilized. For both types of injury, similarities in HA responses in the epidermis and in the dermis were noted, as follows. In normal epidermis, large accumulations of HA were observed, both after TPA (Fig. 1e) and after wounding (Fig. 3c). In Has1/3 null epidermis, HA accumulations were smaller than in WT epidermis, but some HA induction was still observed. Levels of CD44 (the HA receptor) on keratinocyte plasma membranes appeared to correlate with overall HA levels (Fig. 1), consistent with suggestions that CD44 levels are controlled by the receptor internalization rate; this rate is slowed when CD44 is retained on the surface through binding to extracellular HA ligand (Knudson et al., 2002). In the dermis, changes were more difficult to interpret. In WT skin, dermal HA was increased after wounding (up ~145%) but not after TPA (Fig. 3a, b). In Has1/3 null skin, on the other hand, either wounding or TPA exposure led to a decline in dermal HA of ~40-50% (Fig. 3b). We can only speculate that this decline is the result of dermal hyaluronidase activity, which becomes unopposed by HA synthesis in mice that lack both Has1 and Has3.
Our data also provide information on the relative contribution that changes in Has1, 2, and 3 expression may be making to the accumulation of HA in epidermal and dermal compartments after injury. Epidermal Has1 expression remains low (essentially unchanged) after TPA exposure in WT mice (Fig. 1n), suggesting that Has2 and Has3 (which are significantly induced after TPA) are the principal enzymes responsible for HA accumulation in the epidermis following TPA. Has2 is induced to the same extent in Has1/3 null epidermis as in WT epidermis (8-fold in each case, Fig. 1n) following TPA injury, which appears to explain why significant amounts of HA can still be synthesized in the Has1/3 null mice (Fig. 1m). In the dermis, however, both Has1 and Has3 are highly induced post-wounding, whereas Has2 is not. This indicates that Has3 is a major responder following both types of injury, and in both tissue compartments. Interestingly in Has1/3 null mice, a compensatory increase in dermal Has2 expression is observed after wounding as compared to WT mice (Fig. 3d); this could have functional implications, as discussed further below.
Two novel injury phenotypes are observed in Has1/3 null mice: (i) faster wound closure than in WT mice (Fig. 2), and (ii) an exaggerated neutrophil recruitment following cutaneous injury (Fig. 4). Has1/3 null wounds were smaller at all time points measured. The enhanced population of myofibrobasts beginning at day 5 in null wounds could account for the faster closure at day 5 and beyond. At earlier time points (days 1 and 3), the lower amounts of HA in Has1/3 null wounds could lead to drier, less edematous tissue (since HA is very hydrophilic), and hence to a smaller wound size. Alternatively, cytokines released by neutrophils in Has1/3 null wounds may stimulate premature wound contraction through increased fibroblast cytoskeletal contractility, even in the absence of myofibroblast transformation (Vishwanath et al., 2003).
Neutrophils are important participants in a number of inflammatory responses of the skin. In mice, repeated treatment with topical TPA results in a temporal influx of neutrophils that becomes maximal at 3 days (Alford et al., 1992). After acute wounding, neutrophil influx into the skin is detectable by 4 hr, and plateaus at ~3 days (Dechert et al., 2006; Kim et al., 2008). Because the half-life of neutrophils is only a few hours (Kim et al., 2008), neutrophil accumulation at wound sites must reflect continuous recruitment of circulating neutrophils, regulated in some manner by HA. In Has1/3 null mice, reduced levels of dermal HA are associated with an increase in neutrophils at the injury site. In our search for the mechanistic link between HA and enhanced neutrophil recruitment, we hypothesize that reduced HA in Has1/3 null venular walls (Fig. 4) at sites of injury results in increased neutrophil adhesion. Other studies have shown that HA reduces neutrophil adhesion to human endothelial vein cells in vitro (Alam et al., 2005; Forrester and Wilkinson, 1981), and that adhesion of neutrophils to postcapillary venules in vivo after PMA treatment is inhibited by intravenous administration of HA (Alam et al., 2005). An additional hypothesis to explain enhanced neutrophil influx into HA-deficient Has1/3 null dermis is that HA inhibits neutrophil migration (Alstergren et al., 2004). For example, HA-rich tissues (e.g. cartilage; vitreous of the eye) are resistant to influxes of inflammatory cells (Forrester and Lackie, 1981). Other indirect mechanisms may exist. For instance, the synthesis or release of neutrophil chemotactic factors such IL-6 or IL-8 (Pauloin et al., 2009) might be altered.
Two additional points of interest can be noted regarding functions of Has enzymes in the skin. First, epidermal hyperplasia still occurs in Has1/3 null mice despite a failure to induce high levels of HA. Therefore, massive epidermal accumulation of HA is not required for epidermal hyperplasia following TPA, but at least some HA may still be needed for proper epidermal proliferation and stratification since Has2 is still expressed in Has1/3 null epidermis. Partial functional redundancy was described in mice hemizygous for Has2 and nullizygous for Has3 (Has2 +/−, Has3 −/−) (McDonald and Camenisch, 2002; Spicer et al., 2002), suggesting that partial compensation by Has2 is likely in the Has1/3 null mice. Secondly, our data support a role for all three Has enzymes in cutaneous responses after injury. In the WT epidermis, mRNA expression of Has3 and Has2 was increased more than Has1 after TPA stimulation (Fig. 1n). In wounded WT whole skin, both Has1 and Has3 were induced, whereas Has2 expression actually declined (Fig. 3d). This suggests that the relative contribution of different Has enzymes to HA production is dynamic and cell specific, and might contribute to subsequent events through different mechanisms. First, because the average polymer length of HA synthesized by each type of Has enzyme is different (Has1 = Has2 > Has3), a change in the relative amounts of each Has could differentially affect cellular responses. For example, lower mass HA (<200,000 Da), such as that produced by Has3, can more efficiently activate intracellular signaling via CD44 (Tammi et al., 2002). Second, as mentioned above, different cell types may express distinctly different levels of each Has, leading to different cell-specific responses for keratinocytes, vascular endothelium, and fibroblasts after injury. Other investigators have noted the relative importance of Has3 in keratinocytes under basal conditions (Sayo et al., 2002), in response to inflammatory cytokines (Ohtani et al., 2009), and during epidermal hyperplasia caused by sonophoresis (Lee et al., 2009). TPA can promote phosphorylation of Has3 on a critical serine residue (Goentzel et al., 2006), and phosphorylation regulates Has3 activity (Vigetti et al., 2009). In mice subjected to ventilator induced lung injury, Has3 was required to generate low MW HA, accompanied by induction of MIP-2 and a neutrophilic infiltrate (Bai et al., 2005).
For other tissues, however, Has2 may be of relatively greater importance. In mice, conditional inactivation of Has2 leads to disruption in mesenchymal (as opposed to epithelial) compartments, including defects in skeletal and cartilage development (Matsumoto et al., 2009). In the skin of Has1/3 null mice, we have shown that Has2 expression cannot compensate quantitatively for the loss of dermal HA levels. However, the increased Has2 observed in Has1/3 null wounds may be contributing to a pro-fibrotic phenotype. Has2 has been implicated in fibrosis following lung injury (Heldin et al., 2008), and is thought to have an important role in fibroblast to myofibroblast conversion (Webber et al., 2009). Finally, we cannot rule out the possibilty that Has2 induced in Has1/3 null skin (Fig. 3d) may cause a qualitative change in HA produced at specific locations within the dermis, thus contributing to the pro-inflammatory milieu.
In summary, our study demonstrates that the balance of HA produced by distinct Has enzymes is important for regulating inflammatory responses and wound contraction in the skin after injury. Future studies will address the question of whether altered HA patterns in Has1/3 null mice can affect long-term scar formation and tensile strength, keeping in mind that faster healing does not necessarily mean better quality healing. Although detailed mechanisms remain to be determined, areas to focus on will be the role of HA in and around venules during neutrophil recruitment, and the role that dermal HA plays in the proliferation and differentiation of myofibroblasts. .
Materials and Methods
Animals
C57BL/6J mice were obtained from JAX Laboratories. Has1-/- mice (Kobayashi et al., 2010) and Has3-/- mice (Bai et al., 2005) were generated previously using a strategy that eliminates the catalytic site of each enzyme. Has1-/- and Has3-/- mice were intercrossed to generate animals nullizygous for both alleles (Has1/3 null). All mice were maintained in accordance with guidelines of the American Association for the Accreditation of Laboratory Animal Care, and were approved by our Institutional Animal Care and Use Committee (IACUC).
Injury with 12-o-tetradecanoylphorbol-13-acetate (TPA)
For TPA experiments, dorsal skin of 6-10 wk old mice was shaved 48 h prior to application. TPA (Sigma, St. Louis, MO) was prepared (5 mg, dissolved in 20 ml DMSO and then diluted to 100 ml in acetone) and applied to the animal’s back with a cotton swab and gently rubbed (10 μg per application; twice daily for 3 d). Mice were euthanized 2 h after the last treatment, and the skin harvested, fixed in 4% paraformaldehyde, and embedded in paraffin.
Wound healing experiments
All procedures were pre-approval by our institution’s Animal Care and Use Committee (IACUC). Mice (C57/BL6 wildtype, or Has1/3 null, 9-10 weeks of age) were anesthetized with pentobarbital and fur shaved from the upper back. To create full-thickness excisional wounds (one wound per mouse), a 5-mm circular template of sticky tape (cut with a biopsy punch) was placed ~1 cm posterior to the ears. A 5-mm excisional wound created down to fascia, using fine iris scissors. At specified times (from 0 to 11 days), mice were anesthetized with isofluorane and photographed at a fixed distance using a digital camera on a stand. The area of wounds was determined from the digital photos, using image software (pixel counts).
For histological examination, wounds were harvested at 0, 1, 3, 5, and 10 days post-wounding along with unwounded skin. A ~1 cm square of tissue around the wound and beneath the dermis was collected by careful dissection, placed on 3MM Whatman paper (with the orientation noted by marking the caudal pole with a pen). The tissue was bisected with a razor blade oriented in the cephalad-caudad direction. Tissue was fixed in Histochoice (Amresco, Solon, OH), paraffin-embedded, and stored for later sectioning.
Full-thickness linear incisional wounds (1.5 cm long) were made with iris scissors and closed using 6-0 nylon interrupted sutures. Wounds were harvested at 2, 6, 12, and 24 hr post-wounding (2 mm of tissue on either side of the incision). Tissue was frozen and stored at −80 °C for subsequent RNA isolation.
Histology and Immunohistochemistry
Histochoice-fixed or 4% paraformaldehyde-fixed, 5 μm paraffin sections were stained with hematoxylin and eosin using standard methods. For collagen visualization, the Masson Trichrome staining kit (Thermo Fisher Scientific, Pittsburgh, PA) was used according to the manufacturer’s protocol. For detection of HA, sections were rehydrated and hyaluronan visualized by immunofluorescence using a biotinylated HA-binding probe (bHABP) and streptavidin-Cy3 (Passi et al., 2004). For standard immunohistochemistry, the antibodies and staining conditions are described in Supplemental Materials and Methods.
Quantitation of Hyaluronan by an Enzyme-Linked Immunosorbant Assay (ELISA)-like Assay
Solubilized tissues (either from whole skin adjacent to wounds, or from dispase-separated epidermis) were prepared and analyzed in a competitive ELISA-like assay for HA, modified from Fosang et al. (Fosang et al., 1990). Details are provided in Supplemental Materials and Methods.
Quantitation of Hyaluronan by FACE
Fluorophore-assisted carbohydrate electrophoresis (FACE), a technique that quantifies total HA mass, was done as previously described (Calabro et al., 2000; Passi et al., 2004).
RNA Isolation and qPCR Analysis
RNA was prepared from total skin adjacent to full-thickness incisional wounds, or from dispase-separated epidermis after TPA treatment, then reversed transcribed and subjected to real time quantitative PCR (qPCR) as described in Supplementary Materials and Methods.
Digital image analysis of histological sections
Histological specimens were visualized on an Olympus BX-50 microscope with epifluorescence attachments and a Polaroid DU-DMC2 digital camera. Image processing was done with IPLab Spectrum software as described (Maytin et al., 2004).
Statistical Analysis
Differences between experimental and control groups were evaluated with a two-sided Student t-test, assuming equal variance in each group. A P-value of 0.05 or less was considered significant.
Supplementary Material
Table S1. Time course of appearance of inflammatory cells and markers of fibroplasia during cutaneous wound healing in wildtype (WT) and Has1/3 null mice.
Figure S1. Reductions in HA in the subdermal region in Has1/3 null mice correlate with enhanced neutrophil extravasation and early appearance of myofibroblasts at the wound edge. (a, a’) The abundant levels of HA staining normally seen in the subcutaneous region that contains the subdermal vascular plexus (SVP) of wildtype skin (a), is reduced in Has1/3 null mouse skin (a’). HA was visualized in unwounded skin by staining with biotinylated HA binding protein (bHABP) followed by streptavidin-Cy3. A similar HA reduction in Has1/3 null skin is observed at 1 day after wounding; see Fig. 4 of the manuscript. (b, b’) Abundant neutrophil extravasation appears to correspond to an increased efflux of neutrophils from vessels in Has1/3 null wounds in the HA-expressing SVP region, shown here at 24 h(Masson-Trichrome stain). (c, c’) Immunoperoxidase staining (RB6/85c neutrophil-specific antibody) shows dense aggregates of neutrophils (arrows) within vessels (dotted lines) at 24 h post-wounding, compared to wildtype. (d, d’) Immunoperoxidase staining for alpha-smooth muscle actin reveals abundant myofibroblasts in 5-day old wounds from Has1/3 null mice (d’). In contrast, myofibroblasts are still undetectable at 5 days in wildtype mice (d). (e, e’) Myofiboblasts can be appreciated by morphological criteria (spindle-shaped cells embedded in a “young” collagen matrix that is light blue in color, by MT staining) in 5-day old wounds in Has1/3 null mice (e’) but not in 5-day old wounds from wildtype mice (e). Scale bars, 50 μm.
ACKNOWLEDGMENTS
We thank Jamie Monslow, PhD for help in developing the qPCR assays. This work was supported in part by grants from NIH/NIAMS grants R01 AR049249 (to EVM) and R56 AR049249 (to EVM), NIH/NHLBI grant P01 HL107147 (to VCH), and from the Women’s Dermatologic Society (to RJF).
Abbreviations
- bHABP
biotinylated HA-binding protein
- ELISA
Enzyme linked immunosorbent assay
- FACE
Fluorophore assisted carbohydrate electrophoresis
- HA
Hyaluronic Acid
- Hyaluronan Has
HA synthase
- Hyal
Hyaluronidase
- TPA
12-o-tetradecanoylphorbol-13-acetate
- WT
wildtype
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- Alam CA, Seed MP, Freemantle C, Brown J, Perretti M, Carrier M, et al. The inhibition of neutrophil-endothelial cell adhesion by hyaluronan independent of CD44. Inflammopharmacology. 2005;12:535–50. doi: 10.1163/156856005774382733. [DOI] [PubMed] [Google Scholar]
- Alford JG, Stanley PL, Todderud G, Tramposch KM. Temporal infiltration of leukocyte subsets into mouse skin inflamed with phorbol ester. Agents and Actions. 1992;37:260–7. doi: 10.1007/BF02028118. [DOI] [PubMed] [Google Scholar]
- Alstergren P, Zhu B, Glogauer M, Mak TW, Ellen RP, Sodek J. Polarization and directed migration of murine neutrophils is dependent on cell surface expression of CD44. Cell Immunol. 2004;231:146–57. doi: 10.1016/j.cellimm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, et al. The role of hyaluronan synthase 3 in ventilator-induced lung injury. Amer J Resp Crit Care Med. 2005;172:92–8. doi: 10.1164/rccm.200405-652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro A, Benavides M, Tammi M, Hascall VC, Midura RJ. Microanalysis of enzyme digests of hyaluronan and chondroitin/dermatan sulfate by fluorophore-assisted carbohydrate electrophoresis (FACE) Glycobiology. 2000;10:273–81. doi: 10.1093/glycob/10.3.273. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr., et al. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–60. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataisson C, Pearson AJ, Torgerson S, Nedospasov SA, Yuspa SH. Protein kinase C alpha-mediated chemotaxis of neutrophils requires NF-kappa B activity but is independent of TNF alpha signaling in mouse skin in vivo. J Immunol. 2005;174:1686–92. doi: 10.4049/jimmunol.174.3.1686. [DOI] [PubMed] [Google Scholar]
- Dechert TA, Ducale AE, Ward SI, Yager DR. Hyaluronan in human acute and chronic dermal wounds. Wound Repair Regen. 2006;14:252–8. doi: 10.1111/j.1743-6109.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Lackie JM. Effect of hyaluronic acid on neutrophil adhesion. J Cell Sci. 1981;50:329–44. doi: 10.1242/jcs.50.1.329. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Wilkinson PC. Inhibition of leukocyte locomotion by hyaluronic acid. J Cell Sci. 1981;48:315–31. doi: 10.1242/jcs.48.1.315. [DOI] [PubMed] [Google Scholar]
- Fosang AJ, Hey NJ, Carney SL, Hardingham TE. An ELISA plate-based assay for hyaluronan using biotinylated proteoglycan G1 domain (HA-binding region) Matrix. 1990;10:306–13. doi: 10.1016/s0934-8832(11)80186-1. [DOI] [PubMed] [Google Scholar]
- Goentzel BJ, Weigel PH, Steinberg RA. Recombinant human hyaluronan synthase 3 is phosphorylated in mammalian cells. Biochem J. 2006;396:347–54. doi: 10.1042/BJ20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, Skandalis SS. Importance of hyaluronan-CD44 interactions in inflammation and tumorigenesis. Connective Tiss Res. 2008;49:215–8. doi: 10.1080/03008200802143323. [DOI] [PubMed] [Google Scholar]
- Itano N. Simple primary structure, complex turnover regulation, and multiple roles of hyaluronan. J Biochem. 2008;144:131–7. doi: 10.1093/jb/mvn046. [DOI] [PubMed] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–92. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exper Med. 2005;201:1269–79. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvinen S, Pasonen-Seppanen S, Hyttinen JM, Pienimaki JP, Torronen K, Jokela TA, et al. Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J Biol Chem. 2003;278:49495–504. doi: 10.1074/jbc.M310445200. [DOI] [PubMed] [Google Scholar]
- Kim MH, Liu W, Borjesson DL, Curry FR, Miller LS, Cheung AL, et al. Dynamics of neutrophil infiltration during cutaneous wound healing and infection using fluorescence imaging. J Invest Dermatol. 2008;128:1812–20. doi: 10.1038/sj.jid.5701223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson W, Chow G, Knudson CB. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002;21:15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, et al. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 2010;70:7073–83. doi: 10.1158/0008-5472.CAN-09-4687. [DOI] [PubMed] [Google Scholar]
- Lee SE, Jun JE, Choi EH, Ahn SK, Lee SH. Stimulation of epidermal calcium gradient loss increases the expression of hyaluronan and CD44 in mouse skin. Clin Exper Dermatol. 2009;35:650–7. doi: 10.1111/j.1365-2230.2009.03699.x. [DOI] [PubMed] [Google Scholar]
- Marks F, Furstenberger G. Proliferative responses of the skin to external stimuli. Environ Health Perspect. 1993;101(Suppl 5):95–101. doi: 10.1289/ehp.93101s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, et al. Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development. 2009;136:2825–35. doi: 10.1242/dev.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maytin EV, Chung HH, Seetharaman VM. Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. American J Pathol. 2004;165:1331–41. doi: 10.1016/S0002-9440(10)63391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Camenisch TD. Hyaluronan: genetic insights into the complex biology of a simple polysaccharide. Glycoconjugate J. 2002;19:331–9. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- Monslow J, Sato N, Mack JA, Maytin EV. Wounding-induced synthesis of hyaluronic acid in organotypic epidermal cultures requires the release of heparin-binding egf and activation of the EGFR. J Invest Dermatol. 2009;129:2046–58. doi: 10.1038/jid.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani T, Memezawa A, Okuyama R, Sayo T, Sugiyama Y, Inoue S, et al. Increased hyaluronan production and decreased E-cadherin expression by cytokine-stimulated keratinocytes lead to spongiosis formation. J Invest Dermatol. 2009;129:1412–20. doi: 10.1038/jid.2008.394. [DOI] [PubMed] [Google Scholar]
- Pasonen-Seppanen S, Karvinen S, Torronen K, Hyttinen JM, Jokela T, Lammi MJ, et al. EGF upregulates, whereas TGF-beta downregulates, the hyaluronan synthases Has2 and Has3 in organotypic keratinocyte cultures: correlations with epidermal proliferation and differentiation. J Invest Dermatol. 2003;120:1038–44. doi: 10.1046/j.1523-1747.2003.12249.x. [DOI] [PubMed] [Google Scholar]
- Passi A, Sadeghi P, Kawamura H, Anand S, Sato N, White LE, et al. Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exper Cell Res. 2004;296:123–34. doi: 10.1016/j.yexcr.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Pauloin T, Dutot M, Liang H, Chavinier E, Warnet JM, Rat P. Corneal protection with highmolecular-weight hyaluronan against in vitro and in vivo sodium lauryl sulfate-induced toxic effects. Cornea. 2009;28:1032–41. doi: 10.1097/ICO.0b013e3181a0a3f8. [DOI] [PubMed] [Google Scholar]
- Rilla K, Siiskonen H, Spicer AP, Hyttinen JM, Tammi MI, Tammi RH. Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J Biol Chem. 2005;280:31890–7. doi: 10.1074/jbc.M504736200. [DOI] [PubMed] [Google Scholar]
- Sayo T, Sugiyama Y, Takahashi Y, Ozawa N, Sakai S, Ishikawa O, et al. Hyaluronan synthase 3 regulates hyaluronan synthesis in cultured human keratinocytes. J Invest Dermatol. 2002;118:43–8. doi: 10.1046/j.0022-202x.2001.01613.x. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RAF. Cutaneous wound healing. New Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Tien JL, Joo A, Bowling RA. Investigation of hyaluronan function in the mouse through targeted mutagenesis. Glycoconjugate J. 2002;19:341–5. doi: 10.1023/A:1025321105691. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Shimada A, Sayo T, Sakai S, Inoue S. Putative hyaluronan synthase mRNA are expressed in mouse skin and TGF-beta upregulates their expression in cultured human skin cells. J Invest Dermatol. 1998;110:116–21. doi: 10.1046/j.1523-1747.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277:4581–4. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- Tammi R, Pasonen-Seppanen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005;124:898–905. doi: 10.1111/j.0022-202X.2005.23697.x. [DOI] [PubMed] [Google Scholar]
- Tammi R, Ripellino JA, Margolis RU, Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90:412–4. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- Tammi R, Saamanen AM, Maibach HI, Tammi M. Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture. J Invest Dermatol. 1991;97:126–30. doi: 10.1111/1523-1747.ep12478553. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–39. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Vigetti D, Genasetti A, Karousou E, Viola M, Clerici M, Bartolini B, et al. Modulation of hyaluronan synthase activity in cellular membrane fractions. J Biol Chem. 2009;284:30684–94. doi: 10.1074/jbc.M109.040386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath M, Ma L, Otey CA, Jester JV, Petroll WM. Modulation of corneal fibroblast contractility within fibrillar collagen matrices. Invest Ophthalmol Vis Sci. 2003;44:4724–35. doi: 10.1167/iovs.03-0513. [DOI] [PubMed] [Google Scholar]
- Wang A, de la Motte C, Lauer M, Hascall V. Hyaluronan matrices in pathobiological processes. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08069.x. E-pub ahead of print, Epub date 2011/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–85. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Smart RC. Overexpression of protein kinase C-alpha in the epidermis of transgenic mice results in striking alterations in phorbol ester-induced inflammation and COX-2, MIP-2 and TNF-alpha expression but not tumor promotion. J Cell Sci. 1999;112(Pt 20):3497–506. doi: 10.1242/jcs.112.20.3497. [DOI] [PubMed] [Google Scholar]
- Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284:9083–92. doi: 10.1074/jbc.M806989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Itano N, Hata K, Ueda M, Kimata K. Differential regulation by IL-1beta and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol. 2004;122:631–9. doi: 10.1111/j.0022-202X.2004.22332.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Time course of appearance of inflammatory cells and markers of fibroplasia during cutaneous wound healing in wildtype (WT) and Has1/3 null mice.
Figure S1. Reductions in HA in the subdermal region in Has1/3 null mice correlate with enhanced neutrophil extravasation and early appearance of myofibroblasts at the wound edge. (a, a’) The abundant levels of HA staining normally seen in the subcutaneous region that contains the subdermal vascular plexus (SVP) of wildtype skin (a), is reduced in Has1/3 null mouse skin (a’). HA was visualized in unwounded skin by staining with biotinylated HA binding protein (bHABP) followed by streptavidin-Cy3. A similar HA reduction in Has1/3 null skin is observed at 1 day after wounding; see Fig. 4 of the manuscript. (b, b’) Abundant neutrophil extravasation appears to correspond to an increased efflux of neutrophils from vessels in Has1/3 null wounds in the HA-expressing SVP region, shown here at 24 h(Masson-Trichrome stain). (c, c’) Immunoperoxidase staining (RB6/85c neutrophil-specific antibody) shows dense aggregates of neutrophils (arrows) within vessels (dotted lines) at 24 h post-wounding, compared to wildtype. (d, d’) Immunoperoxidase staining for alpha-smooth muscle actin reveals abundant myofibroblasts in 5-day old wounds from Has1/3 null mice (d’). In contrast, myofibroblasts are still undetectable at 5 days in wildtype mice (d). (e, e’) Myofiboblasts can be appreciated by morphological criteria (spindle-shaped cells embedded in a “young” collagen matrix that is light blue in color, by MT staining) in 5-day old wounds in Has1/3 null mice (e’) but not in 5-day old wounds from wildtype mice (e). Scale bars, 50 μm.