Abstract
Scaffold–cartilage integration is critical for the clinical success of a scaffold used for the repair of a focal cartilage defect. In this study, a macroporous polyvinyl alcohol (PVA) scaffold was found to facilitate chondrocyte infiltration and interfacial matrix formation in a juvenile bovine in vitro cartilage defect model. These results were found to depend on the press-fit between the scaffold and the cartilage, pretreatment of the cartilage with collagenase prior to scaffold insertion, and chondrocyte preseeding of the scaffold. Infiltrated and preseeded chondrocytes in the scaffold survived for 6 weeks in culture and resulted in sufficient matrix at the interface to significantly increase the interface shear strength 30-fold that compared favorably with the interface shear strength of cartilage–cartilage constructs. The ability of this macroporous PVA scaffold to form a stable interface with articular cartilage demonstrates the potential use of this scaffold design for focal cartilage defect repair.
Introduction
Localized articular cartilage defects can arise from mechanical trauma1 or pathology, such as osteochondritis dissecans.2 The degree of damage can range from superficial abrasion to deep fissures that extend to the underlying bone.3 Even small defects can progressively increase in size as a function of time and may be a key instigating factor for the onset of osteoarthritis.4 Epidemiological studies estimate that 16% of all adults in the United States have a focal cartilage defect in the knee, with a threefold increase in athletes.5 The prevalence of existing and latent disease and the impact on quality of life is substantial, with associated healthcare costs of up to $185 billion annually.6 Current surgical treatments for focal cartilage defects include microfracture,7 mosaicplasty,8 and autologous chondrocyte implantation (ACI).9 While all of these techniques can minimize pain and restore joint motion in the short term (≤2 years postoperatively), new studies have revealed deteriorating clinical outcomes at longer follow-up times (>5 years postoperatively) for all techniques.10–12 The unsatisfactory long-term performance of currently used surgical techniques has motivated the research into and development of engineered scaffolds as another potential treatment for the treatment of focal cartilage defects.
The presence of a focal cartilage defect alters the mechanical properties of the tissue thus impairing its ability to distribute joint loads and provide normal joint lubrication during activities of daily living.13 Given the substantial mechanical forces that occur during simple activities such as walking,14,15 any construct intended to fill a cartilage defect should have the ability to carry mechanical loads similar to the native tissue and to elicit a beneficial host response. To achieve these goals we developed a nonbiodegradable porous hydrogel (polyvinyl alcohol [PVA]) scaffold, the composition of which is stable over time and whose mechanical properties can be varied by changing the PVA polymer content.16,17 The use of a nonbiodegradable porous hydrogel scaffold for cartilage repair may provide initial properties similar to the native cartilage extracellular matrix (ECM) while allowing for tissue ingrowth to facilitate long-term stability, function, and integration. In orthopedic applications, this concept is embodied in the use of porous metal scaffolds for bone repair.18,19 However, this concept has not been successfully applied in the area of cartilage repair. Our porous nondegradable hydrogel scaffold was designed to function as a cell-free device that would be press-fit into a debrided cartilage defect site, providing initial fill and reinforcement in vivo. We hypothesized that the scaffold would be infiltrated by host cells for eventual de novo matrix generation and integration with surrounding cartilage. We have previously reported that this type of scaffold facilitated cell seeding and that seeded chondrocytes can survive and proliferate.16 However, there remained the question of whether chondrocytes would migrate into the scaffold from surrounding cartilage and synthesize ECM at the scaffold–cartilage interface, thereby creating a mechanically strong scaffold–cartilage integration.
The objective of this study was to optimize and measure the mechanical strength of the integration between articular cartilage and a macroporous PVA scaffold. Using an in vitro cartilage defect model, we tested the following hypotheses: (1) increased press-fit of the scaffold would increase chondrocyte migration and scaffold–cartilage interface strength, (2) partial digestion of articular cartilage would increase chondrocyte migration and resulting interfacial strength, and (3) preseeding the scaffold with chondrocytes would increase the matrix generated at the interface and the resulting scaffold–interface strength. We found that increasing the press-fit, digesting the cartilage, and preseeding the scaffold significantly increased the interface shear strength to levels similar to the interface shear strength of cartilage–cartilage implants. These results will help to advance the design goals of this and similar type scaffolds as a treatment for cartilage lesions.
Materials and Methods
Overview of experimental design
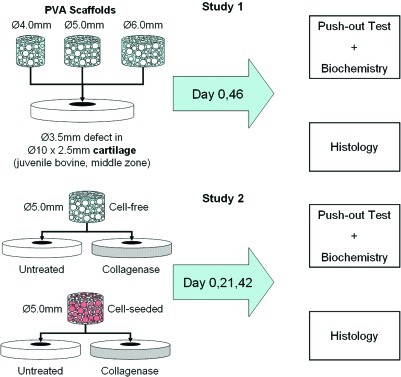
There are two studies presented here (schematically outlined in Fig. 1). In Study 1, to study the effect of increasing press-fit on scaffold–cartilage integration, macroporous PVA scaffolds of three different diameters (Ø4.0, 5.0, and 6.0 mm) were fabricated and placed into Ø3.5-mm cylindrical defects in calf, middle-zone cartilage disks and cultured in vitro. In Study 2, the optimal scaffold size was used from Study 1 to determine the effects of collagenase treatment to the cartilage tissue as well as cell seeding on the scaffold–cartilage interface formation.
FIG. 1.
Schematic of study designs. Study 1 sought to optimize the scaffold diameter for press-fit implantation in an in vitro cartilage defect model. After determining the optimized scaffold size, Study 2 sought to determine the effects of cell seeding and collagenase treatment on the scaffold–cartilage interface over time in culture. Color images available online at www.liebertonline.com/tea
Manufacture of macroporous PVA scaffolds16
Surgical gelatin sponges (Ethicon–Johnson & Johnson, Somerville, NJ) were saturated with deionized water and incubated in PVA (Sigma Aldrich, St. Louis, MO) solutions (1% and 5%) in deionized water with a final incubation at the desired concentration of 10% PVA. The PVA-soaked sponges were frozen to −20°C for 20 h and then thawed at 25°C for 4 h, with this freeze-thaw process repeated 5 additional times (6 total freeze-thaw cycles). Cylinders were cored out of the sponges while still frozen and the cylinders were digested for 14 h with 500 units/mL of collagenase (type II; Worthington Biochemical Corp., Lakewood, NJ) at 37°C to completely degrade the gelatin sponge. The resulting macroporous PVA scaffolds were washed 3 times in deionized water, and transferred to 70% ethanol for 20 min and then 100% ethanol for 20 min. Scaffolds were incubated at 37°C under vacuum for 30 min and then left under a laminar flow hood to completely dry. It was noted that upon rehydrating, the disinfected and dried scaffolds decreased in overall height and diameter ∼10%. To compensate, the scaffold cylinders prior to disinfection and dehydration were cut slightly taller and larger in diameter than the desired final size (e.g., using a Ø5.5-mm biopsy punch for final Ø5-mm scaffold). This loss in size may be due to the formation of additional physical cross-links that is the hypothesized mechanism of PVA hydrogel formation.20
Study 1: effect of press-fit
Cartilage explants (Ø10×2.5 mm) were cored out of the trochlear groove and femoral condyles of calf knee joints (n=6 joints; Max Insel Cohen, Livingston, NJ) with the superficial and deep zones removed via sharp dissection to create uniform, middle-zone-only cartilage disks. Defects (Ø3.5 mm) were created using a sterile biopsy punch. Scaffolds (final size Ø4.0, 5.0, or 6.0 mm×2.0 mm) were manufactured, disinfected, and completely dried as described previously. As the scaffold diameter is larger than the defect diameter, the scaffolds were cut shorter than the thickness of the cartilage disk axially to compensate for extrusion based on Poisson's ratio calculations16 (i.e., Ø5.0×2.0 mm scaffold →Ø3.5×2.5 mm defect).
Cartilage annuli were briefly tamped dry onto sterile gauze and placed into 12-well tissue culture plates. The dried scaffolds were placed into the defects of the cartilage disks and were rehydrated by pipetting 400 μL of advanced Dulbecco's modified Eagle's medium (DMEM)/F12 culture media (Invitrogen, Carlsbad, CA) along the bottom of the well, allowing for capillary action to rehydrate the scaffold, creating a press-fit between the scaffold and cartilage ring. After absorption of the culture media, an additional 2 mL of media was pipetted to completely cover the scaffold–cartilage construct and the samples were incubated for 30 min at 37°C and 5% CO2. Scaffold–cartilage constructs were cultured in 30 mL Advanced DMEM (ADMEM)/F12 with 100 nM dexamethasone, 50 μg/mL ascorbate-2-phosphate, and antibiotics (Sigma Aldrich) for 46 days. On days 0 and 46 the maximum interface strength was determined for scaffold–cartilage constructs (n=4 per diameter group) via a push-out test, followed by biochemical analysis (see “Outcome measures” section). Intact scaffold–cartilage constructs that did not undergo push-out testing (n=3 per group) were also removed at these time points for histological analysis (see below).
Study 2: effect of cell seeding and collagenase treatment
Middle-zone-only cartilage disks (Ø10×2.5 mm) were prepared as stated earlier from calf knee cartilage. Additional cartilage from the same knee joints was digested overnight in 500 U/mL of collagenase type II (Worthington Biochemical Co., Lakewood, NJ) in DMEM (Invitrogen) with 5% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and antibiotics to obtain a mixed population of primary chondrocytes. The resulting solution was then filtered into 50 mL conical tubes using cell strainers (BD Falcon, Bedford, MA) to remove any undigested cartilage, centrifuged and washed in DMEM with 10% FBS to neutralize any remaining collagenase, and then resuspended at 20×106 cells/mL in ADMEM/F12 media.
Defects (Ø3.5 mm) were created in the middle-zone cartilage disks using a sterile biopsy punch and cartilage rings were left untreated or fully immersed in 10 U/mL collagenase solution in ADMEM/F1221,22 for 15 min at 37°C followed by 3 washes of ADMEM/F12. Controls were kept in culture media without collagenase for this length of time. Defects were then filled with disinfected and completely dried 10% PVA scaffolds (Ø 5.0×2.0 mm, based on Study 1). Four hundred microliters of ADMEM/F12 media with or without chondrocytes (20×106/mL) was pipetted directly to the dehydrated scaffold to rehydrate the scaffold into a press-fit as described previously. An additional 2 mL of either cell suspension or cell-free media was pipetted to completely cover the scaffold–cartilage construct and the samples were incubated for 30 min at 37°C and 5% CO2. Scaffold–cartilage constructs were cultured in 30 mL ADMEM/F12 with 100 nM dexamethasone, 50 μg/mL ascorbate-2-phosphate, and antibiotics (Sigma Aldrich). This created the following groups: cell-free + untreated cartilage (“cell−/col−”), cell-free + collagenase-treated cartilage (“cell−/col+”), cell-seeded + untreated cartilage (“cell+/col−”), and cell-seeded + collagenase-treated cartilage (“cell+col+”).
On days 0, 21, and 42, constructs (n=5–6 per group) were removed for push-out testing followed by biochemical analysis. In addition, intact constructs that did not undergo push-out testing were removed for histological analysis (n=3 per group).
Outcome measures
Push-out test and biochemical analysis
Scaffold–cartilage interface strength was determined via push-out test using a Ø2.75-mm stainless steel indenter that was advanced at 10 μm/s.22 An indenter smaller than the defect was used due to limitations in the alignment needed for the mechanical test—any misalignment would result in indenter–cartilage contact that would skew the test results. The maximum load was recorded and normalized to the interfacial surface area for each sample to compute the maximum stress. The tested scaffolds and cartilage rings were frozen and stored at −20°C for biochemical testing for glycosaminoglycan (GAG) content via the DMMB assay23 and DNA content via the Picogreen assay (Invitrogen).
Histological analysis
Untested scaffold–cartilage constructs were fixed in neutral buffered formalin + 0.5% cetylpyridinium chloride for 4 h at room temperature, washed briefly in phosphate-buffered saline (PBS) to remove residue formalin, cryoprotected in a 30% sucrose solution at 4°C overnight, incubated for 2 h in 5% gelatin + 5% sucrose embedding medium, and then embedded in the gelatin–sucrose medium.24 Blocks were cryotomed for histological analysis of cell infiltration via hematoxylin and Picrosirius Red histological stains (8 μm sections). Maximum cell migration distance for cell-free scaffolds was determined by drawing a circular perimeter from the edge of the scaffold and then extending a radius from the circle to the cell that was judged to have infiltrated the furthest. These measurements were averaged from 5 sections per sample.
For Study 2, type II collagen immunohistochemistry was performed as follows: sections were digested with 1 mg/mL pepsin in 0.2 N HCl at 37°C for 10 min, rinsed with PBS, blocked with DAKO Dual Enzyme Block (DAKO, Carpinteria, CA) to remove endogenous peroxidases, rinsed again with PBS + 0.05% TWEEN (Sigma), blocked with DAKO blocking buffer, and then labeled with a 1:500 dilution of monoclonal primary antibody for type II collagen (CIIC1; Developmental Studies Hybridoma Bank, Iowa City, IA). Secondary labeling and DAB staining reaction was performed using the anti-mouse Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Samples were counterstained with hematoxylin.
Statistics
Two-way analysis of variance was performed with maximum push-out stress, GAG content, and DNA content as the dependent variables, and culture time and experimental group (diameter for Study 1, cell/collagenase treatment for Study 2) as the independent variables. Scheffe post hoc analyses were performed with α=0.05 to determine significance between groups.
Results
In vitro cartilage defect Study 1: effect of press-fit
Scaffolds recovered from push-out testing were found to expand to their original size after testing and rehydration for all testing time points (not shown). On day 0, the larger Ø6.0-mm scaffold group possessed the highest interface strength (1.39±0.17 kPa) compared with Ø4.0-mm (0.16±0.03 kPa) and Ø5.0-mm (0.63±0.13 kPa) scaffolds (Table 1, p<0.05). On day 21, no significant change was observed in the Ø4.0- or 6.0-mm group compared with their day 0 values. However, at this time point, the push-out stress of the Ø5.0-mm scaffold–cartilage constructs had increased to be statistically comparable to the Ø6.0-mm group value (Table 1). After 46 days in culture, however, only the Ø5.0-mm scaffold resulted in a significant increase in interfacial strength (10.40±1.54 kPa) compared with all tested other scaffold sizes (p<0.05). Similar trends were observed in GAG content and DNA content (Table 1), with Ø4.0- and Ø6.0-mm scaffolds demonstrating no increases in biochemical content, whereas Ø5.0-mm scaffolds increased significantly to GAG content of 0.035%±0.012%ww and DNA content of 0.00037%±0.00011%ww. Analysis of cartilage rings that held the Ø6.0-mm scaffolds showed decreased GAG on day 21 and DNA content on day 46 relative to starting day 0 values, with no changes with time noted for the other scaffold groups (Table 1).
Table 1.
Interface Strength and Biochemical Composition for Study 1
| GAG%ww | DNA%ww | Maximum push-out stress (kPa) | ||
|---|---|---|---|---|
| Cartilage | d00 | 5.875±0.570 | 0.03000±0.01470 | N/A |
| d21 4.0 mm | 5.641±0.271 | 0.03147±0.01497 | N/A | |
| d21 5.0 mm | 5.747±0.347 | 0.03083±0.01287 | N/A | |
| d21 6.0 mm | 4.011±0.314a,b | 0.02442±0.00611 | N/A | |
| d46 4.0 mm | 5.784±0.625 | 0.03335±0.00235 | N/A | |
| d46 5.0 mm | 5.738±0.360 | 0.02949±0.00884 | N/A | |
| d46 6.0 mm | 3.214±0.441a,b | 0.01700±0.00702a,b | N/A | |
| Scaffold | d00 4.0 mm | 0.000±0.000 | 0.00000±0.00000 | 0.16±0.03 |
| d00 5.0 mm | 0.000±0.000 | 0.00000±0.00000 | 0.63±0.13 | |
| d00 6.0 mm | 0.000±0.000 | 0.00000±0.00000 | 1.39±0.17b | |
| d21 4.0 mm | 0.005±0.017 | 0.00003±0.00015 | 0.25±0.37b | |
| d21 5.0 mm | 0.017±0.021 | 0.00011±0.00024 | 1.25±0.15a | |
| d21 6.0 mm | 0.001±0.015 | 0.00004±0.00018 | 1.29±0.25 | |
| d46 4.0 mm | 0.001±0.011 | 0.00001±0.00010 | 0.15±0.05 | |
| d46 5.0 mm | 0.035±0.012a | 0.00037±0.00011a | 10.40±1.54a–c | |
| d46 6.0 mm | 0.001±0.018 | 0.00001±0.00008 | 1.48±0.50 |
p<0.05 versus day 0 values.
p<0.05 versus other groups of the same time point.
p<0.05 versus previous time points.
Implanted 6.0-mm scaffolds had the highest value for maximum push-out stress, but this value did not change over time. By day 46, implanted scaffolds of diameter 5.0 mm had significant increases in maximum push-out stress and biochemical composition compared with 4.0- and 6.0-mm scaffolds. The cartilage ring of 6.0-mm scaffolds had significant decreases in GAG by day 21 and in DNA after 46 days in culture.
GAG, glycosaminoglycan; NA, not applicable.
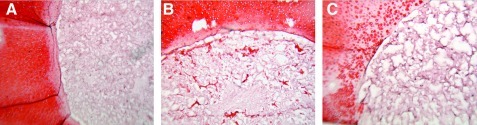
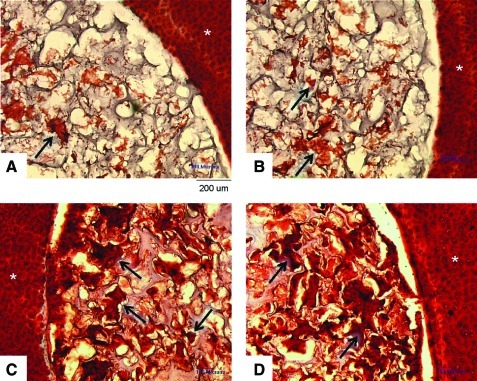
Histological analysis showed that all scaffolds were empty when inserted on day 0 (not shown). Defect creation leads to matrix loss along the cut surface for some specimens, consistent with previous cartilage defect models reported in the literature.25 On day 46, clusters of chondrocytes with a collagen-rich matrix were present in the Ø5.0-mm scaffolds up to ∼400 μm deep from the scaffold-cartilage interface (Fig. 2). Cells did not migrate into the Ø4.0- or Ø6.0-mm scaffold groups. It was also noted that the cartilage rings containing the Ø6.0-mm scaffold exhibited greater matrix loss at the defect edge after time in culture compared with those with Ø4.0-mm or Ø5.0-mm scaffold.
FIG. 2.
Representative day 46 histology for Study 1. Consistent with the quantitative data, implanted 4.0-mm-diameter scaffolds showed no cellular infiltration or matrix formation (A). Implanted 5.0-mm scaffolds showed numerous matrix-rich chondrocyte clusters (B). For the 6.0-mm scaffolds, no chondrocytes were found in the scaffold but also the cartilage showed collagen degradation at the interface region (C). Scale bar=200 μm. Color images available online at www.liebertonline.com/tea
In vitro cartilage defect Study 2: effect of cell seeding and collagenase treatment
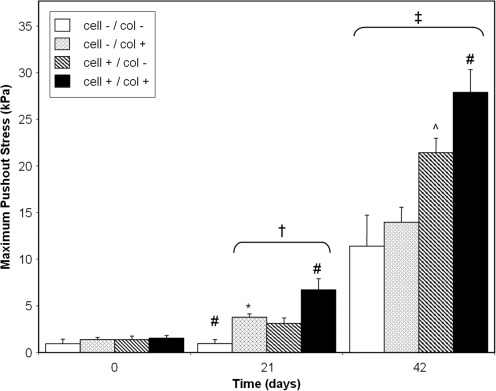
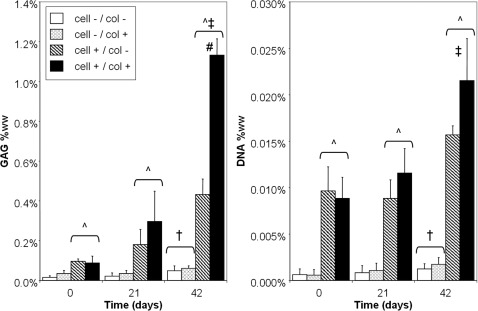
For cell-free scaffolds, collagenase treatment led to a significant increase in the maximum push-out stress on day 21 compared with nontreated controls (3.77±0.38 vs. 0.95±0.44 kPa, p<0.05). After 42 days in culture, the interfacial strength of both control (11.40±3.32 kPa) and collagenase-treated (13.96±1.63 kPa) scaffolds increased significantly compared with day 0 and day 21 values (p<0.05), but no statistical difference was detected between the groups at this time point (Fig. 3). Similarly, GAG content and DNA content were not significantly different on day 21, but did significantly increase after 42 days in culture with no differences between the cell-free scaffold groups (Fig. 4A, B).
FIG. 3.
Maximum push-out stress for Study 2 experimental groups. On day 21, collagenase treatment significantly increased the maximum push-out stress (representative of interface strength) for both cell-free and cell-seeded scaffolds. On day 42, cell-seeded scaffolds in collagenase-treated cartilage (cell+/col+) had the highest values for interface strength. *p<0.05 versus cell-/col- group of same time point; ^p<0.05 versus both cell-free (cell−) groups of same time point; #p<0.05 versus all other groups of the same time point; †p<0.05 versus respective day 0 group; ‡p<0.05 versus respective day 0 and day 21 groups.
FIG. 4.
Biochemical composition for Study 2 scaffold–cartilage constructs. For all culture times, cell-seeded scaffolds possessed significantly higher glycosaminoglycans (GAG, left) and DNA (right) values than cell-free scaffolds. On day 42, cell-free scaffolds for both cartilage groups had significantly higher GAG and DNA values than day 0. For cell-seeded scaffolds, GAG values for both groups significantly increased on day 42 but only the collagenase-treated group (cell+/col+) had a significant increase in DNA content. ^p<0.05 versus both cell-free (cell-) groups of same time point; #p<0.05 versus all other groups of the same time point; †p<0.05 versus respective day 0 group; ‡p<0.05 versus respective day 0 and day 21 groups.
For cell-seeded scaffolds, collagenase treatment significantly enhanced the interface strength on day 21 compared with noncollagenase-treated scaffolds (6.72±1.20 vs. 3.12±0.61 kPa, p<0.05) and both cell-free scaffold groups at this time point (Fig. 3). After 42 days in culture, collagenase-treated cell-seeded scaffolds attained the highest interface strength (27.88±2.44 kPa) of all groups, with both cell-seeded scaffold groups exhibiting significantly higher interfacial strength compared with their cell-free counterparts at this time (Fig. 3). GAG content of cell-seeded scaffolds significantly increased on day 42, with the highest values reached by collagenase-treated, cell-seeded scaffold (Fig. 4A; 1.13%±0.08%ww, p<0.05). DNA values of cell-seeded scaffolds significantly increased on day 42 for only the collagenase-treated group (p<0.05; Fig. 4B).
Histological staining revealed the presence of chondrocytes infiltrating into cell-free scaffolds and forming a collagen-rich matrix by day 21, with a greater number of chondrocytes and matrix staining observed in the collagenase-treated groups, similar to the quantitative biochemistry (Fig. 5, arrows). Chondrocyte migration distance was increased with collagenase treatment on day 21 (324±51 vs. 505±42 μm, p<0.05). This trend was maintained through day 42, though no significant changes in migration distance were noted at that time. Cell-seeded scaffolds remained cellular throughout the experimental time and formed a collagen-rich matrix that appeared more dense than the cell-free scaffolds on day 42.
FIG. 5.
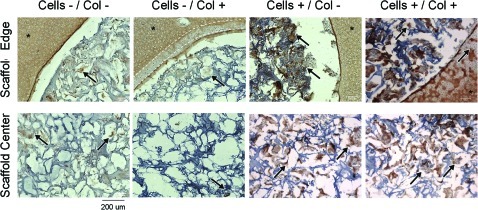
Representative day 42 histology for Study 2. Consistent with the quantitative data, day 42 cell-free scaffolds in either untreated (A) or collagenase-treated (B) cartilage rings (*) showed chondrocyte infiltration and collagen-rich matrix. For both cell-seeded scaffolds (untreated cartilage, C; collagenase treated, D), chondrocytes in the scaffold were found to form dense collagen-rich clusters (arrows). Scale bar=200 μm. Color images available online at www.liebertonline.com/tea
Immunohistochemistry (Fig. 6, only day 42 shown) indicated that, as expected, the cartilage explants possessed extensive type II collagen. Interestingly, collagenase-treated cartilage rings showed reduced staining at the treated edges (dotted lines), suggesting the explants have not fully recovered their type II collagen matrix after 42 days in culture. The ECM formed by the chondrocytes in the scaffold (either migrated in or preseeded) was composed of type II collagen, indicating a hyaline chondrocyte phenotype (arrows).
FIG. 6.
Representative day 42 type II collagen immunohistochemistry for Study 2. The cartilage explants (*) showed positive staining for type II collagen. For the collagenase-treated samples (cells-/col+ and cells +/col+), the edge of the cartilage rings had decreased staining (dotted lines), indicating that type II collagen had not recovered after enzymatic treatment. Cells that had migrated in or were preseeded (arrows) formed clusters that were rich in type II collagen, indicating a hyaline chondrocyte phenotype. Scale bar=200 μm. Color images available online at www.liebertonline.com/tea
Discussion
Scaffold–tissue integration and the creation of a mechanically strong interface is a critical aspect of the success or failure of scaffolds for focal cartilage repair. We have presented a novel nonbiodegradable PVA-based scaffold that is capable of facilitating inward chondrocyte migration from surrounding articular cartilage tissue. Results were sensitive to the degree of press-fit between the scaffold and the cartilage, pretreatment of the cartilage with collagenase, and whether or not the scaffold was preseeded with cells. Chondrocytes in the scaffold (both migrated and preseeded) survived for a 6-week culture period and generated sufficient matrix to impart a significant increase in interface strength as a function of time. The combination of chondrocyte preseeding and collagenase treatment yielded the highest scaffold–cartilage interface strength, with a 30-fold increase in maximum push-out stress over time in culture (from ∼1 kPa to ∼30 kPa). The scaffold construct compares favorably to the interface strength of calf cartilage–cartilage constructs (∼25 kPa) that were cultured in vitro for similar times and using similar push-out testing configurations.26 In addition, this value was superior to the interface strength between a chondrocyte-seeded agarose hydrogel and cartilage (∼2 kPa after 21 days) in in vitro culture.27 This latter scaffold possessed 3 times as many cells and similar matrix content as our presented PVA scaffolds. Taking our results in light of the literature demonstrates that our porous PVA scaffold appears to be well-suited for cartilage integration and warrants continued research and development.
While previous research has demonstrated that biological glues or gels (e.g., Puramatrix)28,29 can be used to improve the interface of scaffold–cartilage and cartilage–cartilage constructs, we sought to create an initially stable interface with a simpler press-fit approach that could be reinforced with time through the inward migration of chondrocytes and de novo matrix to span the interface. For our porous, relatively soft scaffold, the degree of press-fit required to achieve an initially stable interface was unclear. To explore this question, an in vitro defect model was created by creating a Ø3.5-mm hole in a Ø10-mm cartilage disk and scaffolds ranging in diameter from 4 to 6 mm were implanted. At time zero, the 4.0-mm scaffold had the lowest initial interfacial strength and exhibited no changes in interface strength or cellularity over time. This is likely due to poor contact with the cartilage as indicated by the extremely low interface strength. In contrast, the largest scaffold diameter tested led to the highest initial interface strength, but also led to cell death and matrix loss in the surrounding cartilage and a lack of chondrocyte migration into the scaffold. Though it is not clear as to whether the inhibition of chondrocyte migration precedes or follows chondrocyte death in the Ø6.0-mm scaffold–cartilage construct, given the slow rate of migration observed in the Ø5.0-mm scaffold group, it is likely that cell death is the dominant mechanism. This is likely due to excessive static compression of the cartilage tissue in the transverse direction leading to chondrocyte death.30 The progression and rate of chondrocyte death can be studied in the future using TUNEL staining and will be a concern in determining the proper scaffold size for different defect sizes. The Ø5.0-mm scaffold demonstrated an interfacial strength that was in between that of the 4- and 6-mm-diameter scaffolds at time zero, but the interface was reinforced with time due to the inward migration of chondrocytes that resulted in a 10-fold increase in interface strength after 42+ days. This result was surprising given that there were no chemoattractant or chemotactic factors in the culture media or in the scaffold. It has been previously demonstrated that cells do not strongly attach to PVA without some initial adsorption of matrix proteins on the PVA surface.31,32 This may have hampered chondrocyte initial migration of cells into the scaffold and is a target for future research, but our data nonetheless suggest that robust migration occurred with time in culture.
In cell-free scaffolds, collagenase treatment was found to increase scaffold–cartilage interface strength, biochemical content, and penetration of chondrocytes into the scaffold by day 21. Though no significant difference was detected at day 42, it could be that collagenase treatment increased the speed at which the scaffold–cartilage interface attained that final value between the day 21 and day 42 time points. The likely mechanism of these results is in the combined increased cell migration and proliferation from the articular cartilage surface due to the cutting of the cartilage to prepare the annuli model25 combined with the collagenase treatment.33 The stimulation of cell proliferation may be clinically beneficial given the lower number of chondrocytes in adult cartilage.34 In comparison, cell-seeded scaffolds exhibited continued matrix elaboration over time in culture and cell proliferation by day 42, indicating that chondrocytes can survive and thrive within the scaffold environment. The cell-seeded scaffolds with collagenase-treated cartilage resulted in the highest GAG content and interface strength. These results combined indicate that cells can migrate into the scaffold and that they can survive over extended culture times to elaborate matrix that can bridge the gap. As the chondrocytes continued to synthesize type II collagen, the chondrocytes in the scaffold appear to maintain their proper phenotype.
We encountered several technical challenges in analyzing the cartilage–scaffold constructs used in this study. The presence of dissolved PVA in the sample digests for biochemical analysis resulted in high background values in the standard colorometric, orthohydroxyproline assay for total collagen quantification.35 Therefore, we had to rely on histology for the detection of collagen in our scaffold for this presented work. To preserve the cartilage–scaffold interface for histology, we found that we could not use standard paraffin or resin embedding due to differences in the dehydration rates between the highly porous scaffold and cartilage tissue. Therefore, we had to pursue cryosectioning of our specimens using specialized gelatin embedding24 as opposed to the typical optical cutting temperature compound (OCT) embedding that resulted in poor adhesion of the PVA scaffold to microscope slides. Our laboratory is currently working on removing the dissolved PVA from the sample digests to allow for collagen assessment via the orthohydroxyproline (OHP) assay and more type-specific enzyme-linked immunosorbent assays.
In translating this polymer scaffold from the bench to the bedside, our promising in vitro results need to be tempered with the limitations of our experimental model. In deciding on the culture conditions, we decided to use a relatively simple, but established, static culture system with a well-defined culture media.36 Though this media maintain chondrocyte phenotype and cartilage tissue properties, the lack of growth factors is not representative of a physiologic condition. Studies in the literature have demonstrated that although in vitro cartilage–cartilage interface strength is low, the interface strength can increase 10-fold in vivo in the presence of cytokines and growth factors.22,37 In addition, dynamic mechanical loading that is present in articulating joints in vivo will likely impact scaffold–cartilage integration.
In both our experimental model and in clinical debridement of cartilage defects, the act of cutting articular cartilage leads to some cell death and matrix loss at the cut edges,25 though the cells at the margins maintain their normal phenotype.38 In our experimental model, however, as the superficial and deep zones were also cut, these top and bottom surfaces represent additional areas for chondrocyte migration out of the tissue and into the scaffold. Though an optimal scaffold size was found for this particular defect size, it is not clear whether it is the ratio of the scaffold-to-defect diameter or whether it is the stress and resulting friction at the scaffold–cartilage interface that would be the major determining component of our results. Future experiments are planned to hold the scaffold-to-defect diameter ratio constant and vary the stress on the cartilage by changing the PVA concentration (and therefore the mechanical properties) of the scaffold.
Implantation of a cell-seeded scaffold resulted in the best integration results for our in vitro model. To adopt this in a clinical setting would require 2 surgeries—first to harvest cells for expansion and second to implant the cell-seeded scaffold—similar to ACI.9 Given the associated increased medical costs and risk of complication, an optimized 1-step, cell-free procedure that could invite host cell recruitment would be more ideal (senior orthopedic surgeon opinion). Finally, though collagenase treatment was used in this study to promote cell migration and proliferation, it is known that adult human cartilage composition may be different and a different enzymatic digestion procedure, such as combined hyaluronidase/collagenase, may be necessary.21 The immunohistochemistry indicated that for our model, the loss of type II collagen in the cartilage explant that is induced by the collagenase treatment was not recovered after 42 days in culture. Though this may be due to the lack of anabolic growth factors in the culture medium as discussed previously, this finding does highlight a potential pitfall that might be magnified if used in patients with degenerative cartilage pathology.
In conclusion, our macroporous PVA scaffold shows great potential as a clinical treatment for focal cartilage repair. In a well-controlled laboratory setting, it facilitates chondrocyte infiltration and matrix formation that results in a significant increase in interfacial strength over a period of 42 days. In translating this device toward clinical use, future laboratory studies will improve our analysis techniques, study the effects of growth factors on tissue formation in the scaffold, and explore methods to improve the PVA surface for cellular/tissue attachment.
Acknowledgments
This research was supported by the NIH (TL1RR024998- KW Ng, Core Center AR046121, Research Facilities Improvement Program C06-RR12538-01), Clark Foundation, Kirby Foundation, Leo Rosner Foundation, and the Russell Warren Chair for Tissue Engineering.
Disclosure Statement
No competing financial interests exist.
References
- 1.Buckwalter J.A. Mechanical Injuries of Articular Cartilage. Iowa Orthop J. 1992;12:50. [Google Scholar]
- 2.Pappas A.M. Osteochondrosis dissecans. Clin Orthop Relat Res. 1981;158:59. [PubMed] [Google Scholar]
- 3.ICRS Cartilage Evaluation Package. Presented at the International Cartilage Repair Society Workshop. Munchenwilder; Switzerland: 2000. [Google Scholar]
- 4.Davies-Tuck M.L. Wluka A.E. Wang Y. Teichtahl A.J. Jones G. Ding C., et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:337. doi: 10.1016/j.joca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Flanigan D.C. Harris J.D. Trinh T.Q. Siston R.A. Brophy R.H. Prevalence of Chondral Defects in Athletes' Knees - A Systematic Review. Med Sci Sports Exerc. 2010;42:1795. doi: 10.1249/MSS.0b013e3181d9eea0. [DOI] [PubMed] [Google Scholar]
- 6.Kotlarz H. Gunnarsson C.L. Fang H. Rizzo J.A. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 7.Steadman J.R. Rodkey W.G. Rodrigo J.J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(Suppl):S362. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 8.Hangody L. Kish G. Karpati Z. Udvarhelyi I. Szigeti I. Bely M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics. 1998;21:751. doi: 10.3928/0147-7447-19980701-04. [DOI] [PubMed] [Google Scholar]
- 9.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 10.Solheim E. Hegna J. Oyen J. Austgulen O.K. Harlem T. Strand T. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee. 2010;17:84. doi: 10.1016/j.knee.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Moseley J.B., Jr. Anderson A.F. Browne J.E. Mandelbaum B. Micheli L.J. Fu F., et al. Long-term durability of autologous chondrocyte implantation: a multicenter, observational study in U.S. patients. Am J Sports Med. 2010;38:238. doi: 10.1177/0363546509348000. [DOI] [PubMed] [Google Scholar]
- 12.Mithoefer K. McAdams T. Williams R.J. Kreuz P.C. Mandelbaum B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 13.Gratz K.R. Wong B.L. Bae W.C. Sah R.L. The effects of focal articular defects on cartilage contact mechanics. J Orthop Res. 2009;27:584. doi: 10.1002/jor.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuster M.S. Wood G.A. Stachowiak G.W. Gachter A. Joint load considerations in total knee replacement. J Bone Joint Surg Br. 1997;79:109. doi: 10.1302/0301-620x.79b1.6978. [DOI] [PubMed] [Google Scholar]
- 15.van den Bogert A.J. Read L. Nigg B.M. An analysis of hip joint loading during walking, running, and skiing. Med Sci Sports Exerc. 1999;31:131. doi: 10.1097/00005768-199901000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Ng K.W. Torzilli P.A. Warren R.F. Maher S.A. Biomechanical characterization of a macroporous polyvinyl alcohol scaffold for the repair of focal articular cartilage defects. J Tissue Eng Regen Med Rev. 2011 doi: 10.1002/term.1510. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholten P.M. Ng K.W. Joh K. Serino L.P. Warren R.F. Torzilli P.A., et al. A semi-degradable composite scaffold for articular cartilage defects. J Biomed Mater Res A. 2011 doi: 10.1002/jbm.a.33005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop (Belle Mead NJ) 2002;31:216. [PubMed] [Google Scholar]
- 19.Maccauro G. Iommetti P.R. Muratori F. Raffaelli L. Manicone P.F. Fabbriciani C. An overview about biomedical applications of micron and nano size tantalum. Recent Pat Biotechnol. 2009;3:157. doi: 10.2174/187220809789389153. [DOI] [PubMed] [Google Scholar]
- 20.Cho S.H. Oh S.H. Lee J.H. Fabrication and characterization of porous alginate/polyvinyl alcohol hybrid scaffolds for 3D cell culture. J Biomater Sci Polym Ed. 2005;16:933. doi: 10.1163/1568562054414658. [DOI] [PubMed] [Google Scholar]
- 21.Bos P.K. DeGroot J. Budde M. Verhaar J.A. van Osch G.J. Specific enzymatic treatment of bovine and human articular cartilage: implications for integrative cartilage repair. Arthritis Rheum. 2002;46:976. doi: 10.1002/art.10208. [DOI] [PubMed] [Google Scholar]
- 22.van de Breevaart Bravenboer J. In der Maur C.D. Bos P.K. Feenstra L. Verhaar J.A. Weinans H., et al. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6:R469. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farndale R.W. Sayers C.A. Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 24.Brown D.A. Chou Y.F. Beygui R.E. Dunn J.C. Wu B.M. Gelatin-embedded cell-polymer constructs for histological cryosectioning. J Biomed Mater Res B Appl Biomater. 2005;72:79. doi: 10.1002/jbm.b.30116. [DOI] [PubMed] [Google Scholar]
- 25.Archer C.W. Redman S. Khan I. Bishop J. Richardson K. Enhancing tissue integration in cartilage repair procedures. J Anat. 2006;209:481. doi: 10.1111/j.1469-7580.2006.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam H.K. Srivastava A. Colwell C.W., Jr. D'Lima D.D. In vitro model of full-thickness cartilage defect healing. J Orthop Res. 2007;25:1136. doi: 10.1002/jor.20428. [DOI] [PubMed] [Google Scholar]
- 27.Kelly T.A. Fisher M.B. Lima E.G. Ateshian G.A. Hung C.T. Integrative properties of chondrocyte-seeded agarose constructs. Presented at the Trans Orthop Res Soc. Washington, D.C.: 2005. [Google Scholar]
- 28.Wang D.A. Varghese S. Sharma B. Strehin I. Fermanian S. Gorham J., et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6:385. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher S.A. Mauck R.L. Rackwitz L. Tuan R.S. A nanofibrous cell-seeded hydrogel promotes integration in a cartilage gap model. J Tissue Eng Regen Med. 2010;4:25. doi: 10.1002/term.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chahine N.O. Ateshian G.A. Hung C.T. The effect of finite compressive strain on chondrocyte viability in statically loaded bovine articular cartilage. Biomech Model Mechanobiol. 2007;6:103. doi: 10.1007/s10237-006-0041-2. [DOI] [PubMed] [Google Scholar]
- 31.Nuttelman C.R. Mortisen D.J. Henry S.M. Anseth K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J Biomed Mater Res. 2001;57:217. doi: 10.1002/1097-4636(200111)57:2<217::aid-jbm1161>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Zajaczkowski M.B. Cukierman E. Galbraith C.G. Yamada K.M. Cell-matrix adhesions on poly(vinyl alcohol) hydrogels. Tissue Eng. 2003;9:525. doi: 10.1089/107632703322066705. [DOI] [PubMed] [Google Scholar]
- 33.Lee D.A. Bentley G. Archer C.W. The control of cell division in articular chondrocytes. Osteoarthritis Cartilage. 1993;1:137. doi: 10.1016/s1063-4584(05)80029-9. [DOI] [PubMed] [Google Scholar]
- 34.Barbero A. Grogan S. Schafer D. Heberer M. Mainil-Varlet P. Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 36.Bian L. Lima E.G. Angione S.L. Ng K.W. Williams D.Y. Xu D., et al. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech. 2008;41:1153. doi: 10.1016/j.jbiomech.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gratz K.R. Wong V.W. Chen A.C. Fortier L.A. Nixon A.J. Sah R.L. Biomechanical assessment of tissue retrieved after in vivo cartilage defect repair: tensile modulus of repair tissue and integration with host cartilage. J Biomech. 2006;39:138. doi: 10.1016/j.jbiomech.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Hunziker E.B. Quinn T.M. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am. 2003;85-A(Suppl 2):85. doi: 10.2106/00004623-200300002-00011. [DOI] [PubMed] [Google Scholar]