Abstract
Oncolytic vesicular stomatitis virus (VSV) has potent antitumor activity, but infects a broad range of cell types. Here, we used the measles virus (MV) hemagglutinin (H) and fusion (F) envelope glycoproteins to redirect VSV entry and infection specifically to tumor-associated receptors. Replication-defective VSV, deleted of its glycoprotein gene (VSVΔG), was pseudotyped with MV-F and MV-H displaying single-chain antibodies (scFv) specific for epidermal growth factor receptor (EGFR), folate receptor (FR), or prostate membrane-specific antigen (PSMA). Viral titers were ∼105 PFU/ml, but could be concentrated to 107 PFU/ml. Immunoblotting confirmed incorporation of the MV-H-scFv and MV-F into functional VSV virions. Although VSV-G was able to infect all tumor cell lines tested, the retargeted VSV infected only cells that expressed the targeted receptor. In vivo specificities of the EGFR-, FR-, and PSMA-retargeted VSV were assessed by intratumoral injection into human tumor xenografts. Analysis of green fluorescent protein reporter gene expression indicated that VSV infection was restricted to receptor-positive tumors. In summary, we have demonstrated for the first time that VSV can be efficiently retargeted to different cellular receptors using the measles display technology, yielding retargeted VSV vectors that are highly specific for tumors that express the relevant receptor.
Ayala-Breton and colleagues use the measles virus (MV) hemagglutinin (H) and fusion (F) envelope glycoproteins to redirect vesicular stomatitis virus (VSV) entry and infection specifically to tumor-associated receptors such as epidermal growth factor receptor, folate receptor, and prostate membrane-specific antigen. In vivo expression of the all retargeted VSV was restricted to receptor-positive human tumor xenografts.
Introduction
Vesicular stomatitis virus (VSV) is a new promising oncolytic agent due to its preferential replication in tumor cells, fast replication cycle, and high burst size (Balachandran and Barber, 2000, 2004). However, one of the main concerns with using VSV for treatment of cancer is toxicity, as the virus presents broad tropism for different types of cells, including neurons (Clarke et al., 2006; Johnson et al., 2007). Various approaches have been used to engineer a VSV that replicates preferentially in tumor cells, but is attenuated in normal cells (Obuchi et al., 2003; Stojdl et al., 2003; Bergman et al., 2007; Edge et al., 2008; Kelly et al., 2010), including exploiting the defective interferon (IFN) response pathways in tumor cells (Garcia-Sastre and Biron, 2006). As the virus is exquisitely sensitive to the antiviral effects of IFN produced by infected cells, VSV expressing murine IFN-β (VSV-mIFNβ) or human IFN-β (VSV-hIFNβ) have been generated to obtain viruses that replicate selectively in tumor cells, and they are significantly less toxic than wild-type VSV (Obuchi et al., 2003). Despite this improvement, it was reported in a toxicology study that BALB/c mice given high intravenous doses of VSV-mIFNβ (1010 TCID50) showed signs of neurotoxicity, occurring between days 2 and 7, manifested as seizures, whole-body tremors, and weight loss of 10% on day 1 (Jenks et al., 2010). BALB/c mice (n=16) given 108 or 109 TCID50 of VSV-mIFNβ intravenously did not exhibit neurological symptoms, although animals appeared scruffy and lost 6–16% of initial body weight between days 1 and 7 (Jenks et al., 2010).
Hence, there is a strong rationale to include strategies, other than intracellular restriction, to achieve tumor-selective replication of the virus (Russell and Peng, 2007). Here, we explored the feasibility of using transductional targeting to control the tropism of VSV, with the aim of maintaining the high infectivity of VSV in tumor cells and ablating VSV infection of nontarget cells. This is especially important in the context of cancer therapy, where VSV could be used to treat patients with an immune system compromised by the disease (such as in multiple myeloma) or by repeat cycles of chemotherapy.
Previous works have reported the specific retargeting of measles virus (MV) to different cellular receptors overexpressed in tumor cells (Hadac et al., 2004). This change in the specificity of virus entry and infection is achieved by modifying the measles hemagglutinin protein (MV-H) to bear a single-chain antibody (scFv) directed to the desired tumor-associated cellular receptors (Nakamura et al., 2005). The retargeting of MV can include ablation of MV-H tropism for CD46 and SLAM, the natural cellular receptors of the Edmonston vaccine strain of measles virus (Dörig et al., 1993; Tatsuo et al., 2000b; Hadac et al., 2004). Some examples of retargeted MV include those redirected to cellular receptors such as prostate-specific membrane antigen (PSMA), epidermal growth factor receptor (EGFR), α-folate receptor (αFR), CD38, and αvβ3 integrins (Peng et al., 2003; Nakamura et al., 2005; Hasegawa et al., 2006; Liu et al., 2009; Ong et al., 2009).
In this work, we demonstrated the feasibility of pseudotyping VSV with measles fusion protein (MV-F) and MV-H bearing scFvs directed to three well characterized tumor-associated cellular receptors EGFR, αFR, and PSMA. The resulting viruses exhibited very specific tropism for receptor-positive cells, whereas infection of receptor-negative cells was reduced at least 50-fold compared with the parental VSV vector. The specificity of the pseudotyped VSV was also confirmed in subcutaneous tumors established in mice.
Materials and Methods
Cells and viruses
Human cortical neuronal cells HCN-1A [American Type Culture Collection (ATCC), Manassas, VA; CRL-10442] were maintained in medium as recommended by ATCC. CHO cell lines stably expressing CD46 (CHO-CD46), FR (CHO-FR), and EGFR (CHO-EGFR) have been described previously (Nakamura et al., 2004). The PC3 cells stably expressing PSMA (PC3-PSMA, originally named PC3-PIP) were a kind gift from Dr. Michel Sadelain (Memorial Sloan-Kettering Cancer Center) (Chang et al., 1999). KAS 6/1 multiple myeloma cells were a gift from Dr. Diane Jelinek (Mayo Clinic), and SKOV3ip.1 ovarian tumor cells were a gift from Dr. Ellen Vitetta (University of Texas Southwestern Medical Center). KAS 6/1 cells are positive for CD46 and EGFR, but not αFR or PSMA. SKOV3ip.1 cells express CD46, EGFR, and αFR, but not PSMA. VSV (Indiana strain) with a deleted glycoprotein gene that was replaced by a green fluorescent protein (GFP) cDNA (VSVΔG) was described previously (Majid et al., 2006). The Vero-αHis cells express a membrane-anchored single-chain antibody that recognizes a six-histidine peptide (Nakamura et al., 2005).
Preparation of VSVΔG pseudotypes
For pseudotyping VSVΔG with MV glycoproteins, three different types of plasmids were used: pCGF encoding parental Edmonston strain MV-F protein, pCGH encoding parental Edmonston strain MV-H protein, or pTNHaa (Nakamura et al., 2004) encoding a mutated MV-H protein, with two point mutations, Y481A and R533A, that block the interaction of MV-H with MV receptors CD46 and SLAM, respectively (Vongpunsawad et al., 2004). The plasmids encoding MV-H bearing an scFv directed against either αFR, EGFR, or PSMA (pTNHaa-αFR, pTNHaa-αEGFR, or pTNHaa-αPSMA) were used in this study (Nakamura et al., 2005; Hasegawa et al., 2006; Liu et al., 2009). HEK-293T cells (107) were seeded in a 150-mm plate. Next day, 30 μg of pMD-G (plasmid encoding VSV-G protein) or 30 μg of a plasmid encoding MV-H protein (pCGH or pTNHaa) and 30 μg of a plasmid encoding MV-F protein (pCGF) were transfected into the cells using the calcium phosphate method. To avoid cell fusion due to the intracellular expression of MV-F and MV-H, 6.6 μg of fusion inhibitory peptide (FIP; Bachem, Americas Inc., Torrance, CA) per milliliter of culture medium was added to the cells 5 hr post transfection. The following day, transfected cells were infected for 3 hr with VSVΔG-G (VSVΔG pseudotyped with VSV-G protein) at a multiplicity of infection (MOI) of 3 in the presence of FIP. The virus inoculum was then removed, and cells were washed five times and incubated in OptiMEM (Invitrogen, Carlsbad, CA) plus FIP. After 24 hr of infection, cells and supernatant were freeze-thawed two times; then the supernatant was clarified (5 min at 1,600 rpm) and stored at −80°C. Titer for each virus was determined in Vero-αHIS cells using the standard TCID50 titration method as described previously for MV (Hadac et al., 2004). The viral supernatants were also concentrated by centrifugation for 5 min at 2,500 rpm in an Amicon Ultra-15 device with a 100,000 molecular weight cutoff (Millipore, Billerica, MA). The supernatant that did not pass through the filter was collected and stored at −80°C.
Plaque reduction neutralization (PRN) assay
Two hundred TCID50 of MV expressing GFP (MVG), VSVΔG-G, or VSV-H/F pseudotypes in 50 μl of OptiMEM was incubated for 1 hr at 37°C in 5% CO2 with twofold dilutions of anti-VSV serum (rat anti-VSV pooled serum) or anti-measles serum (pooled human measles immune serum; Valley Biomedical, lot no. C80553). Vero-αHis cells (1.5×104) resuspended in OptiMEM were then added to each mixture and incubated at 37°C. After 2 hr, 50 μl of Dulbecco's modified Eagle's medium/5% fetal bovine serum was added, and cells were incubated for 1–2 days. The PRN antibody titer was reported as the dilution of serum that reduced numbers of plaques by at least 80%.
Immunoblotting for viral proteins
Protein lysates were fractionated by PAGE in 10% Tris-HCl Criterion precast gels (Bio-Rad, Hercules, CA) and transferred to a polyvinylidene difluoride membrane (Bio-Rad). Membranes were blocked with 5% nonfat milk in Tris-buffered saline (TBS)–Tween for 1 hr at room temperature, incubated with primary antibodies [polyclonal rabbit αMV-H (Hadac et al., 2004), polyclonal αVSV structural proteins (Jenks et al., 2010)], washed five times with TBS-Tween, incubated with secondary antibody conjugated to peroxidase, and washed again five times. Signal was developed using Pierce ECL western blotting substrate kit (Thermo Scientific, Waltham, MA) following the conditions recommended by the manufacturer.
In vivo experiments
All procedures involving animals were approved by and performed according to guidelines of the Institutional Animal Care and Use Committee of Mayo Foundation. Six-week-old female CB17 ICR SCID mice (n=3 per group; Taconic Farms, Germantown, NY) were irradiated with 150 Gy; 24 hr later, human myeloma KAS 6/1 cells were injected in the right flanks of the mice. When tumors reached 0.5 cm in diameter, mice received one intratumoral injection of VSV pseudotypes (106 TCID50/100 μl). Two days post injection, mice were euthanized and tumors were harvested. Tumor 5-μm cryosections were stained with DAPI, and GFP expression was analyzed using a Zeiss LSM 510 confocal microscope to detect areas of viral infection.
Results
Optimization the pseudotyping of VSV with MV-F and MV-H-scFv proteins
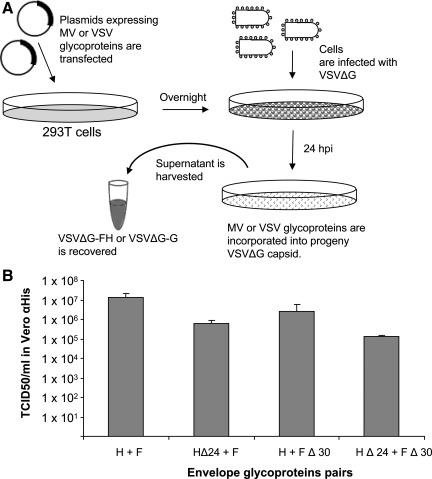
To pseudotype VSV with MV-F and MV-H proteins, 293T cells were first transfected with plasmids expressing MV-H and MV-F proteins and then were infected with a mutant VSV lacking the glycoprotein gene (Fig. 1). Progeny VSV were harvested from the supernatant and used in the study. Due to deletion of the G gene from its genome, infectivity of the progeny VSV is driven exclusively by the incorporated MV-F and MV-H or H-scFv proteins. It was reported that the truncation of the cytoplasmic tails of native MV glycoproteins is required to enable their incorporation into HIV-1 lentiviral vectors (Frecha et al., 2008; Funke et al., 2008). To determine if MV-F or MV-H with shorter cytoplasmic tail could enhance their incorporation into VSV, we pseudotyped VSV vectors with parental MV-F/H or two mutant MV glycoproteins: MV-HΔ24 (MV-H with an N-terminal deletion of 24 amino acids) and MV-FΔ30 (with a complete deletion of the cytoplasmic tail except for the three membrane-proximal residues RGR). Results in Fig. 1 show a 10–100-fold reduction in the viral titers of VSV pseudotyped with the truncated glycoproteins. Hence, for all subsequent studies, MV-F and MV-H with parental cytoplasmic tails were used.
FIG. 1.
(A) Schematic representation of the protocol for pseudotyping VSVΔG with MV or VSV glycoproteins. The recovered supernatant contains VSVΔG-FH or VSVΔG-G vectors that can infect target cells and express viral proteins, but cannot produce viral progeny due to deletion of the VSV-G gene from the viral genome. (B) Titers of VSV pseudotyped with MV F/H glycoproteins bearing parental MV-H and F proteins or with truncated cytoplasmic tails (MV-HΔ24 or MV-FΔ30). Viral titers were determined on Vero-αHis cells. Results show representative data from two experiments.
VSV can be pseudotyped with MV-F and MV-H bearing a single-chain antibody
To study the possible retargeting of VSV, the virus was pseudotyped with MV-F and either one of the following different versions of MV-H: parental Edmonston strain MV-H, MV-H bearing a single-chain antibody (H-scFv) directed to EGFR, αFR, and PSMA. These tumor-associated receptors are overexpressed in tumor cells (Ekstrand et al., 1991; Toffoli et al., 1997; Wang et al., 2007) and have been used as targets in studies using retargeted MV (Hasegawa et al., 2006; Paraskevakou et al., 2007; Liu et al., 2009).
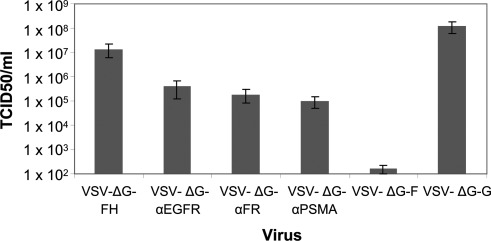
Viral titers from the pseudotyped VSV are shown in Fig. 2. To demonstrate that the infection was due to MV-F/H pseudotyped VSV and not because of residual input VSVΔG-G virus, MV-H plasmid was not transfected into the cells to generate VSVΔG-F, and that virus had minimal infectivity (Fig. 2). In contrast, there was robust infection for other viral vectors, with titers ranging from 107 TCID50 for VSV pseudotyped with MV-H/F to 105 TCID50 for VSV pseudotyped with MV-H-scFv. Viral titers of pseudotyped vectors bearing retargeted envelopes could be increased to 1×107 when concentrated by ultrafiltration or sucrose cushion.
FIG. 2.
Titers of retargeted VSV vectors. VSV were pseudotyped with VSV-G, MV-F alone, or a combination of MV-F and MV-H with and without an scFv, and titers were determined on Vero-αHis cells. Results show average of four independent experiments.
To prove that the infection of pseudotyped VSV was dependent on the incorporation of MV-F/H proteins, viruses were analyzed by PRN assays (Table 1). It was observed that the infection of MVG and VSVΔG-G were only neutralized when viruses were incubated with antimeasles or anti-VSV serum, respectively. On the other hand, pseudotyped VSV was not neutralized by anti-VSV serum, but only by antimeasles serum, indicating that the infectivities of VSV pseudotypes were due to the incorporated MV F and H proteins.
Table 1.
Plaque Reduction Neutralization (PRN) Titers of Pseudotyped VSV
| |
Reciprocal PRN titer |
|||
|---|---|---|---|---|
| |
αMV-serum |
αVSV-serum |
||
| Virus | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 |
| MVG | 128 | 128 | <2 | <2 |
| VSVΔG-FH | 512 | 256 | <2 | <2 |
| VSVΔG-αEGFR | 512 | 256 | <2 | <2 |
| VSVΔG-αFR | 512 | 512 | <2 | <2 |
| VSVΔαPSMA | 512 | 1,024 | <2 | <2 |
| VSVΔG-G | <2 | <2 | 2,048 | 2,048 |
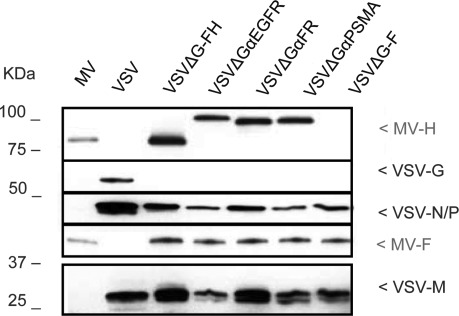
To confirm the incorporation of the MV-H/F proteins into VSV virions, proteins lysates from the purified vector stocks were analyzed by immunoblot. As shown in Fig. 3, VSV-G was detected only in VSVΔG-G lysates; MV proteins, on the other hand, were observed in the lanes where lysates of VSVΔG-FH (and retargeted versions) were loaded.
FIG. 3.
Immunochemical analysis of VSV pseudotypes. Viral supernatants were purified, and proteins were fractionated by SDS-PAGE. MV and VSV proteins were detected with polyclonal anti-MV or anti-VSV antibodies.
VSV infection can be specifically retargeted by MV glycoproteins
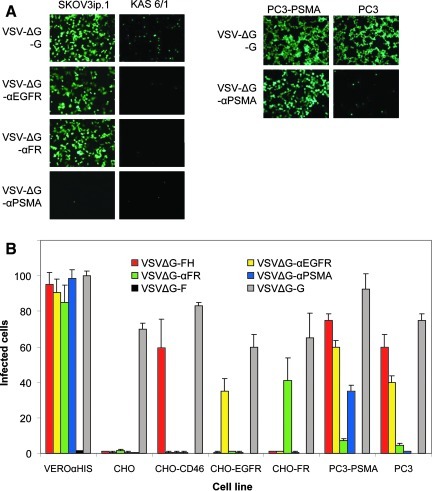
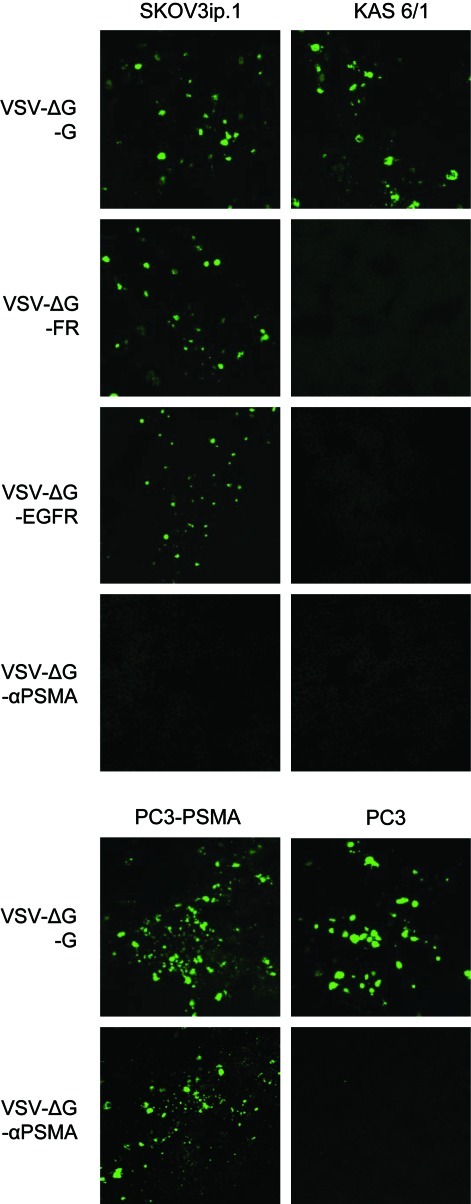
After confirming that VSV can be pseudotyped with MV-H bearing an scFv, the next step was to determine if its infectivity can indeed be retargeted to cells expressing the corresponding receptor. To test the specificity of VSV pseudotypes, virus infection was performed on an array of CHO cells expressing the specific receptors. As shown in Fig. 4A, virus entry and infection, as shown by the presence of GFP expression, were restricted to receptor-positive cells, and not in receptor-negative cells, for each of the respective retargeted VSV vectors. It is important to note that MV-H-scFv contained two point mutations at residues 481 and 533, rendering them unable to interact with MV natural receptors, CD46 and SLAM (Nakamura et al., 2004). Therefore, VSVΔG-αEGFR, VSVΔG-αFR, and VSVΔG-αPSMA were not able to infect CHO-CD46 or CHO-SLAM cells. The numbers of GFP-positive cells were counted, demonstrating the specificity of these pseudotyped vectors (Fig. 4B).
FIG. 4.
Retargeted VSV pseudotypes preferentially transduced receptor-positive cells. (A) Photographs of SKOV3.ip.1, KAS 6/1, PC3, and PC3-PSMA cells at 24 hr post transduction by retargeted VSV pseudotypes (MOI 3.0). GFP signal was observed under an epifluorescence microscope. (B) Quantitation of the numbers of VSV-transduced GFP-expressing cells at 24 hr post infection (MOI 0.1). Data show the average of three independent experiments.
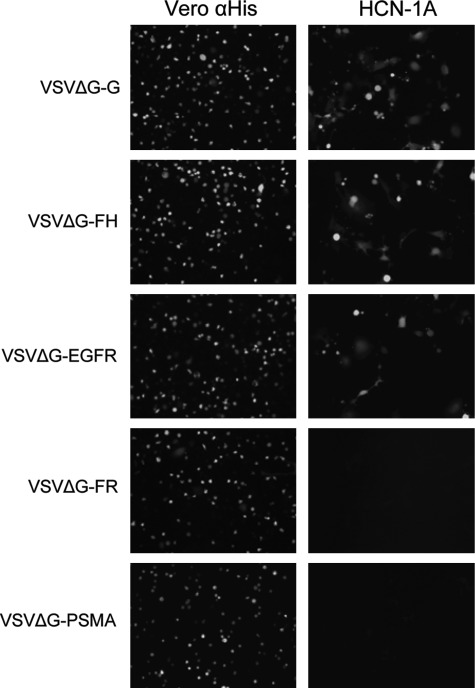
To evaluate the tropism of the VSV vectors in neurons, human cortical neuronal cells HCN-1A were transduced with VSVΔG-G or the VSVΔG-FH-retargeted vectors. As expected, these CD46, EGFR-positive human HCN-1A cells were transduced by VSVΔG-G, VSVΔG-FH, and VSVΔG-αEGFR, but not by αFR- or PSMA-specific vectors (Fig. 5).
FIG. 5.
Human neuronal cells were not transduced by αFR- or αPSMA-retargeted VSVΔG pseudotypes (MOI 1.0). Representative photographs of GFP-expressing cells taken at 48 hr post transduction under an epifluorescence microscope (100× magnification) are shown.
Specificity of retargeted VSV pseudotypes is conserved in vivo
After confirming that the retargeted VSV vectors specifically infected receptor-positive cells, we wanted to know if the same specificity is conserved when theses viruses are injected in mice. Human tumor cell lines, KAS 6/1 (EGFR-, αFR-, and PSMA-negative cells), SKOV3ip.1 (EGFR- and αFR-positive cells), PC3 (PSMA-negative cells), and PC3-PSMA cells were injected subcutaneously in the flanks of either SCID or athymic mice. Once the tumors reached 0.5 cm in diameter, 106 infectious viruses were injected intratumorally. The tumors were harvested 2 days later for analysis. As shown in Fig. 6, there was robust GFP expression in the receptor-positive tumor, but not in receptor-negative tumors. Hence, we confirmed that the corresponding retargeted VSV vectors were stable and maintained their tropism in vivo and could efficiently infect receptor-positive tumors (Fig. 6).
FIG. 6.
Specificity of retargeted VSV pseudotypes was retained in vivo. Subcutaneous SKOV3.ip.1, KAS 6/1, PC3, or PC3-PSMA tumors were injected intratumorally with one dose of 106 retargeted VSV vectors. Tumors were harvested 48 hr later, and GFP signals were detected using a fluorescence microscope. Representative images are shown (100× magnification). Color images available online at www.liebertonline.com/hum
Discussion
In this article, we have demonstrated that VSV can be retargeted by the incorporation of MV F and H proteins displaying scFv against EGFR, αFR, and PSMA. The pseudotyped VSV preferentially infected cells expressing the corresponding receptor of the displayed scFv. This specificity was conserved in vitro and in vivo. One of the main concerns with the use of VSV as a modality for cancer therapy is its neurotoxic effects when injected into experimental animals (Clarke et al., 2006; Jenks et al., 2010). Several groups have thus engineered VSV to restrict virus replication in normal cells while conserving its oncolytic properties selectively in tumor cells. Strategies include insertion of transgenes such as IFN-β (Obuchi et al., 2003), which restricts VSV replication in normal but not in cancer cells, or mutations in the matrix protein, which interferes with transport of mRNA from the nucleus to the cytoplasm (Stojdl et al., 2003), and insertion of miRNA sequences to restrict viral replication in specific cell types (Edge et al., 2008; Kelly et al., 2010). However, a 100% protection has not yet been achieved when animals were given very high doses of these viruses. For example, BALB/c mice tolerated 109 TCID50 VSV-mIFNβ, but 1010 TCID50 VSV-mIFNβ injected intravenously resulted in encephalitis in the animals (Jenks et al., 2010). BALB/c mice injected intracranially with VSV incorporating neuronal miRNA (VSV-mir125L) target sequences tolerated significantly higher doses of virus (intracranial injection of 1×104 VSV). Although all mice had to be euthanized due to neurotoxicity from parental VSV, only one of 10 mice eventually succumbed to VSV-mir125L-induced encephalitis (Kelly et al., 2010).
An additional layer of targeting could be added by transductional targeting through control of VSV infection and cell entry. So far, there have been no published reports on engineering the G protein of the virus for retargeting, but a number of VSV have been pseudotyped with envelope glycoproteins from various viruses, including MV, lymphocytic choriomeningitis virus, and Sindbis virus (Tatsuo et al., 2000a; Bergman et al., 2004; Muik et al., 2011). By using VSV vectors pseudotyped with H and F proteins from the Edmonston vaccine and KA wild-type strains of MV, it was demonstrated that the H protein of MV controls virus tropism (Tatsuo et al., 2000a). In that study, a mutant VSV lacking the glycoprotein gene (VSVΔG) was used to infect cells transiently expressing MV-H and MV-F to generate a VSV that incorporated MV-H/F proteins. The tropism of the resulting virus was limited to cells expressing CD46 and SLAM, which are the cellular receptors of MV. Recently, it was demonstrated that targeting ligands, including single-chain antibodies, could be displayed as C-terminal extensions on the hemagglutinin attachment protein of MV coat proteins of MV, resulting in exquisite control of MV tropism (Nakamura et al., 2005; Lech and Russell, 2010). Using this MV display platform, the titers obtained for the MV pseudotyped vectors were 107 and 105 TCID50/ml for VSVΔG-FH and VSVΔG-FH-scFv, respectively, which were lower than that obtained for the parental VSVΔG-G at 108 TCID50/ml. This reduction in the titer of viral yield could be due to less efficient incorporation of the foreign capsid proteins incorporated into the VSV virions. Further increases in titers (50-fold) could be obtained through ultrafiltration of the vectors. We also tested if modification of the cytoplasmic tails of the glycoproteins could increase vector yield. In another study, where the MV envelope glycoproteins were used successfully to pseudotype lentiviral vectors, thus permitting infection of quiescent cells, the MV proteins were truncated to enable more efficient incorporation into HIV-1 vectors (Frecha et al., 2008, 2010; Funke et al., 2009). In contrast to the HIV-1 vectors, we determined that VSV vectors more efficiently incorporated H/F with native cytoplasmic tails than with the truncated tails, giving higher titer vectors when tested on indicator cells.
As observed with MV, the tropisms of the VSV/MV pseudotypes were controlled very tightly by the displayed scFv, both in vitro and in vivo by intratumoral injection of the vectors into subcutaneous tumors in mice. There was a clear difference in the presence or absence of GFP-positive cells in receptor-positive tumors and receptor-negative tumors, confirming that the specificity of VSV, given by MV-F and MV-H proteins, is still conserved in vivo. Importantly, the VSV pseudotypes ablated for binding to CD46 and SLAM, but retargeted to tumor-associated receptors such as αFR or PSMA, did not infect human neuronal cells. In contrast, both the parental VSVΔG-G and VSVΔG-FH were able to transduce the neuronal cells. Hence, transductional targeting of VSV may be an effective approach to minimize the neurotoxicity associated with experimental inoculation of replication-competent VSV into research animals (Clarke et al., 2006; Jenks et al., 2010).
This demonstration that it is feasible to redirect the infection of VSV to tumor-associated receptors via an scFv in the MV-H protein is the first step to obtaining an oncolytic VSV whose replication and spread are dependent on the H-scFv attachment protein. A potential weakness in using the MV H and F envelopes is the prevalence of preexisting antimeasles antibodies in the patient population that could neutralize the systemically administered virus, thus decreasing the antitumor potential of this virus. Although first it is necessary to test if a replicative VSV-FH has efficacy against tumor cells, it is important to note that there are several strategies to avoid virus neutralization by the immune system. One of them is to use VSV-FH to treat malignancies, such as multiple myeloma, where the patients have no or low antimeasles antibodies (Dingli et al., 2005). Other strategies include shielding viral envelope proteins with polymers or hiding the viruses in cell carriers that home to the tumor sites (Russell and Peng, 2009; Fisher and Seymour, 2010). However, the prevalence of antimeasles antibodies in the population also implies that the chimeric virus VSV-FH (if transmissible) would not likely represent a risk for the caregivers of the cancer patients.
In conclusion, we have demonstrated, as proof of concept, that VSV infection can be specifically retargeted using MV proteins bearing an scFv in the H protein and that the FH pseudotyped VSV infects only cells expressing the corresponding receptor both in vitro and in vivo. This work represents an advance in controlling the specificity of VSV vectors with the goal of increasing the safety of VSV when used as an anticancer therapy.
Acknowledgments
We thank Suzanne Greiner for expert technical assistance with the animal experiments. This work is funded by grants from the NIH/NCI (CA118488, CA129193, and CA129966).
Author Disclosure Statement
No competing financial interests exist for all authors.
References
- Balachandran S. Barber G.N. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- Balachandran S. Barber G.N. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Bergman I. Whitaker-Dowling P. Gao Y. Griffin J.A. Preferential targeting of vesicular stomatitis virus to breast cancer cells. Virology. 2004;330:24–33. doi: 10.1016/j.virol.2004.06.048. [DOI] [PubMed] [Google Scholar]
- Bergman I. Griffin J.A. Gao Y. Whitaker-Dowling P. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to her2/neu. Int. J. Cancer. 2007;121:425–430. doi: 10.1002/ijc.22680. [DOI] [PubMed] [Google Scholar]
- Chang S.S. Reuter V.E. Heston W.D., et al. Five different anti-prostate-specific membrane antigen (psma) antibodies confirm psma expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- Clarke D.K. Cooper D. Egan M.A., et al. Recombinant vesicular stomatitis virus as an hiv-1 vaccine vector. Springer Semin. Immunopathol. 2006;28:239–253. doi: 10.1007/s00281-006-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingli D. Peng K.W. Harvey M.E., et al. Interaction of measles virus vectors with Auger electron emitting radioisotopes. Biochem. Biophys. Res. Commun. 2005;337:22–29. doi: 10.1016/j.bbrc.2005.08.261. [DOI] [PubMed] [Google Scholar]
- Dörig R.E. Marcil A. Chopra A. Richardson C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Edge R.E. Falls T.J. Brown C.W., et al. A let-7 microRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol. Ther. 2008;16:1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- Ekstrand A.J. James C.D. Cavenee W.K., et al. Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- Fisher K.D. Seymour L.W. HPMA copolymers for masking and retargeting of therapeutic viruses. Adv. Drug Deliv. Rev. 2010;62:240–245. doi: 10.1016/j.addr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Frecha C. Costa C. Negre D., et al. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood. 2008;112:4843–4852. doi: 10.1182/blood-2008-05-155945. [DOI] [PubMed] [Google Scholar]
- Frecha C. Levy C. Cosset F.L. Verhoeyen E. Advances in the field of lentivector-based transduction of T and B lymphocytes for gene therapy. Mol. Ther. 2010;18:1748–1757. doi: 10.1038/mt.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke S. Maisner A. Muhlebach M.D., et al. Targeted cell entry of lentiviral vectors. Mol. Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke S. Schneider I.C. Glaser S., et al. Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity. Gene Ther. 2009;16:700–705. doi: 10.1038/gt.2009.11. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A. Biron C.A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Hadac E.M. Peng K.W. Nakamura T. Russell S.J. Reengineering paramyxovirus tropism. Virology. 2004;329:217–225. doi: 10.1016/j.virol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Hasegawa K. Nakamura T. Harvey M., et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin. Cancer Res. 2006;12:6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- Jenks N. Myers R. Greiner S.M., et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-β in rodents and nonhuman primates. Hum. Gene Ther. 2010;21:451–462. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.E. Nasar F. Coleman J.W., et al. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007;360:36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E.J. Nace R. Barber G.N. Russell S.J. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J. Virol. 2010;84:1550–1562. doi: 10.1128/JVI.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech P.J. Russell S.J. Use of attenuated paramyxoviruses for cancer therapy. Expert Rev. Vaccines. 2010;9:1275–1302. doi: 10.1586/erv.10.124. [DOI] [PubMed] [Google Scholar]
- Liu C. Hasegawa K. Russell S.J., et al. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate. 2009;69:1128–1141. doi: 10.1002/pros.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid A.M. Ezelle H. Shah S. Barber G.N. Evaluating replication-defective vesicular stomatitis virus as a vaccine vehicle. J. Virol. 2006;80:6993–7008. doi: 10.1128/JVI.00365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A. Kneiske I. Werbizki M., et al. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J. Virol. 2011;85:5679–5684. doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T. Peng K.W. Vongpunsawad S., et al. Antibody-targeted cell fusion. Nat. Biotechnol. 2004;22:331–336. doi: 10.1038/nbt942. [DOI] [PubMed] [Google Scholar]
- Nakamura T. Peng K.W. Harvey M., et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- Obuchi M. Fernandez M. Barber G.N. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong H.T. Trejo T.R. Pham L.D., et al. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol. Ther. 2009;17:1012–1021. doi: 10.1038/mt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevakou G. Allen C. Nakamura T., et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRviii expressing gliomas. Mol. Ther. 2007;15:677–686. doi: 10.1038/sj.mt.6300105. [DOI] [PubMed] [Google Scholar]
- Peng K.W. Donovan K.A. Schneider U., et al. Oncolytic measles viruses displaying a single-chain antibody against cd38, a myeloma cell marker. Blood. 2003;101:2557–2562. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- Russell S.J. Peng K.W. Viruses as anticancer drugs. Trends Pharmacol. Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.J. Peng K.W. Measles virus for cancer therapy. Curr. Top. Microbiol. Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojdl D.F. Lichty B.D. Tenoever B.R., et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Tatsuo H. Okuma K. Tanaka K., et al. Virus entry is a major determinant of cell tropism of Edmonston and wild-type strains of measles virus as revealed by vesicular stomatitis virus pseudotypes bearing their envelope proteins. J. Virol. 2000a;74:4139–4145. doi: 10.1128/jvi.74.9.4139-4145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuo H. Ono N. Tanaka K. Yanagi Y. [the cellular receptor for measles virus: Slam (cdw 150)] Uirusu. 2000b;50:289–296. [PubMed] [Google Scholar]
- Toffoli G. Cernigoi C. Russo A. Gallo A., et al. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Vongpunsawad S. Oezgun N. Braun W. Cattaneo R. Selectively receptor-blind measles viruses: identification of residues necessary for slam- or cd46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 2004;78:302–313. doi: 10.1128/JVI.78.1.302-313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Yin L. Rao P., et al. Targeted treatment of prostate cancer. J. Cell. Biochem. 2007;102:571–579. doi: 10.1002/jcb.21491. [DOI] [PubMed] [Google Scholar]