Abstract
Pompe disease can be treated effectively, if immune tolerance to enzyme replacement therapy (ERT) with acid α-glucosidase (GAA) is present. An adeno-associated viral (AAV) vector carrying a liver-specific regulatory cassette to drive GAA expression (AAV-LSPhGAA) established immune tolerance in GAA knockout (KO) mice, whereas ubiquitous expression with AAV-CBhGAA provoked immune responses. Therefore, we investigated the hypothesis that immune tolerance induced by hepatic-restricted expression was dominant. AAV-LSPhGAA and AAV-CBhGAA were administered singly or in combination to groups of adult GAA-KO mice, and AAV-LSPhGAA induced immune tolerance even in combination with AAV-CBhGAA. The dual vector approach to GAA expression improved biochemical correction of GAA deficiency and glycogen accumulations at 18 weeks, and improved motor function testing including wire-hang and grip-strength testing. The greatest efficacy was demonstrated by dual vector administration, when both vectors were pseudotyped as AAV8. T cells from mice injected with AAV-LSPhGAA failed to proliferate at all after an immune challenge with GAA and adjuvant, whereas mock-treated GAA-KO mice mounted vigorous T cell proliferation. Unlike AAV-LSPhGAA, AAV-CBhGAA induced selective cytokine and chemokine expression in liver and spleen after the immune challenge. AAV-CBhGAA transduced dendritic cells and expressed high-level GAA, whereas AAV-LSPhGAA failed to express GAA in dendritic cells. The level of transduction in liver was much higher after dual AAV8 vector administration at 18 weeks, in comparison with either vector alone. Dual vector administration failed to provoke antibody formation in response to GAA expression with AAV-CBhGAA; however, hepatic-restricted expression from dual vector expression did not prevent antibody formation after a strong immune challenge with GAA and adjuvant. The relevance of immune tolerance to gene therapy in Pompe disease indicates that hepatic expression might best be combined with nonhepatic expression, achieving the benefits of ubiquitous expression in addition to evading deleterious immune responses.
Zhang and colleagues investigate the mechanism for immunomodulatory gene therapy by evaluating two AAV vectors encoding human acid α-glucosidase (hGAA), including both a tolerogenic vector containing the liver-specific promoter (LSP) and an immunogenic vector containing the ubiquitously active cytomegalovirus enhancer and chicken β-actin promoter (CB) regulatory cassette. The authors find that simultaneous administration of tolerogenic and immunogenic vectors induces immune tolerance in mice and leads to higher levels of liver transduction, in comparison with either vector alone.
Introduction
If untreated by enzyme replacement therapy (ERT), infantile-onset Pompe disease (glycogen storage disease type II; MIM 232300) causes death early in childhood from cardiorespiratory failure related to an underlying hypertrophic cardiomyopathy (Hirschhorn and Reuser, 2001; Kishnani et al., 2006). The deficiency of acid α-glucosidase (GAA; acid maltase; EC 3.2.1.20) in Pompe disease affects the heart and skeletal muscle primarily, and infants with Pompe disease develop profound weakness and hypotonia. Late-onset forms of Pompe disease feature progressive weakness without significant cardiomyopathy, and patients with juvenile-onset Pompe disease typically become ventilator-dependent due to respiratory and/or cardiac muscle involvement. The histopathology of Pompe disease includes marked lysosomal accumulation of glycogen in cardiac and skeletal muscle, which culminates with the leakage/rupture of lysosomes and cytoplasmic pooling of glycogen. GAA is normally widely expressed from a promoter with resemblance to a “housekeeping” gene (Martiniuk et al., 1990; Ponce et al., 1999), and GAA deficiency causes lysosomal glycogen accumulation in virtually all tissues.
The presence of immune tolerance to a therapeutic protein, such as recombinant human (rh) GAA in Pompe disease, correlated with efficacy in several animal models for genetic disease. In contrast, antibody formation has often reduced the efficacy of protein replacement therapy or ERT. Animal and human studies have suggested that the development of humoral immunity against the infused enzyme presents an obstacle to successful ERT in Pompe disease. For instance, formation of anti-GAA antibodies and associated infusion reactions prevented continuation of ERT beyond 3 weeks in GAA-knockout (KO) mice. Long-term ERT could be tested in a Pompe disease mouse model only by the generation of liver-expressing transgenic Pompe disease mice that were immune tolerant to GAA (Raben et al., 2003).
Patients with Pompe disease who lack any residual GAA protein are deemed cross-reacting immune material negative (CRIM-negative). CRIM-negative subjects with Pompe disease produced high anti-hGAA antibodies and demonstrated markedly reduced efficacy from ERT (Amalfitano et al., 2001). In the first pilot study of ERT in Pompe disease using Chinese hamster ovary cell-derived rhGAA, the two patients who were CRIM-negative produced higher titers of anti-hGAA antibodies than the third patient, who was CRIM-positive. This corresponded with a markedly reduced efficacy of the treatment in the CRIM-negative patients (Amalfitano et al., 2001). Tolerization therapy, including administration of high-dose rhGAA with immune suppressant drugs, failed to improve the clinical response to ERT in those CRIM-negative subjects. Indeed, high-dose rhGAA therapy precipitated nephrotic syndrome in one of the CRIM-negative subjects, possibly related to effects of antibody complexes on the glomerular basement membrane (Hunley et al., 2004). The similarity of the antibody response between GAA-KO mice and CRIM-negative patients with Pompe disease can be explained by the presence by two underlying null mutations in the GAA gene, because in the complete absence of GAA expression the immune system will recognize rhGAA as a foreign protein.
ERT has encountered antibody and hypersensitivity reactions in GAA-KO mice, which demonstrated immunity to repeat administration of the therapeutic protein. Fortunately, vector-mediated hGAA expression has avoided hypersensitivity reactions; however, the efficacy of gene therapy in these experiments was inversely related to the presence of antibody responses (Koeberl and Kishnani, 2009). The appearance of anti-GAA antibodies correlated with the disappearance of secreted hGAA precursor from the plasma and a lack of efficacy from viral vectors (Ding et al., 2001; Franco et al., 2005). An adeno-associated viral (AAV) vector containing a unique liver-specific promoter (LSP) to drive GAA expression did not provoke antibody formation in response to GAA expression (Franco et al., 2005; Sun et al., 2007); moreover, the LSP-containing vector induced immune tolerance to GAA and ameliorated hypersensitivity reactions and mortality in response to an immune challenge with rhGAA (Sun et al., 2007, 2010). An AAV vector containing the LSP to drive coagulation factor IX (FIX) expression similarly prevented an antibody response against FIX in mice and dogs with hemophilia B (Wang et al., 1999, 2000). Several factors determine the ability to avoid antibody responses against foreign protein through liver-specific expression. Higher circulating levels of infused FIX were associated with the induction of tolerance to liver-specific FIX expression (Mingozzi et al., 2003). Acquisition of tolerance to FIX required induction of regulatory CD4+ T cells, most likely CD4+CD25+ regulatory T (Treg) cells, which suppressed neutralizing antibody formation (Mingozzi et al., 2003). The role of CD4+CD25+ Treg cells in immune tolerance has been evaluated in other genetic disease models. Adoptive transfer of CD4+CD25+ cells to naive recipient mice, after administration of an AAV vector to donor mice, prevented antibody formation in response to an immune challenge with human FIX in hemophilia B mice (Cao et al., 2007). An AAV2/8 vector containing a liver-specific regulatory cassette to drive α-galactosidase expression induced immune tolerance to α-galactosidase in Fabry disease mice, and the transfer of splenocytes from vector-treated mice prevented the antibody response against an α-galactosidase challenge in recipient Fabry mice (Ziegler et al., 2007). Finally, anti-CD25 administration prevented the induction of immune tolerance with the LSP-containing vector in GAA-KO mice, presumably be depleting Treg cells (Sun et al., 2010). Taken together, these data strongly support the ability of an AAV vector containing a liver-specific regulatory cassette to induce immune tolerance to an introduced foreign protein, which comprises immunomodulatory gene therapy.
We have investigated the mechanism for immunomodulatory gene therapy by evaluating two AAV vectors encoding hGAA, including both a tolerogenic vector containing the LSP and an immunogenic vector containing the ubiquitously active cytomegalovirus enhancer and chicken β-actin promoter (CB) regulatory cassette. The each vector was analyzed, first singly and then in combination, with regard to ability to transduce dendritic cells, associated T cell and antibody responses, and efficacy as reflected by functional testing. These experiments established the immunodominance of liver-specific expression with regard to inducing immune tolerance, thereby enhancing the efficacy from AAV vectors in mice with Pompe disease.
Materials and Methods
Preparation AAV 2/8 vectors
Briefly, 293 cells were transfected with the pAAV-LSPhGAA vector or pAAV-CBhGAA vector plasmid (Franco et al., 2005), the AAV8 or AAV9 packaging plasmid (Gao et al., 2002) (courtesy of J.M. Wilson, University of Pennsylvania, Philadelphia, PA), and pAdHelper (Stratagene, La Jolla, CA). The LSP regulatory cassette (subcloned from pAV-LSP-cFIX, courtesy of I. Verma [Salk Institute, La Jolla, CA]; sequence available on request) contains a thyroid hormone-binding globulin promoter sequence downstream from two copies of a α1-microglobulin/bikunin enhancer sequence, and previously achieved long-term efficacy in hemophilia B mice within an AAV vector encoding coagulation factor IX (Wang et al., 1999). Cell lysate was harvested 48 hr after infection and freeze–thawed three times, and isolated by sucrose cushion pelleting followed by two cesium chloride gradient centrifugation steps. AAV stocks were dialyzed against three changes of Hanks' buffer with 5% sorbitol added to the third dialysis, and aliquots were stored at −80°C. The number of vector DNA containing-particles was determined by DNase I digestion, DNA extraction, and Southern blot analysis. All viral vector stocks were handled according to Biohazard Safety Level 2 guidelines published by the National Institutes of Health (Bethesda, MD).
In vivo analysis of AAV vector
The AAV vector stocks were administered intravenously (via the retroorbital sinus) in 3-month-old GAA-KO mice. At the indicated time points postinjection, plasma or tissue samples were obtained and processed as described below. GAA activity and glycogen content were analyzed as described (Amalfitano et al., 1999). All animal procedures were done in accordance with Duke University (Durham, NC) Institutional Animal Care and Use Committee-approved guidelines.
Flow cytometry
Splenocytes were isolated from individual spleens by mashing them through a cell strainer, and red blood cells were lysed with red blood cell lysing buffer (Sigma-Aldrich, St. Louis, MO). After washing with phosphate-buffered saline (PBS) twice, cells were resuspended in 1% bovine serum albumin (BSA)–PBS. Cells were surface-stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 (RM4-5) and allophycocyanin (APC)-conjugated anti-mouse CD25 (PC61.5), and subsequently with phycoerythrin (PE)-conjugated anti-mouse FoxP3 monoclonal antibody (FJK-16s) using a mouse regulatory T cell staining kit (eBioscience, San Diego, CA) according to the manufacturer's instructions. Cells were analyzed with a FACSCalibur equipped with CellQuest software (BD Biosciences, San Jose, CA).
T cell proliferation assay
Splenocytes were resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), and were put into 96-well flat-bottomed plates (500,000 cells per well). Recombinant GAA protein was added (final concentrations: 0.1, 1, 10, and 100 μg/ml) to the wells. After 4 days of incubation, 1 μCi of [3H]thymidine (PerkinElmer, Boston, MA) was added to each well, and 18 hr later, uptake was counted by liquid scintillation counter (TRILUX; Wallac, Turku, Finland). The stimulation index was calculated by dividing the [3H]thymidine uptake values by the value of the control (without GAA antigen).
Quantification of vector RNA and DNA
Real-time PCR was performed with SYBR green in a LightCycler 480 II (Roche, Indianapolis, IN) in accordance with the manufacturer's instructions. For RT-PCR total RNA was isolated from spleen or liver, using TRIzol. The RNA was reverse transcribed with Moloney-murine leukemia virus (M-MLV) reverse transcriptase (Life Technologies, Gaithersburg, MD) and random hexamers (Invitrogen) in accordance with the manufacturer's protocol. One microliter of cDNA was used for RT-PCR. Primers used were as follows: mouse CXCL-1, interleukin (IL)-1β, FoxP3, GM-CSF (granulocyte-macrophage colony-stimulating factor), and β-actin (Table 1). Relative mRNA expression was normalized with β-actin and calculated by the ΔΔCt method. Quantification of vector DNA was performed as follows, using primers for human GAA and mouse β-actin (Table 1). Plasmid DNA corresponding to 0.01 to 10 copies of human GAA gene (in 50 ng of genomic DNA) was used in a standard curve. To determine the viral copy number, the ΔΔCt values of samples were compared with the standard curve.
Table 1.
Primer Sequences for Real-Time RT-PCR
| Primer | Sequence |
|---|---|
| FoxP3 F | 5′-TCTTGCCAAGCTGGAAGACT-3′ |
| FoxP3 R | 5′-TTCGCAGGTCCCGACCTTGG-3′ |
| GM-CSF F | 5′-AGCCCTGAACCTCCTGGATGAC-3′ |
| GM-CSF R | 5′-TGGTGAAATTGCCCCGTAGAC-3′ |
| IFN-γ F | 5′-CCACGGCACAGTCATTGAAAG-3′ |
| IFN-γ R | 5′-AATCTGGCTCTGCAGGATTT-3′ |
| IL-1β F | 5′-CGTGCTGTCGGACCCATATGAG-3′ |
| IL-1β R | 5′-GCCCAAGGCCACAGGTATTT-3′ |
| IL-12β F | 5′-AGTTTGGCCAGGGTCATTCC-3′ |
| IL-12β R | 5′-TCTCTGGCCGTCTTCACCAT-3′ |
| β-Actin F | 5′-AGAGGGAAATCGTGCGTGAC-3′ |
| β-Actin R | 5′-CAATAGTGATGACCTGGCCGT-3′ |
| GAA F | 5′-AGTGCCCACACAGTGCGACGT-3′ |
| GAA R | 5′-CCTCGTAGCGCCTGTTAGCTG-3′ |
GAA, acid α-glucosidase.
Antibody quantification
The ELISA for anti-GAA IgG was performed as described (Ding et al., 2002). Briefly, rhGAA (5 μg) in carbonate buffer was coated onto each well of a 96-well plate (Costar 3596; Corning Life Sciences, Lowell, MA) at 4°C overnight. After a wash with PBS containing 0.05% Tween 20, serial dilutions of plasma samples were added in duplicate to rhGAA-coated plates and incubated at room temperature. The wells were washed with 0.05% Tween 20–PBS, incubated with a 1:2,500 dilution of alkaline phosphatase-conjugated sheep anti-mouse IgG1 at room temperature for 1 hr, and washed, and alkaline phosphatase substrate (p-nitrophenyl phosphate) was added. The absorbance at 411 nm was measured with a Tecan SpectraFluor (MTX Lab Systems, Vienna, VA) microplate reader. All samples yielded absorbance values that were within the linear range of the assay at their respective dilutions. Absorbance values were deemed positive if both values at any given serial dilution were >0.1.
Isolation and transduction of dendritic cells
Dendritic cells were obtained by culturing GAA-KO mouse bone marrow cells with GM-CSF and IL-4 for 7 days, and the phenotype was validated by flow cytometry. Typically 80–90% cells were positive for CD11c, CD80, and MHC class II and less than 10% CD14 positive. On day 10, 2×106 cells were transduced with 1×105 vector particles (VP)/cell of each vector, respectively, and control cells were left untransduced. Cells were harvested 72 hr later and GAA activity was analyzed for both suspended and attached populations
Motor function testing
Rotarod testing was performed at 6 months of age as described (Sun et al., 2005b). Motor function was assessed by wire-hang tests, grip-strength, and open field testing, using methods previously described (Ribar et al., 2000; Allen et al., 2009). Vector-treated mice were 9 months of age for motor function testing by these three methods, whereas control GAA-KO mice were 7 months of age. On the first day of testing mice were examined for wire-hang duration and grip strength; 24 hr later all animals were placed in a novel open field for 30 min. For wire hang, mice were allowed to grip a horizontal 2-mm wire with four paws up to 60 sec and the latency to fall from the wire was scored. Grip strength was examined with a San Diego Instruments mouse grip-strength meter. Animals were given three trials and the average grip-strength response for the three trials was reported as grams force. Spontaneous locomotor activity was assessed in an automated AccuScan open field apparatus (AccuScan Instruments, Columbus, OH). Mice were singly placed into 21×21×30 cm acrylic arenas where locomotion was monitored with infrared diodes interfaced to a computer running Omnitech Digiscan software (AccuScan Instruments).
Statistical analyses
The significance of differences between groups was tested using a two-sided Wilcoxon rank sum test for continuous variables. A p value less than 0.05 was considered to be statistically significant. All statistical analyses were conducted with Stata 10 (StataCorp, College Station, TX). The figures illustrate mean values, with error bars depicting the range above and below the mean of its standard error.
Results
Immunodominant, liver-specific expression enhances efficacy from ubiquitous transgene expression
Liver-specific expression of hGAA with an adeno-associated vector (AAV-LSPhGAA) has established immune tolerance in GAA-KO mice (Sun et al., 2007, 2010); however, an AAV vector containing the universally active CB regulatory cassette (AAV-CBhGAA) provoked both T cell and antibody responses against hGAA and failed to achieve biochemical correction (Franco et al., 2005). Therefore, we investigated the hypothesis that a dual vector strategy that added hepatic-restricted expression along with ubiquitous expression might achieve higher efficacy than either vector alone. Given the established lack of efficacy from AAV-CBhGAA by itself, we coadministered AAV-CBhGAA with AAV-LSPhGAA, in comparison with AAV-LSPhGAA administration alone, to groups of adult GAA-KO mice. The two AAV2/8 vectors were administered at different doses; AAV-LSPhGAA was administered at a low dose that prevented antibody formation without achieving efficacy (2×1010 VP) (Sun et al., 2007), and AAV-CBhGAA was administered at a higher dose that might achieve efficacy in the setting of immune tolerance to GAA (1×1011 VP). Dual vector treatment achieved higher efficacy as demonstrated at 18 weeks by improved biochemical correction of GAA deficiency and glycogen accumulations (Fig. 1). The GAA activity of liver, heart, quadriceps, gastrocnemius, extensor digitorum longus (EDL), and soleus was significantly elevated by dual vector administration, in comparison with AAV-LSPhGAA administration alone (Fig. 1A and B; p=0.01 for each tissue). The glycogen content was reduced for heart (p=0.01) and quadriceps (p=0.03) after dual vector administration, in comparison with AAV-LSPhGAA administration alone (Fig. 1C), confirming the improved efficacy of the dual vector treatment.
FIG. 1.
Biochemical correction of striated muscle after single or dual administration of AAV vectors containing the liver-specific promoter (LSP) or chicken β-actin promoter (CB) regulatory cassettes. Acid α-glucosidase (GAA) activity, glycogen content, and vector genome quantification were analyzed 18 weeks after AAV vector administration. Means±standard error are shown. (A) GAA activity of highly transduced tissues, after administration of AAV-LSPhGAA (2×1010 VP/mouse; n=5), both AAV-LSPhGAA (2×1010 VP) and AAV-CBhGAA (1×1011 VP; n=4), AAV2/8-LSPhGAA (1×1011 VP/mouse; n=4), both AAV2/8-LSPhGAA and AAV2/9-CBhGAA (n=5), or AAV2/9-CBhGAA (1×1011 VP; n=3). Vectors were injected intravenously into mice at 3 months of age. Mock-treated GAA-knockout (KO) mice were negative controls (n=4). (B) GAA activity of skeletal muscle from groups of GAA-KO mice detailed previously. (C) Glycogen content for striated muscles from groups of GAA-KO mice detailed previously. The p values are indicated as follows: *p=0.03; **p<0.03. EDL, extensor digitorum longus; Gastroc., gastrocnemius.
The dose and serotype of the AAV vectors was next modified by administering a higher amount of AAV-LSPhGAA pseudotyped as AAV2/8 (1×1011 VP) together with AAV-CBhGAA pseudotyped as AAV2/9 (1×1011 VP) to GAA-KO mice, in comparison with each vector administered by itself. The number of AAV-LSPhGAA vector particles was increased in this experiment to allow direct comparison with AAV-CBhGAA, by administering an equivalent number of vector particles of either vector. AAV-CBhGAA was pseudotyped as AAV2/9 in this experiment to achieve higher transduction of striated muscle and liver, and potentially the central nervous system, in comparison with the AAV2/8 pseudotype (Duque et al., 2009). Administration of AAV-LSPhGAA with AAV-CBhGAA increased the GAA activity of both the heart and liver simultaneously to high levels, and the elevation of GAA activity in both tissues was not observed with either vector alone (Fig. 1A). Administration of both vectors significantly increased the GAA activity of all skeletal muscles analyzed except for the EDL, in comparison with AAV-CBhGAA alone (Fig. 1B; p=0.03 for each muscle). The GAA activity and glycogen content of the brain were not affected by administration of either dual vectors or AAV-CBhGAA alone, suggesting that too few particles were administered to achieve transduction within the central nervous system (data not shown). Administration of both vectors did significantly lower the glycogen content of all skeletal muscles analyzed, in comparison with AAV-CBhGAA alone (Fig. 1C; p=0.03 for each muscle).
The lack of efficacy in the absence of immune tolerance was emphasized by the significantly higher heart glycogen content after administration of AAV2/9-CBhGAA by itself (Fig. 1C), in comparison with AAV-LSPhGAA alone (p=0.02). GAA expressed by AAV-CBhGAA did not correct glycogen storage in the heart, even though GAA activity was significantly higher after administration of AAV-CBhGAA than after administration of AAV-LSPhGAA (Fig. 1A; p=0.03). Treatment with dual vectors significantly increased GAA activity in the heart, in comparison with administration of AAV-LSPhGAA alone (1300±1000 vs. 25±nmol·hr–1·mg–1; p=0.02). Similarly, dual vector administration significantly decreased glycogen content in the quadriceps, in comparison with administration of AAV-LSPhGAA alone (1.2±0.4 vs. 2.0±0.6; p=0.05).
A significant difference in antibody formation between the aforementioned groups of vector-treated mice correlated with efficacy (Fig. 2). Our hypothesis predicted that hepatic expression with AAV2/8-LSPhGAA would induce dominant immune tolerance to human GAA, and thereby prevent immune responses against ubiquitous expression from coadministered AAV2/9-CBhGAA. Antibody responses against GAA were prevented by administration of AAV2/8-LSPhGAA in addition to AAV2/9-CBhGAA (Fig. 2), whereas AAV-CBhGAA by itself provoked antibody formation as described (Franco et al., 2005). Mice did not form antibodies after administration of AAV2/8-LSPhGAA and AAV2/9-CBhGAA, and the highest degree of glycogen clearance in the heart was demonstrated in GAA-KO mice that received both vectors (Fig. 1C).
FIG. 2.
Humoral response after systemic single or dual AAV vector administration in GAA-KO mice. ELISA of GAA-KO mouse plasma (1:200 dilution) for anti-GAA IgG1 was performed on plasma samples obtained 6 weeks after AAV vector administration. Means and standard error are shown for the absorbance at 411 nm. The p values indicated as follows: *p=0.03.
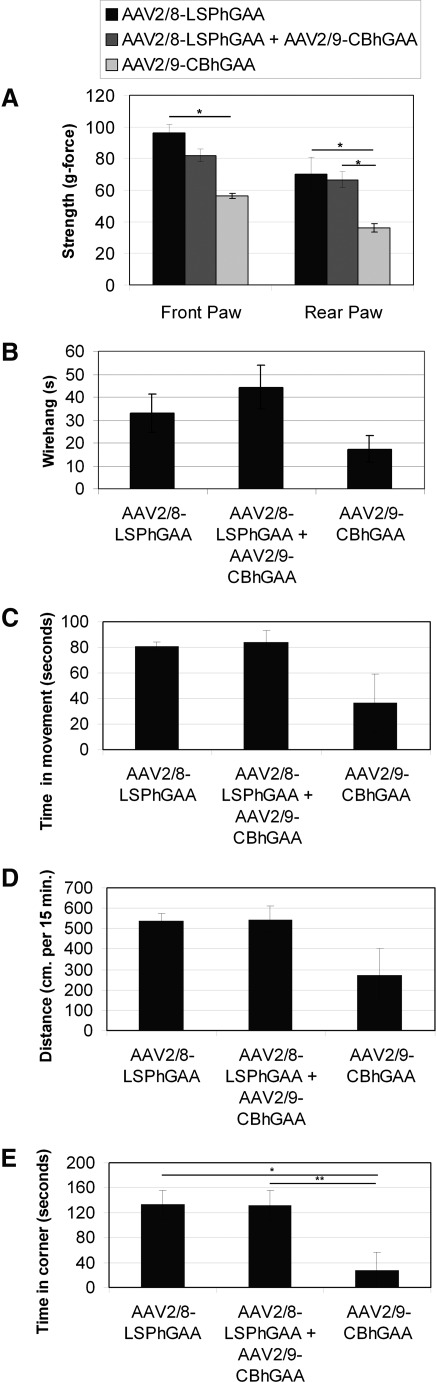
The functional relevance of biochemical correction was demonstrated by motor testing. Functional muscle testing, including grip-strength (Fig. 3A) and wire-hang (Fig. 3B) testing, revealed advantages for the administration of both vectors in comparison with mice treated with AAV2/9-CBhGAA alone. Rear grip strength after dual vector or AAV2/8-LSPhGAA administration was increased in comparison with AAV-CBhGAA alone (p=0.01), and front grip strength was increased after AAV2/8-LSPhGAA administration, in comparison with AAV2/9-CBhGAA alone (p=0.01). Wire-hang latency trended higher in AAV-LSPhGAA-treated mice, in comparison with AAV-CBhGAA alone (p=0.07). Untreated 7-month-old GAA-KO mice performed slightly better than AAV-CBhGAA-treated mice that were 9 months of age when this testing was performed (data not shown). Rotarod latency was significantly increased for all groups of vector-treated GAA-KO mice, in comparison with untreated GAA-KO mice at 6 months of age (data not shown; p=0.01 for each group).
FIG. 3.
Muscle function testing after AAV vector administration. GAA-KO mice were treated with AAV2/8-LSPhGAA (n=4), AAV/2/9-CBhGAA (n=3), or dual vector administration (n=5). Means and standard error are shown. (A) Grip-force at 9 months of age for vector-treated mice. (B) Wire-hang latency at 9 months of age for vector-treated mice. (C) Open field testing of 15-min duration was analyzed at 9 months of age for vector-treated mice. GAA-KO mice were treated with AAV2/8-LSPhGAA (n=4), AAV/2/9-CBhGAA, or dual vector administration (n=5). Shown is time in motion during open field testing. (D) Distance traveled during open field testing. (E) Time in cage corners during open field testing.
Functional muscle testing revealed additional efficacy in the absence of immune responses against GAA. Open field testing revealed that improved mobility correlated with the biochemical correction of striated muscle. Dual vector-treated mice trended toward a higher time in motion (Fig. 3C; p=0.08) and demonstrated increased distance traveled (Fig. 3D; p=0.05), in comparison with mice treated with AAV-CBhGAA. Time in the corner was increased in AAV-LSPhGAA-treated mice (Fig. 3E; p=0.03) and dual vector-treated mice (p=0.02), in comparison with AAV-CBhGAA-treated mice, consistent with the greater activity of mice after biochemical correction. Although the time in the cage corner might be interpreted as a reflection of anxiety in normal mice, most likely it indicated that GAA-KO mice were more active after the induction of immune tolerance to GAA in association with the biochemical correction of striated muscle. Untreated 7-month-old GAA-KO mice performed slightly better than AAV-CBhGAA-treated mice that were 9 months of age when this testing was performed, whereas AAV-LSPhGAA and dual vector-treated mice performed as well as untreated 7-month-old GAA-KO mice (data not shown). The latter data indicated that liver-restricted hGAA expression prevented the progression of Pompe disease. Thus, AAV-CBhGAA-treated mice lost muscle strength between 7 and 9 months of age, whereas AAV-LSPhGAA and dual vector-treated mice had preserved muscle strength.
Liver-specific GAA expression suppresses T cell responses and prevents dendritic cell activation against transgene expression
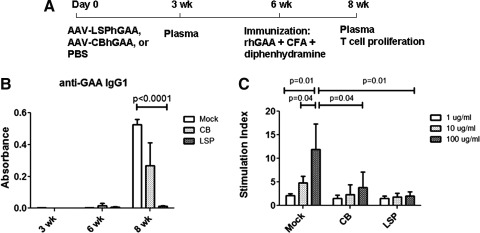
We next investigated the immunologic basis for the difference between immune responses, based on selection of regulatory cassette. Groups of GAA-KO mice were injected intravenously with AAV-LSPhGAA or AAV-CBhGAA, or were uninjected as mock control. Six weeks later, mice were immunized by intraperitoneal injection with rhGAA and complete Freund's adjuvant. GAA-KO mice injected with AAV-CBhGAA formed anti-GAA IgG1 by 2 weeks after immunization, whereas mice injected with AAV-LSPhGAA did not form anti-GAA antibodies. As expected, mock-treated GAA-KO mice demonstrated a vigorous anti-GAA IgG1 response after immunization (Fig. 4A). Mice were killed for tissue analysis 2 weeks after immunization, which corresponded to 8 weeks after vector administration. In accordance with the anti-GAA IgG1 response, T cells from the mock-treated mice showed a vigorous proliferation after in vitro stimulation with rhGAA, whereas T cells from AAV-LSPhGAA-injected mice showed no proliferation to GAA (Fig. 4B). However, T cells from the AAV-CBhGAA-injected mice did not demonstrate higher proliferation, in comparison with the AAV-LSPhGAA-injected mice (Fig. 4C).
FIG. 4.
AAV-LSPhGAA induced systemic tolerance. (A) Groups of GAA-KO mice were injected intravenously with AAV-LSPhGAA or AAV-CBhGAA, or were left untreated as control (four per group). Six weeks later, mice were immunized by intraperitoneal injection with rhGAA and complete Freund's reagent. Two weeks after immunization, spleens were collected for T cell proliferation and FACS. (B) Serum was collected at 3, 6, and 8 weeks for analysis of anti-GAA IgG1. (C) Splenocytes were cultured with rhGAA at 1, 10, or 100 μg/ml for 4 days. [3H]Thymidine was added during the last 5 hr of cell culture.
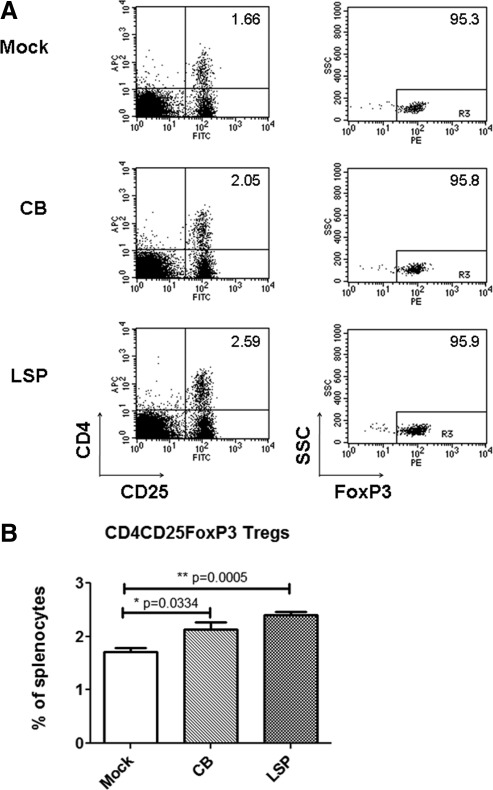
To determine the role of immunosuppressive Tregs in determining immune responses to each vector, we examined by FACS the population of Tregs in the spleens from the above-described experiment (Fig. 5). In the spleens from AAV-LSPhGAA-injected mice CD4+CD25+FoxP3+ Tregs were not significantly elevated among CD4+ splenocytes (Fig. 5A). However, CD4+CD25+FoxP3+ T cells from AAV-LSPhGAA-injected mice were significantly increased among total splenocytes (2.41±0.12%), in comparison with the mock-treated group (1.71±0.16%) (Fig. 5B). AAV-CBhGAA-injected mice also developed increased Tregs, in comparison with the mock-treated control mice (2.14±0.26%). Real-time RT-PCR did not reveal increased FoxP3 expression in the liver or spleen, indicating that any increase in total Tregs was slight (data not shown).
FIG. 5.
Increased FoxP3+ Tregs are associated with AAV-LSPhGAA-induced systemic tolerance. Splenocytes were stained with anti-CD4–FITC, anti-CD25–PE, permeabilized, fixed, and then stained with anti-FoxP3–APC. Representative plots from each group are shown (four per group). (A) Percentage of CD4+CD25+FoxP3+ T cells in CD4+ lymphocytes from spleen. (B) Percentage of CD4+CD25+FoxP3+ T cells among total splenocytes. Means and standard deviation are shown.
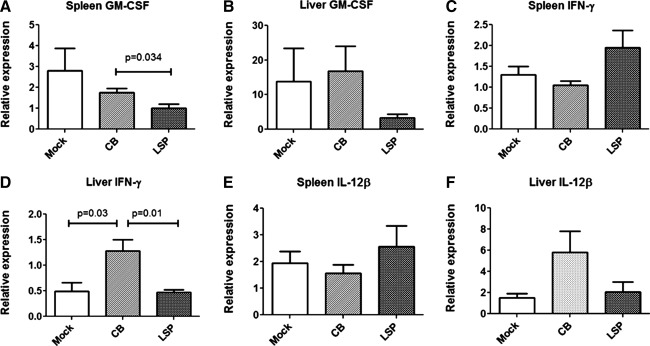
The expression of cytokines in the spleen and liver were analyzed by real-time RT-PCR to better understand the adaptive response to each vector. AAV-CBhGAA stimulated increased GM-CSF in the spleen, in comparison with AAV-LSPhGAA (Fig. 6A). However, GM-CSF was expressed in the liver at similar levels for all three groups (Fig. 6B). Interferon (IFN)-γ was not elevated in the spleen (Fig. 6C), but it was significantly elevated in the liver after AAV-CBhGAA administration, in comparison with AAV-LSPhGAA (Fig. 6D). Furthermore, IL-12β was not elevated in the spleen (Fig. 6E), but it trended higher in the liver after AAV-CBhGAA administration, in comparison with AAV-LSPhGAA (p=0.08; Fig. 6F). IL-1β and FoxP3 were not significantly altered by vector administration in either liver of spleen (data not shown). Thus, AAV-CBhGAA administration was associated with unique increases in cytokine expression in the liver and spleen.
FIG. 6.
Differential activation of inflammatory genes in liver by AAV-LSPhGAA or AAV-CBhGAA. Mice were injected intravenously with AAV-LSPhGAA or AAV-CBhGAA or left uninjected as controls (four per group). At 8 weeks of age, liver and spleen were collected and snap-frozen. Total RNA was extracted for analysis of expression of select cytokine and chemokine genes by real-time PCR. (A) Expression of GM-CSF in spleen. (B) Expression of GM-CSF in liver. (C) Expression of IFN-γ in spleen. (D) Expression of IFN-γ in liver. (E) Expression of IL-12β in spleen. (F) Expression of IL-12β in liver. Means and standard deviation are shown.
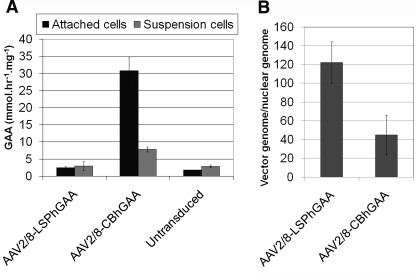
To further investigate how the two AAV2/8 vectors provoked different adaptive immune responses, we evaluated the transduction of bone marrow-derived mature dendritic cells (Fig. 7A). Attached dendritic cells transduced with AAV-LSPhGAA expressed low GAA activity (2.5±0.2 nmol/hr/mg), similar to uninfected cells (1.8±0.1 nmol/hr/mg). In contrast, attached dendritic cells transduced with AAV-CBhGAA expressed significantly higher GAA activity (31±2 nmol/hr/mg) than those infected with AAV-LSPhGAA or naive cells. Suspended dendritic cells transduced with AAV-CBhGAA similarly demonstrated significantly higher GAA activity, in comparison with suspended cells transduced with AAV-LSPhGAA (7.8±0.4 vs. 3.0±0.7 nmol/hr/mg, respectively). Real-time PCR quantification revealed that both AAV vectors transduced dendritic cells efficiently (Fig. 7B).
FIG. 7.
Transduction of dendritic cells with AAV-CBhGAA. (A) Bone marrow-derived dendritic cells from 3-month-old GAA-KO mice were prepared by culturing with GM-CSF and IL-4 for 7 days. FACS analysis showed that 80–90% cells were positive for CD11c, CD80, and MHC class II and less than 10% CD14 positive (see Supplementary Fig. S1; supplementary data are available online at www.liebertonline.com/hum). On day 10, cells were infected with each vector, respectively, and control cells were left untreated. Cells were harvested 72 hr later and GAA activity was measured. The experiment was performed twice, and means±standard error are shown. (B) Real-time quantification of vector genomes in transduced dendritic cells. Means±standard deviation are shown.
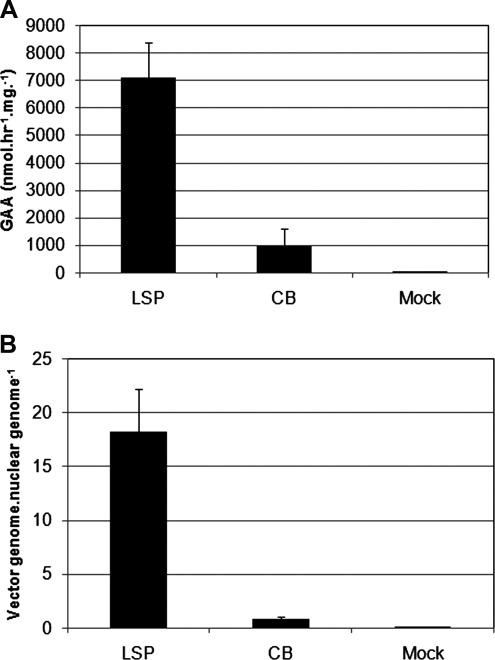
Liver transduction was evaluated 8 weeks after vector administration (2 weeks after immunization with rhGAA), given elevations of IFN-γ that suggested inflammation in response to AAV2/8-CBhGAA (Fig. 6D). GAA activity was 7-fold higher by 8 weeks after AAV-LSPhGAA administration, in comparison with AAV-CBhGAA (Fig. 8A). Vector genome quantification revealed a 22-fold higher number of vector genomes by 8 weeks after AAV-LSPhGAA, in comparison with AAV-CBhGAA (Fig. 8B).
FIG. 8.
Liver transduction and IFN-γ expression 8 weeks after administration of AAV-LSPhGAA or AAV-CBhGAA. (A) Real-time PCR quantification of vector genomes in the liver from groups of GAA-KO mice 8 weeks after administration of AAV-LSPhGAA or AAV-CBhGAA (1×1011 VP/mouse); age-matched, mock-treated GAA-KO mice were negative controls (n=4 per group). Vectors were injected intravenously at 3 months of age. (B) RT-PCR quantification of IFN-γ in the liver of groups of GAA-KO mice detailed previously.
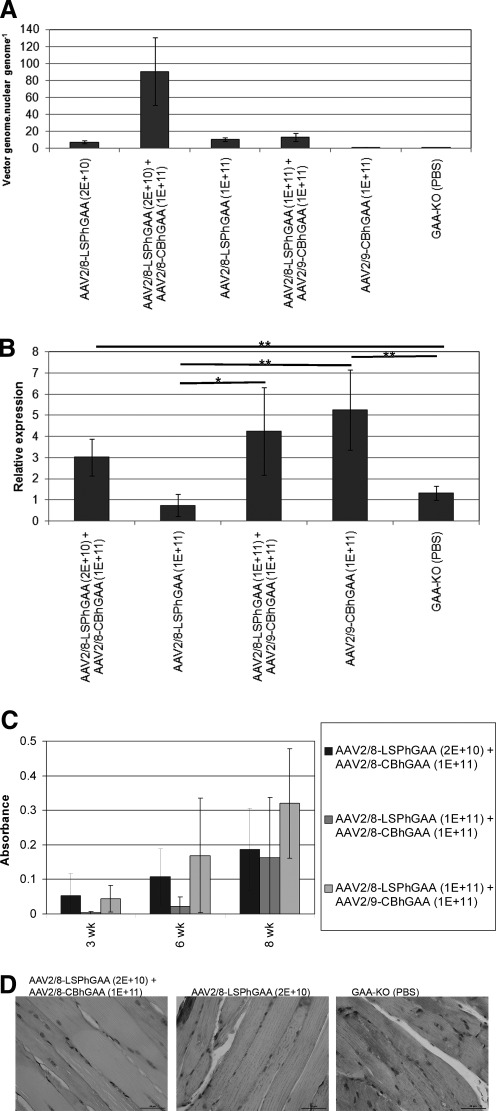
Liver transduction was evaluated 18 weeks after single and dual vector administration to reveal the basis for differences in efficacy between the vector treatments. Vector genome quantification revealed a 13-fold increase in vector genomes in the liver from dual vector-treated mice, in comparison with AAV-LSPhGAA alone (Fig. 9A). Administration of AAV2/9-CBhGAA elevated vector genomes to low levels, in comparison with dual vectors or AAV2/8-LSPhGAA (Fig. 9A). Thus, the lack of biochemical correction from AAV2/9-CBhGAA alone correlated with low vector genomes in the liver.
FIG. 9.
Liver transduction and immune responses after single or dual vector administration. (A) Real-time PCR quantification of vector genomes in the liver from groups of GAA-KO mice after administration of AAV-LSPhGAA (2×1010 VP/mouse; n=5), both AAV-LSPhGAA (2×1010 VP) and AAV-CBhGAA (1×1011 VP; n=4), AAV2/8-LSPhGAA (1×1011 VP/mouse; n=4), both AAV2/8-LSPhGAA and AAV2/9-CBhGAA (n=5), or AAV2/9-CBhGAA (1×1011 VP; n=3). Vectors were injected intravenously into mice at 3 months of age. Mock-treated GAA-KO mice were negative controls (n=4). (B) RT-PCR quantification of IFN-γ in the liver of groups of GAA-KO mice detailed previously. The p values are as follows: *p=0.03; **p<0.03. (C) Groups of GAA-KO mice were injected intravenously with AAV-LSPhGAA and AAV-CBhGAA as indicated before an immune challenge with rhGAA and adjuvant as shown (Fig. 4A; n=5 per group). (D) Periodic acid–Schiff staining of glycogen in the quadriceps (original magnification, ×400).
The difference in transduction was attributed to cellular immune responses associated with the clearance of AAV-CBhGAA vector genomes from the liver (Franco et al., 2005), and elevated IFN-γ has been associated with helper T cell type 1 (Th1) responses in the liver (Mays and Wilson, 2011). Therefore, IFN-γ RNA was quantified in the liver of AAV vector-injected GAA-KO mice 18 weeks after high-dose single or dual vector administration (≥1×1011 VP). AAV2/9-CBhGAA elevated IFN-γ in comparison with either AAV2/8-LSPhGAA or mock treatment (Fig. 9B; p<0.03). AAV2/9-CBhGAA trended higher than the combination of low-dose AAV2/8-LSPhGAA with AAV2/8-CBhGAA (p=0.08). However, dual vector therapy with AAV2/8-LSPhGAA and AAV2/9-CBhGAA also elevated IFN-γ RNA in the liver, in comparison with AAV2/8-LSPhGAA alone (Fig. 9B; p=0.01). Vaccination with rhGAA (Fig. 4A) revealed that dual vector administration failed to completely establish immune tolerance, because antibodies were formed within 2 weeks after an immune challenge with rhGAA plus adjuvant (Fig. 9C). Dual vector therapies were evaluated with the immune challenge of rhGAA and adjuvant 6 weeks after vector administration (either low- or high-dose AAV2/8-LSPhGAA with AAV2/8-CBhGAA, and high-dose AAV2/8-LSPhGAA with AAV2/9-CBhGAA), and none prevented antibody formation at 8 weeks (Fig. 9C). Despite the lack of complete immune tolerance to rhGAA, the combination of AAV2/8-LSPhGAA with AAV2/9-CBhGAA transduced liver with the highest level of vector genomes and achieved the greatest degree of biochemical correction by significantly reducing the glycogen content of both the quadriceps and EDL, in comparison with each of the other four treatment groups (Fig. 1C; p≤0.01 for each comparison). Dual vector administration reduced glycogen storage in the quadriceps, in comparison with AAV2/8-LSPhGAA alone, demonstrating the increased efficacy from AAV2/9-CBhGAA administration (Fig. 9D).
Discussion
The AAV-LSPhGAA vector has achieved greater efficacy than the AAV-CBhGAA vector with respect to persistent GAA expression and clearance of glycogen storage in striated muscle (Franco et al., 2005). Both of the aforementioned vectors initially produced high-level GAA detected in blood; thereafter, GAA expression from AAV-CBhGAA subsequently became undetectable, and vector genomes were eliminated from the liver by 12 weeks after vector administration. The transience of transduction with AAV-CBhGAA was associated with CD8+ T cell responses and antibody formation, whereas AAV-LSPhGAA evaded both types of immune response and stably transduced the liver at high levels (Franco et al., 2005). The immune basis for this difference has been emphasized by the lack of cellular and humoral responses in multiple studies, when AAV-LSPhGAA was administered (Franco et al., 2005; Sun et al., 2007, 2010). Current data confirmed that the induction of immune tolerance by liver-specific expression of GAA with AAV-LSPhGAA occurred even in the presence of simultaneous, ubiquitous expression of GAA with AAV-CBhGAA. These data compellingly demonstrated the dominant effect of immune tolerance mediated by AAV-LSPhGAA, because the administration of both AAV2/8-LSPhGAA and AAV2/8-CBhGAA prevented antibody formation and reduced elevations of IFN-γ in the liver. The immune responses directed toward AAV2/8-CBhGAA have been well described (Franco et al., 2005; Sun et al., 2005a,b), and current data confirmed the activation of T cells, transduction of dendritic cells, and stimulation of Th1 cytokine responses by administration of that vector to GAA-KO mice. However, dual vector administration failed to prevent antibody formation in response to vaccinating with rhGAA plus adjuvant, despite preventing antibody formation in the absence of a rigorous immune challenge. Thus, hepatic expression of human GAA with low-dose AAV2/8-LSPhGAA established functional immune tolerance over the humoral and cellular immune responses provoked by ubiquitous GAA expression with AAV2/8-CBhGAA, while achieving the highest degree of biochemical correction in skeletal muscle among the gene therapy regimens currently evaluated.
Administration of low-dose AAV2/8-LSPhGAA along with AAV2/8-CBhGAA significantly increased efficacy, whereas AAV2/8-CBhGAA was previously inefficacious in immunocompetent GAA-KO mice (Franco et al., 2005). Administration of both AAV2/8 vectors significantly enhanced the biochemical correction of striated muscle in GAA-KO mice, as demonstrated by increased GAA activity and decreased glycogen content, in comparison with AAV2/8-LSPhGAA alone (Fig. 1). The combination of high vector particle numbers of AAV2/8-LSPhGAA and AAV2/9-CBhGAA, in a dual vector treatment, revealed few significant differences from high-dose AAV2/8-LSPhGAA alone; however, that dual vector treatment simultaneously increased GAA activity in both the liver and heart to high levels (Fig. 1A). Each vector by itself elevated GAA highly only in the heart or liver, not in both tissues. The glycogen content in EDL was significantly reduced by dual vector treatment, in comparison with administration of AAV2/8-LSPhGAA alone (Fig. 1C). AAV2/9-CBhGAA and the dual vector treatment both elevated GAA content to high levels (Fig. 1A), but glycogen content was reduced only by dual vector treatment with AAV2/8 and AAV2/9 (Fig. 1C). We surmise that cellular immune responses interfered with the function of GAA in the heart, preventing the clearance of glycogen storage from AAV2/9-CBhGAA alone despite the high levels of GAA achieved. The lack of efficacy from an AAV vector containing a ubiquitously active regulatory cassette concurs with experiments by Ziegler and colleagues, which demonstrated little efficacy from ubiquitous expression of α-galactosidase in Fabry disease mice (Ziegler et al., 2007). Perhaps most significantly, when the immunogenic AAV2/8-CBhGAA vector was coadministered with a small number of tolerogenic AAV2/8-LSPhGAA vector particles it achieved a degree of biochemical correction equivalent to that from a high dose of the tolerogenic AAV2/8 vector. The reduction of glycogen in the quadriceps from the current dual AAV2/8 vector treatment was approximately 70%, equivalent to the effect from an equivalent number of tolerogenic vector particles, whereas AAV2/8-CBhGAA by itself had no impact on the glycogen content of skeletal muscle (Franco et al., 2005; Ziegler et al., 2008).
The coadministration of a tolerogenic vector, AAV-LSPhGAA, along with an immunogenic vector, AAV-CBhGAA, was sufficient to prevent antibody responses against GAA and to promote efficacy in GAA-KO mice. In the absence of anti-GAA antibodies it is likely that GAA is secreted from transduced liver and muscle cells, and taken up by cardiomyocytes from the blood via mannose 6-phosphate receptors. The mechanism for inducing immune tolerance to GAA in mice with Pompe disease involves Treg cells, because Treg cells can be depleted to prevent the establishment of immune tolerance to GAA in GAA-KO mice with the tolerogenic vector (Sun et al., 2010). The adoptive transfer of Treg cells to naive recipient mice after administration of an AAV vector to donor mice has prevented antibody formation in response to an immune challenge with foreign proteins in hemophilia B mice (Cao et al., 2007) and Fabry mice (Ziegler et al., 2007), thereby confirming the role of Treg cells in mediating immune tolerance to an introduced therapeutic protein. A previous experiment demonstrated that immune tolerance to sphingomyelinase could be established by hepatic transgene expression in mice with Niemann-Pick disease, followed by transduction of the central nervous system with a second vector delivering a ubiquitously active transgene (Passini et al., 2007). The highly efficacious combination of these dual vectors foreshadowed the benefits observed in the current study of liver-specific and ubiquitous transgene expression in GAA-KO mice. Whether AAV vectors confer unique advantages in their ability to induce immune tolerance through hepatic expression remains unclear; however, it has been suggested that the nonpathogenic nature of AAV facilitates the tolerogenic capabilities of AAV vectors (Loduca et al., 2009). Intriguingly, we found that administration of both AAV vectors studied here did not increase Treg cells in the spleen, in comparison with mock treatment, as analyzed by quantifying Tregs among CD4+ splenocytes or by RT-PCR quantification of FoxP3 in the spleen. This observation does not exclude the previously demonstrated role of CD25+ Tregs specific for GAA in inducing immune tolerance (Sun et al., 2010).
The high-level expression of GAA in dendritic cells transduced with AAV-CBhGAA was associated with a lack of efficacy in GAA-KO mice after administration of this vector; moreover, ubiquitous GAA expression with AAV-CBhGAA has provoked cellular and humoral immune responses in this model previously and in the current study (Franco et al., 2005; Sun et al., 2005b). AAV-LSPhGAA vector was transcriptionally silent in dendritic cells, because vector DNA was present in dendritic cells that failed to express GAA (Fig. 7). These data were consistent with the hepatic-specific pattern of expression of the LSP regulatory cassette (Wang et al., 1999, 2000; Franco et al., 2005). The transduction of dendritic cells with DNA vectors has been implicated as a mechanism for the activation of cytotoxic T lymphocyte (CTL) responses in rodent models and for the attenuation of transgene expression (Jooss et al., 1998; Zhang et al., 2000). Adoptive transfer of dendritic cells transduced with an adenovirus vector encoding β-galactosidase provoked robust T cell activation and elimination of transgene expression, in contrast with adoptive transfer of dendritic cells transduced with an AAV vector containing a similar transgene (Jooss et al., 1998). The lack of transgene-directed responses after transfer of AAV-transduced dendritic cells and lack of β-galactosidase staining in those cells supported the view that AAV vectors failed to transduce and activate dendritic cells. Subsequent experiments revealed that immature dendritic cells were transducible and capable of provoking CTL responses after adoptive transfer, whereas mature dendritic cells lacked those capabilities (Zhang et al., 2000) More recently, canine dendritic cells were transduced with an AAV2/8 vector in vivo (Ohshima et al., 2009), indicating that other serotypes than AAV2 could potentially activate dendritic cells with accompanying risk for CTL responses directed against the transgene.
The simultaneous administration of tolerogenic and immunogenic vectors induced immune tolerance in the current study, which is novel and somewhat unexpected. Previous examples of liver-specific transgene expression that induced immune tolerance have introduced the vector carrying the hepatic-specific transgene several weeks before the vector containing a ubiquitously expressing transgene (Hoffman et al., 2007; Passini et al., 2007; Breous et al., 2009). We have demonstrated that ERT can begin simultaneously or even 3 weeks before tolerogenic vector administration, but this might be understandable if the injection of recombinant protein does not represent an effective immune challenge (Sun et al., 2010). However, we currently administered an immunogenic vector that induced vigorous antibody and CTL responses (AAV-CBhGAA), simultaneously with the tolerogenic vector (AAV-LSPhGAA), without abrogating immune tolerance to GAA. We further demonstrated that systemic immune tolerance induced by AAV-LSPhGAA could suppress activation of the immune system on subsequent active immunization. We showed that mice receiving AAV-LSPhGAA failed to form anti-GAA IgG1 and lacked T cell proliferation on immunization with rhGAA (Fig. 4B and C). In addition, these mice had high expression of liver GAA and high vector genomes in liver (Fig. 9). In contrast, administration of AAV-CBhGAA failed to suppress anti-GAA IgG1 responses and T cell proliferation after rhGAA immunization (Fig. 4B and C). More importantly, the AAV-CBhGAA-injected mice showed much lower liver GAA and liver vector genomes after rhGAA immunization (Fig. 9), indicating the lack of ability to suppress immune activation after immunization. The lower cytokine expression in spleen and liver after immunization correlated with the immunosuppressive character of the AAV vector containing the liver-specific promoter. GM-CSF is an inflammatory cytokine and has been shown to be transiently increased in mouse livers injected with AAV2 (Zaiss et al., 2002). Here we show that increased splenic GM-CSF was linked to an anti-GAA IgG1 antibody response after rhGAA immunization in the GAA-KO mouse model, and that AAV-LSPhGAA suppressed immunization-induced increases in splenic GM-CSF expression, in comparison with AAV-CBhGAA (Fig. 6A). Liver IFN-γ expression was differentially expressed after administration of AAV-CBhGAA or AAV-LSPhGAA, and subsequent immunization. Previously we demonstrated CD8+ lymphocyte infiltrates induced by AAV-CBhGAA (Franco et al., 2005). In the current study AAV-CBhGAA induced significantly increased liver IFN-γ (Fig. 6D), which most probably is related to CTLs in response to ubiquitous GAA expression in GAA-KO mice.
A potential advantage for immunomodulatory gene therapy is that relatively few immunogenic vector particles will suppress immune responses to another vector expressing the therapeutic protein in many tissues, thereby maximizing the efficacy from the lowest effective vector dose. For instance, antibody formation in response to the administration of a ubiquitously expressing, immunogenic vector has decreased efficacy in Pompe disease mice, but administration of a ubiquitously expressing vector is desirable if skeletal muscle and nervous system involvement is to be treated effectively. The relevance of antibody formation to efficacy of therapy in Pompe disease has been emphasized by the poor response of CRIM-negative patients to ERT, which correlated with the onset of high-titer antibodies (Kishnani et al., 2010). The safety of immunomodulatory gene therapy is enhanced by the low number of vector particles required to prevent antibody formation and thereby enhance efficacy, because any risk from AAV vectors should be minimized by reducing the vector dose (Manno et al., 2006; Sun et al., 2010). Furthermore, it is desirable to minimize the vector dose to prevent anti-capsid T cell responses that were encountered in a previous clinical trial of AAV2 vector-mediated gene therapy in hemophilia B (Manno et al., 2006). The clinical relevance of transgene-directed immune responses has been emphasized in a clinical trial in subjects with Duchenne muscular dystrophy, although CTLs in that trial appeared to be related to previous T cell activation that might be resistant to immunomodulatory gene therapy (Mendell et al., 2010).
At present we have evaluated two examples of dual vector therapy by administering a tolerogenic vector and an immunogenic vector. The combination of two AAV2/8 vectors was most efficacious, possibly because of the high degree of liver transduction achieved with AAV2/8 vectors. No significant advantage for combining AAV2/8- and AAV2/9-pseudotyped vectors was demonstrated, in comparison with a high-dose tolerogenic vector by itself, and administering two serotypes risks provoking anti-capsid antibodies against both serotypes AAV8 and AAV9 that would prevent treatment later with either serotype (Gao et al., 2002). The benefit of immune tolerance was reflected by improved muscle function, when either AAV2/8 and AAV2/9 vectors or the tolerogenic AAV2/8 vectors were administered, in comparison with the immunogenic AAV2/9 vector alone (Fig. 3). However, the lack of increased muscle performance by dual AAV2/8 and AAV2/9 vector administration, in comparison with the AAV2/8 vector alone, raises an additional question about that strategy. Overall, the combination of low-dose AAV2/8-LSPhGAA and high-dose AAV2/8-CBhGAA equals the benefit of previous vector treatments in adult GAA-KO mice (Franco et al. 2005; Ziegler et al., 2008), and this combination might have undetected advantages from the transduction of nonmuscle tissue by the ubiquitously expressing CBhGAA cassette. The further development of immunomodulatory gene therapy through liver-specific expression of GAA in Pompe disease can be justified by the improved efficacy of dual vector treatment, potentially achieving higher efficacy than with either vector alone.
Supplementary Material
Acknowledgments
D.D.K. was supported by NIH grants R01 HL081122 from the National Heart, Lung, and Blood Institute and 1R01HD054795 from the National Institute of Child Health and Human Development. B.S. was supported by a Development Grant from the Muscular Dystrophy Association. GAA-KO mice were provided courtesy of Dr. Nina Raben at the National Institutes of Health (Bethesda, MD). The AAV8 and AAV9 packaging plasmids were provided courtesy of Dr. James M. Wilson at the University of Pennsylvania (Philadelphia, PA). The authors thank Caroline J. Kim for technical assistance in behavioral testing of the mice.
Author Disclosure Statement
No competing financial interests exist.
References
- Allen K.D. Griffin T.M. Rodriguiz R.M., et al. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis Rheum. 2009;60:2684–2693. doi: 10.1002/art.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalfitano A. McVie-Wylie A.J. Hu H., et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-α-glucosidase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:8861–8866. doi: 10.1073/pnas.96.16.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalfitano A. Bengur A.R. Morse R.P., et al. Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease type II: Results of a phase I/II clinical trial. Genet. Med. 2001;3:132–138. doi: 10.109700125817-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Breous E. Somanathan S. Vandenberghe L.H. Wilson J.M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. doi: 10.1002/hep.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O. Dobrzynski E. Wang L., et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding E. Hu H. Hodges B.L., et al. Efficacy of gene therapy for a prototypical lysosomal storage disease (GSD-II) is critically dependent on vector dose, transgene promoter, and the tissues targeted for vector transduction. Mol. Ther. 2002;5:436–446. doi: 10.1006/mthe.2002.0563. [DOI] [PubMed] [Google Scholar]
- Ding E.Y. Hodges B.L. Hu H., et al. Long-term efficacy after [E1–, polymerase–] adenovirus-mediated transfer of human acid-α-glucosidase gene into glycogen storage disease type II knockout mice. Hum. Gene Ther. 2001;12:955–965. doi: 10.1089/104303401750195917. [DOI] [PubMed] [Google Scholar]
- Duque S. Joussemet B. Riviere C., et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L.M. Sun B. Yang X., et al. Evasion of immune responses to introduced human acid α-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol. Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R. Reuser A.J.J. Glycogen storage disease type II: Acid α-glucosidase (acid maltase) deficiency. In: Scriver C.R., editor; Beaudet A.L., editor; Sly W.S., editor; Valle D., editor. The Metabolic and Molecular Basis for Inherited Disease. McGraw-Hill; New York: 2001. pp. 3389–3419. [Google Scholar]
- Hoffman B.E. Dobrzynski E. Wang L., et al. Muscle as a target for supplementary factor IX gene transfer. Hum. Gene Ther. 2007;18:603–613. doi: 10.1089/hum.2007.042. [DOI] [PubMed] [Google Scholar]
- Hunley T.E. Corzo D. Dudek M., et al. Nephrotic syndrome complicating α-glucosidase replacement therapy for Pompe disease. Pediatrics. 2004;114:e532–e535. doi: 10.1542/peds.2003-0988-L. [DOI] [PubMed] [Google Scholar]
- Jooss K. Yang Y. Fisher K.J. Wilson J.M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani P.S. Hwu W.L. Mandel H., et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Kishnani P.S. Goldenberg P.C. Dearmey S.L., et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol. Genet. Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberl D.D. Kishnani P.S. Immunomodulatory gene therapy in lysosomal storage disorders. Curr. Gene Ther. 2009;9:503–510. doi: 10.2174/156652309790031094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loduca P.A. Hoffman B.E. Herzog R.W. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr. Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Martiniuk F. Mehler M. Tzall S., et al. Sequence of the cDNA and 5′-flanking region for human acid α-glucosidase, detection of an intron in the 5′ untranslated leader sequence, definition of 18-bp polymorphisms, and differences with previous cDNA and amino acid sequences. DNA Cell Biol. 1990;9:85–94. doi: 10.1089/dna.1990.9.85. [DOI] [PubMed] [Google Scholar]
- Mays L.E. Wilson J.M. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol. Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.R. Campbell K. Rodino-Klapac L., et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F. Liu Y.L. Dobrzynski E., et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima S. Shin J.H. Yuasa K., et al. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol. Ther. 2009;17:73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini M.A. Bu J. Fidler J.A., et al. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc. Natl. Acad. Sci. U.S.A. 2007;104:9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce E. Witte D.P. Hirschhorn R., et al. Murine acid α-glucosidase: cell-specific mRNA differential expression during development and maturation. Am. J. Pathol. 1999;154:1089–1096. doi: 10.1016/s0002-9440(10)65361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raben N. Danon M. Gilbert A.L., et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol. Genet. Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Ribar T.J. Rodriguiz R.M. Khiroug L., et al. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J. Neurosci. 2000;20:RC107. doi: 10.1523/JNEUROSCI.20-22-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B. Zhang H. Franco L.M., et al. Correction of glycogen storage disease type II by an adeno-associated virus vector containing a muscle-specific promoter. Mol. Ther. 2005a;11:889–898. doi: 10.1016/j.ymthe.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Sun B. Zhang H. Franco L.M., et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol. Ther. 2005b;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Sun B. Bird A. Young S.P., et al. Enhanced response to enzyme replacement therapy in Pompe disease after the induction of immune tolerance. Am. J. Hum. Genet. 2007;81:1042–1049. doi: 10.1086/522236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B. Kulis M.D. Young S.P., et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine Pompe disease. Mol. Ther. 2010;18:353–360. doi: 10.1038/mt.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Takabe K. Bidlingmaier S.M., et al. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Nichols T.C. Read M.S., et al. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol. Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- Zaiss A.K. Liu Q. Bowen G.P., et al. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Chirmule N. Gao G. Wilson J. CD40 ligand-dependent activation of cytotoxic T lymphocytes by adeno-associated virus vectors in vivo: Role of immature dendritic cells. J. Virol. 2000;74:8003–8010. doi: 10.1128/jvi.74.17.8003-8010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler R.J. Cherry M. Barbon C.M., et al. Correction of the biochemical and functional deficits in Fabry mice following AAV8-mediated hepatic expression of α-galactosidase A. Mol. Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]
- Ziegler R.J. Bercury S.D. Fidler J., et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid α-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum. Gene Ther. 2008;19:609–621. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.