Abstract
Stem cell therapy for tissue repair is a rapidly evolving field and the factors that dictate the physiological responsiveness of stem cells remain under intense investigation. In this study we hypothesized that the mechanical loading history of muscle-derived stem cells (MDSCs) would significantly impact MDSC survival, host tissue angiogenesis, and myocardial function after MDSC transplantation into acutely infarcted myocardium. Mice with acute myocardial infarction by permanent left coronary artery ligation were injected with either nonstimulated (NS) or mechanically stimulated (MS) MDSCs. Mechanical stimulation consisted of stretching the cells with equibiaxial stretch with a magnitude of 10% and frequency of 0.5 Hz. MS cell-transplanted hearts showed improved cardiac contractility, increased numbers of host CD31+ cells, and decreased fibrosis, in the peri-infarct region, compared to the hearts treated with NS MDSCs. MS MDSCs displayed higher vascular endothelial growth factor expression than NS cells in vitro. These findings highlight an important role for cyclic mechanical loading preconditioning of donor MDSCs in optimizing MDSC transplantation for myocardial repair.
Introduction
Stem cell transplantation is emerging as a promising therapy for the regeneration of numerous tissues, including heart disease, muscular dystrophy, diabetes, hemophilia, multiple sclerosis, and Parkinson's disease.1–3 Cardiac disease, particularly myocardial infarction, is a leading cause of mortality and morbidity worldwide, and the current standard practice cannot reverse the progressive deterioration in cardiac function that typically occurs after injury or disease.4 Cell therapy for cardiac repair is a rapidly expanding area of research, with a wide variety of cell types under investigation.2,5–7 Progenitor and stem cells can be derived from most vascularized tissues in the body, and skeletal muscle-derived cells have shown particular promise for muscle repair due to their autologous origin, in vitro scalability, and high resistance to stress. We have previously isolated a population of early myogenic progenitors called muscle-derived stem cells (MDSCs) based on their selective adherence to collagen-coated flasks.8,9 MDSCs are multipotent; have been shown to differentiate along skeletal, cardiac and smooth muscle, bone, cartilage, tendon, nerve, endothelial, and hematopoietic lineages; and display a high regenerative potential after transplantation.9–13 MDSCs have been shown to regenerate cardiac and skeletal muscle in a more effective manner than myoblasts.13,14
While stem cell transplantation has produced promising initial results, many of the stem cell-mediated improvements in cardiac function are only transient. One main barrier to successful cardiac cell therapy is low cell survival due to initial cell death postimplantation, subsequent biological cell death,15 and immune-mediated cell destruction.16,17 The post-transplantation in vivo cellular response is influenced by many factors in the cellular microenvironment, including the extracellular matrix, growth factors, cytokines, and mechanical loading. For example, improved cardiac repair after myocardial infarction has been shown to occur when vascular endothelial growth factor (VEGF) secretion is increased.18 The activation of signaling pathways by pharmacologically preconditioning skeletal myoblasts promotes their survival by inducing the release of paracrine factors that stimulate angiogenesis in the infarcted heart.19
Mechanical and structural cues have been shown to play a critical role in tissue physiology, including cell growth, migration, gene expression, and cell signaling,20,21 and this may play a role in cell transplantation since most cells and tissues experience biomechanical forces within the body.20 Indeed, it has been recently shown that culturing muscle-derived cells on soft hydrogel substrates that mimic the elasticity of muscle, self-renew in vitro and regenerate skeletal muscle in a more effective manner than muscle-derived cells grown on rigid plastic dishes.22 However, no consensus has been reached as to the tissue-specific roles that these mechanical forces play in healing and regeneration. Evidence is emerging that mechanical preconditioning can impact postimplanted stem cell survival, proliferation, and participation in tissue regeneration.23–26 For example, skeletal muscle is known to respond to mechanical stimulation by regulating the production of structural proteins, including actin and myosin.27 Mechanical stretch also plays an important role in the proliferation of mesenchymal stem cells,28 and a recent report indicates that the application of cyclic stretch can induce the proliferation of satellite cells and inhibit their differentiation into myotubes.29 The effect of mechanical preconditioning on biochemical and structural responses suggests that the mechanical loading history experienced by cells in vitro or in vivo may affect cellular behavior to a greater extent than previously thought.
Our current study investigates the impact of MDSC loading history on the regenerative capacity of MDSCs for cardiac muscle. In a mouse model of acute myocardial infarction, mechanically stimulated (MS) MDSCs attenuated the decline of cardiac function to a greater extent than nonstimulated (NS) MDSCs or phosphate-buffered saline (PBS) alone. We investigated the effects of mechanically preconditioning cells in vitro to ascertain changes in cell behavior. We analyzed paracrine factor secretion, resistance to oxidative stress, antioxidant capacity, proliferation, and differentiation toward skeletal muscle. Through these studies, we confirm that mechanical preconditioning can influence the paracrine effect of MDSCs (angiogenic factor secretion), reduce scar tissue, and consequently improve cardiac recovery after stem cell transplantation.
Materials and Methods
MDSC isolation
MDSCs were isolated from the skeletal muscle of 3-week-old normal C57BL/10J mice (Jackson Laboratories) using the modified preplate technique as previously described.8 MDSCs were cultured in a proliferation medium (PM) containing Dulbecco's modified Eagle's medium (DMEM; Invitrogen), 10% fetal bovine serum (Invitrogen), 10% horse serum (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 0.5% chick embryo extract (Accurate Chemical).
Mechanical stimulation
MDSCs were cultured on bioflex plates, which are flexible bottomed six-well culture plates coated with collagen I (100,000 cells/well; Flexcell Intl. Corp.). After 12 h of culture, an FX-4000T strain unit was used to subject the cells to 10% equibiaxial strain with a 0.5-Hz sine wave for the designated time period as previously described.18 Control MDSCs were cultured on bioflex plates as described without strain. The mechanical stimulation conditions were chosen based on previous studies that indicated increased VEGF secretion under these conditions in vitro.18,30,31
In vitro studies
In vitro MDSC proliferation activity
After exposure to mechanical strain, cells were replated on 24-well collagen-coated plates and then placed onto a previously described32 live cell imaging system (Automated Cell, Inc.), and fluorescent and brightfield images were taken every 10 min in three locations per well. These images were analyzed using ImageViewer software (ACI) and the number of cells was counted at 12-h intervals to track proliferation rates.
Cell survival under oxidative stress
Preconditioned cells were plated in PM at a density of 1000 cells/well in a 24-well collagen-coated plate and then assessed for cell survival as previously described.32 Briefly, 24 h after plating the medium was switched to PM or PM containing 400 μM hydrogen peroxide and propidium iodide (PI, 1 μL/500 μL medium; Sigma). The plates were then placed onto a live cell imaging system (Automated Cell, Inc.) and fluorescent and brightfield images were taken every 10 min in three locations per well. These images were analyzed using ImageViewer software (Automated Cell, Inc.). Cell survival (n=21) was determined by counting the number of PI-positive cells in the fluorescent images and the number of cells present in the brightfield images at the 12-h time point.
In vitro myogenic differentiation
Differentiation toward skeletal muscle was assessed as previously described.33 Cells were plated on 24-well plates after mechanical stimulation, and 24 h later the medium was changed to DMEM containing 2% serum, which induces myotube differentiation. At 10 days, the plates were stained with a mouse primary antibody for anti-fast skeletal myosin heavy chain (fsMHC) (1:500; Sigma), Alexafluor 488 donkey anti-mouse secondary antibody (1:400; Molecular Probes), and 4′,6-diamidino-2-phenylindole (DAPI, for nuclei). We then determined the fusion index of the cells, which is an indicator of myogenic differentiation,34 by calculating the percentage of nuclei in the fused myotubes (n=15) in three images per well in three different wells per treatment group (fusion index=number of nuclei in fused myotubes/total number of nuclei).
In vitro VEGF expression
Cells were MS as described above in DMEM with 1% penicillin/streptomyocin for 24 h (MS). Cells were stimulated for 24 h, allowed to rest for 24 h (MS rest), and then stimulated again (MS rest MS). The conditioned medium was collected for each sample and flash frozen. VEGF secretion was quantified by an enzyme-linked immunosorbant assay (ELISA) for mouse VEGF according to the manufacturer's instructions (R&D Systems) and normalized to cell number.
Animal studies
The use of animals and the surgical procedures performed in this study were approved by the Institutional Animal Care and Use Committee of the Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center (protocol 14/06).
Cell transplantation into infarcted myocardium
Fifty adult male immunodeficient C57BL/6J-Prkdcscid mice (12 weeks old) were obtained from Jackson Laboratories and bred for the current study at the institution's animal facility. An acute infarction model was used to assess the efficacy of mechanical stimulation on cardiac transplantation of the MDSCs. The mice underwent permanent left anterior descending coronary artery ligation using a 7-0 prolene suture to create a myocardial infarction as previously described.13,35,36 Infarcted mice were randomly allocated between the treatment groups (PBS, nonstretch MDSCs [NS], or 24-h stretch MDSCs [MS]). Five minutes after permanent ligation of the artery, 3×105 cells in 30 μL of PBS (three injections of 10 μL each) were injected into the anterior and lateral aspects of the left ventricular free wall bordering the infarct zone, and into the center of the infarct. Male severe combine immunodeficient (SCID) mice 13–16 weeks of age were used following a protocol previously described.13,18
Echocardiography
Two, 6, and 12 weeks after cell injection, echocardiography was performed by a blinded investigator on the left ventricular short axis view at the mid-papillary muscle level as described previously18 to assess cardiac function. Left ventricular end-diastolic area and end-systolic area were measured from short axis images of the left ventricle, and end-diastolic dimensions and end-systolic dimensions were measured from M-mode tracing. After echocardiography the mice were euthanized and the hearts flash-frozen in 2-methylbutane and cryosectioned.13,18
Identification of CD31+ cells within the injected myocardium
The number of CD31+ cells in the engraftment area was determined by double-staining tissue sections with rat anti-CD31 primary antibody (1:300; Sigma) and Alexafluor 555 donkey anti-rat secondary antibody (1:300; Molecular Probes), and mouse anti-fsMHC antibody (1:400; Sigma) and Alexafluor 488 goat anti-mouse (1:200; Sigma). CD31+ vessel formation within the cell-injected areas was determined by counting the number of CD31(+) cells associated with fsMHC(+) cells within the infarct area. fsMHC-positive cells represent donor MDSCs in the cardiac tissue.36 Blood vessel formation in the peri-infarct area was determined by staining with rat anti-CD31 primary antibody (1:300; Sigma) and Alexafluor 555 donkey anti-rat secondary antibody (1:300; Molecular Probes). The number of CD31+ cells in three high-powered images in the peri-infarct area was quantified in each heart.
Identification and quantification of transplanted MDSCs in infarcted myocardium
Cryosections were fixed in 4% paraformaldehyde and then stained to determine donor cell engraftment. Heart sections were stained with mouse anti-fsMHC antibody (1:400; Sigma) and Alexafluor 555 donkey anti-mouse secondary antibody (1:300; Molecular Probes) to examine engraftment of the different cell populations. The number of engrafted cells was determined by assessing the area of fsMHC(+) cells normalized to the area of the entire muscle section.34
Staining for cardiac-specific markers in vivo
Sections from the infarcted samples were stained with mouse anti-fsMHC (1:400; Sigma) and Alexafluor 488 donkey anti-mouse secondary antibody (1:300; Molecular Probes), and goat anti-cardiac troponin I (cTnI, 1:25,000; Scripps) with rabbit anti-goat Alexafluor 555 (1:200; Molecular Probes). The number of double-positive cells was counted in three high powered fields per heart.
Scar tissue area fraction
Infarcted heart sections were fixed with 1% gluteraldehyde for 2 min and stained with the Masson Modified IMEB Trichrome Stain Kit (IMEB). Trichrome staining was performed according to the manufacturer's guidelines as previously described.13,18 The sections were assessed for the percentage area of collagen-positive area normalized to total muscle area within the sections using ImageJ software (NIH).
Microscopy
Fluorescence and brightfield microscopy were performed using either a Nikon Eclipse E800 microscope (Nikon Corp.) or a Leica inverted microscope (Leica Microsystems) with a Retiga EXi digital camera (Q Imaging) and Northern Eclipse software (version 6.0; Empix Imaging, Inc.). Image analysis was performed using Northern Eclipse software.
Statistical analysis
Data are expressed as mean±standard error of the mean. Student's t-test was used to compare the in vitro proliferation and cell survival and CD31 expression in the infarction area (α=0.05; Excel). Echocardiography data were analyzed by using two-way-repeated measure analysis of variance (ANOVA). All other statistical analysis was determined by one-way ANOVA. In the event of a significant ANOVA, the Tukey test was used for post hoc analysis (SPSS v. 16.0; SPSS, Inc.).
Results
In vitro proliferation of MDSCs is increased with mechanical stretch, but differentiation and resistance to oxidative stress is not affected
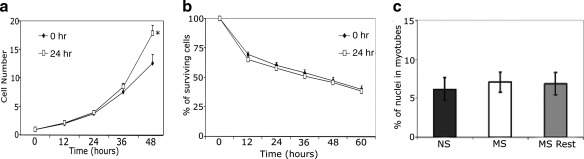
The proliferation rate of MDSCs after mechanical stimulation was initially similar to the proliferation rate of NS cells, but by 48 h, the MS-MDSCs displayed a higher proliferation rate (p=0.04, Fig. 1a). The post-transplantation microenvironment is known to be hostile to transplanted cells with oxidative stress playing a significant role in that process. We therefore evaluated the effect of MS on the MDSCs response to oxidative stress. Mechanically preconditioned cells (n=21) showed similar levels of survival under oxidative stress versus NS controls (n=21, 12 h: p=0.48, 24 h: p=0.68, 24 h: p=0.62, 36 h: p=0.72, 48 h: p=0.71, 60 h: p=0.52, Fig. 1b). Myotube formation, an index of myogenic differentiation capacity, was also unaffected by mechanical stimulation (n=15 per treatment, p=0.89 NS to MS, p=0.94 NS to MS rest, p=0.99 MS to MS rest, Fig. 1c).
FIG. 1.
In vitro mechanical stimulation did not affect differentiation or survival, but increased proliferation. (a) Mechanically stimulated (MS) cells (open square, 24 h stimulation) had significantly higher proliferation than nonstimulated (NS) (closed diamond, 0 h stimulation) at 48 h post-treatment (*p<0.05). (b). Cell survival was similar between the groups (MS cells [open square, 24 h stimulation], and NS [closed diamond, 0 h stimulation]) exposed to oxidative stress. (c) There was no difference in the differentiation capacity after 10 days between the groups (NS, MS, MS rest [24 h of stimulation then 24 h of rest]).
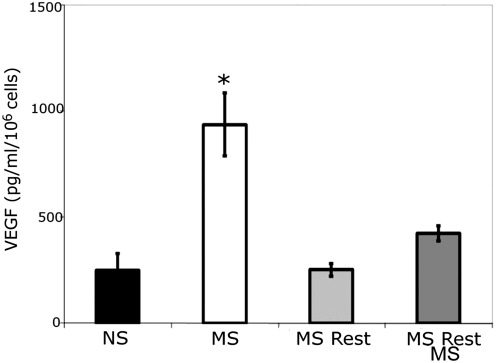
Mechanical stimulation increased the in vitro expression of VEGF by the MDSCs
Previous results have shown that MDSCs exposed to MS resulted in increased VEGF secretion.18 We consequently examined the effect of MS on the time course of VEGF secretion and confirmed that in vitro mechanical stimulation increased VEGF secretion (MS [n=6]: 937±148 pg/mL; p=0.01 MS to NS; NS [n=6]: 248±80 pg/mL; Fig. 2); however, if mechanical stimulation was followed by 24 h of static culture (MS rest), secreted VEGF levels returned to the level of the NS cells (n=6, 254±30 pg/mL, p=0.91 NS to MS rest). We then re-stimulated the cells for 24 h and found that cells re-exposed to MS (MS rest MS) showed an increase in VEGF secretion (n=6, 426±36 pg/mL, p=0.99 NS to MS rest MS), but this increase was much lower than the initial MS levels. These results provide evidence that the effect of mechanical stimulation on MDSCs is time dependent in vitro, as VEGF secretion is shown to be dependent on mechanical loading history.
FIG. 2.
In vitro mechanical stimulation increases vascular endothelial growth factor (VEGF) expression. VEGF was increased after 24 h of mechanical stimulation (MS), *p<0.05. The VEGF overexpression was abolished when the cells were removed from mechanical stimulation for 24 h (MS rest) and slightly increased after a second MS period (MS rest MS).
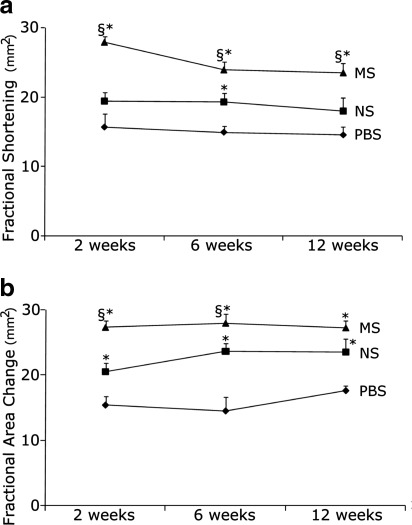
Mechanical preconditioning preserved cardiac function after myocardial infarction
To quantify the efficacy of mechanical preconditioning of donor MDSCs on the regenerative capacity of host myocardium, we compared MS MDSCs with NS MDSCs for their ability to aid in the functional recovery of myocardial infarct injured hearts. The most critical endpoint in cell transplantation for cardiac repair is maintenance or improvement of cardiac contractility and output. Therefore, cardiac function was assessed at 2, 6, and 12 weeks after cell transplantation to determine the effect of preconditioning on the attenuation of postinfarction cardiac functional deterioration. Hearts treated with MS-MDSCs had significantly higher systolic function as indicated by fractional shortening versus NS MDSCs or PBS injected control hearts (Fig. 3a and Table 1). Fractional area change, another index of systolic cardiac function, further confirmed that MS-MDSCs were better able to preserve systolic function after myocardial infarction versus NS MDSCs or PBS-injected control hearts (Fig. 3b and Table 1).
FIG. 3.
Cardiac function was improved by mechanically preconditioning the muscle-derived stem cells (MDSCs). (a) Hearts treated with MS-MDSCs had improved fractional shortening over the NS MDSCs and phosphate-buffered saline (PBS) groups. *p<0.05 to PBS, §p<0.05 to NS. (b) MS MDSCs improved fractional area change over NS cells or PBS. *p<0.05 to PBS, §p<0.05 NS cells.
Table 1.
Fractional Shortening and Fractional Area Data from Echocardiographic Analyses of Function in Hearts at 2, 6, and 12 Weeks After Myocardial Infarction and Cell Injection
| 2 weeks | 6 weeks | 12 weeks | |
|---|---|---|---|
| MS | |||
| FS | 28%±1%, n=13, p=0.01 to NS, p=0.01 to PBS | 24%±1%, n=13, p=0.01 to NS, p=0.01 to PBS | 24%±1%, n=8, p=0.049 to NS, p=0.01 to PBS |
| FAC | 27%±1%, n=13, p=0.01 to NS, p=0.01 to PBS | 28%±1%, n=13, p=0.049 to NS, p=0.01 to PBS | 27%±1%, n=13, p=0.16 to NS, p=0.01 to PBS |
| NS | |||
| FS | 19%±1%, n=13, p=0.16 to PBS | 19%±1%, n=13, p=0.02 to PBS | 18%±1%, n=11, p=0.38 to PBS |
| FAC | 21%±1%, n=13, p=0.03 to PBS | 24%±1%, n=13, p=0.01 to PBS | 24%±1%, n=11, p=0.11 to PBS |
| PBS | |||
| FS | 16%±2%, n=8 | 15%±1%, n=14 | 15%±1%, n=5 |
| FAC | 15%±1%, n=8 | 15%±2%, n=14 | 18%±1%, n=5 |
Data are the mean±SEM.
PBS, phosphate-buffered saline; NS, nonstimulated; MS, mechanically stimulated; FS, fractional shortening; FAC, fractional area change.
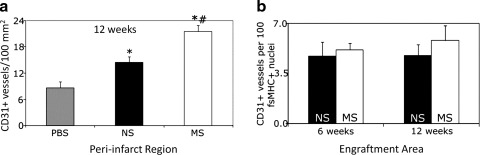
Mechanical stimulation significantly increased the number of CD31+ cells after myocardial infarction
We explored the impact of MS-MDSCs on the number of CD31+ cells within the injected hearts, as blood vessel formation has been previously shown to be critical for cardiac repair.18 A significant difference in the numbers of CD31+ cells in the peri-infarct region at 12 weeks (Fig. 4a) was observed between MS MDSCs (21±1 CD31+ cells/mm2, n=5, p=0.01 to NS, p=0.0004 to PBS), NS MDSCs (14±1 CD31+ cells/mm2, n=5, p=0.03 to PBS), and PBS (9±1 CD31+ cells/mm2, n=4) (12 weeks). A similar trend was found when examining the number of CD31+ cells in the engraftment region (fsMHC+ areas) (p=0.11, Fig. 4b). The data shown are the mean±standard error of the mean.
FIG. 4.
Mechanical preconditioning increases neoangiogenesis. (a) MS-MDSCs have increased angiogenesis above NS cells and PBS, while NS had higher angiogenesis than PBS in the peri-infarct region at 12 weeks (*p<0.05 to PBS, #p<0.05 to NS cells). (b) In the engraftment area, there is a slight increase in angiogenesis in the MS cell group above NS at both 6 and 12 weeks (p=0.34 for 6 weeks, p=0.18 for 12 weeks).
Mechanical stimulation does not affect cell survival and cardiac differentiation in vivo
Despite a positive effect imparted by the MS-MDSCs on cardiac functional recovery, MDSC cell survival in vivo was similar between the MS and NS groups (6 weeks: n=3 per group, p=0.24; 12 weeks: n=5 per group, p=0.47; data not shown). Previously, we found only low levels of in vivo MDSC differentiation toward a cardiac phenotype13,14 that was confirmed in the current study, with less than 1 out of 100 injected cells coexpressing cTnI and fsMHC at 6 or 12 weeks after injection (data not shown). There were no differences observed between the MS and NS cells (6 weeks: n=3 per treatment, p=0.72; 12 weeks: n=5 per treatment, p=0.95; data not shown). These results imply that the increase in cardiac function seen with MS-MDSCs is not related to MDSC differentiation along the cardiac lineage nor is it related to differences in cell survival.
Mechanical stimulation of MDSCs significantly reduced scar tissue area compared with the PBS control
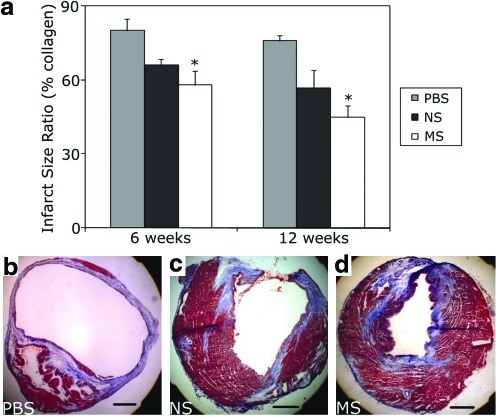
The scar tissue area fraction, defined as the ratio of collagen-rich fibrous tissue to total cardiac muscle area, is known to affect myocardial function after infarction, with lower levels correlating with a greater degree of cardiac contractility.37 A similar degree of scar tissue formation was observed in the MS-MDSC group at both 6 and 12 weeks (6 weeks: 58%±6%, n=3, p=0.98 to NS; 12 weeks: 45%±5%, n=6, p=0.30 to NS) versus the NS cells (6 week: 66%±2%, n=3, p=0.06 to PBS; 12 weeks: 57%±7%, n=5, p=0.07 to PBS; Fig. 5a); however, the MS-MDSC-injection group had significantly lower levels of scar tissue than the PBS controls at both time points tested (6 weeks: p=0.04 to PBS; 12 weeks: p=0.003 to PBS) (6-week PBS: 80%±5%; 12-week PBS: 76%±2%).
FIG. 5.
Mechanical stimulation of MDSCs prior to implantation reduced scar tissue formation. (a) Although no significant difference was observed between the MS and NS-MDSC, PBS-injected hearts had significantly higher levels of scar tissue than the MS group (*p<0.05 to PBS). (b–d) Representative images of hearts injected with PBS, NS, and MS MDSCs, respectively. Color images available online at www.liebertonline.com/tea
Discussion
Examining loading history through mechanical preconditioning of MDSCs increases the understanding of stem cell biology along with providing a potential method to improve cell transplantation for the treatment of disease and injury. The experiments reported here show that the expression of VEGF plays a potential role in the beneficial effect of mechanical stimulation of MDSCs for cellular cardiomyoplasty. In addition, the increase in CD31+ cells and reduction in scar tissue formation at the injection sites, likely play a role in the improved cardiac function after injury seen in the MS-MDSC group compared to the PBS control group.
Cellular behavior in vivo is influenced by a dynamic microenvironment that includes a diverse extracellular matrix, growth factors, cytokines and other soluble factors, and dynamic mechanical forces. The behavior of stem cells, in particular, may be significantly regulated by the cellular microenvironment and local cues that maintain quiescence or can trigger tissue repair and regeneration. Although cells and tissues containing progenitor cells are under constant mechanical stress, few studies have directly investigated the influence of these mechanical forces on stem cells and their regenerative potential. We hypothesized that the transplantation of MDSCs exposed to mechanical loading prior to transplantation would result in increased repair of the myocardium after myocardial infarction. Studying the effects of mechanical stimulation on cardiac muscle was based on a number of previous studies showing mechanically governed responses in cells and cellular components.38–40 On a molecular level a study on actin-network growth in vitro showed that the velocity of growth was a function of the previous loading applied to the network.39 Although this study was on isolated actin filaments, it showed that the loading history of cells may also play an important role in determining future cellular behavior. Application of mechanical stretch to cultured adult rat muscle satellite cells resulted in the release of hepatocyte growth factor and accelerated entry into the cell cycle.40 In addition, in a coculture system with rat neonatal cardiomyocytes, skeletal muscle stem cells can differentiate into cardiomyocytes when mechanical loading is applied.38
We first examined whether using MDSCs exposed to mechanical stimulation in vitro prior to transplantation could improve regeneration in vivo. We found that the transplantation of MS cells into a mouse model of myocardial infarction resulted in significant preservation of cardiac function compared to NS cells and PBS alone. As MDSCs do not appear to contribute directly to cardiomyocyte regeneration through their differentiation into a cardiomyocyte lineage or fusion with existing cardiomyocytes, the likely mechanism for MS-MDSC cardiac recovery is improved angiogenesis, as this has been previously shown to be critical for cardiac repair after injury and disease.18,41
To support our in vivo results, we explored MDSC behavior in vitro under a variety of conditions. We observed an increased secretion of VEGF after mechanical stimulation, which may partially explain the improvement in MS MDSC engraftment and repair and further supports our earlier results.18 Previous reports suggest that VEGF influences myoblast migration and survival and increases skeletal muscle repair during ischemia.42,43 Kasper et al. showed that the conditioned medium from mesenchymal stem cells subjected to mechanical loading increased sprouting of endothelial cells by creating an angiogenic promoting environment.44 Certainly other paracrine factors besides VEGF could also be playing a role in the improvement of cardiac function imparted by the MS MDSCs. For example, the conditioned medium of MS mesenchymal stem cells had an upregulation of MMP-2 and TGF-B1,44 which is an area for future study.
As cells and the body are controlled by tightly regulated spatiotemporal processes, it has been shown by numerous groups that it is not just the addition or removal of stimulation,45,46 but rather the controlled timing of the stimuli that produces distinct results.47 Results from our VEGF secretion study also demonstrate that the effect of MS is time dependent. Interestingly, the increased secretion of VEGF, while being significant at 24 h, required continued mechanical stimulation and was reversible as the growth factor levels returned to close to baseline levels within 24 h after cessation of stretch. Just as spatiotemporal control of chemical factors is critical in regeneration, the time course and duration of the mechanical stimulation could be just as important. The tight regulation and timing demonstrated by these results suggest that the mechanical loading history can predispose the MDSCs toward specific responses that are advantageous for cell transplantation. This is an essential point in this study since it shows that adding mechanical stimulation before transplantation into a mechanical environment is both a valid means of increasing VEGF secretion and improves transplantation results. We speculate that after intracardiac implantation, the MS-MDSCs undergo a mechanical stretching regiment dictated by the host microenvironment that complement the mechanical stimulation in vitro and lead to a constant expression of VEGF. Further study is necessary to fully optimize the mechanical preconditioning regimen.
Another noteworthy finding of our current study is that mechanical preconditioning of MDSCs before transplantation ameliorated the negative remodeling and scar tissue formation of the injected hearts when compared with the PBS group; however, the effect on scar tissue formation was only significant in the MS-MDSC group. These results, taken together, suggest that the paracrine effect imparted by the injected MDSCs plays a major determinant in cardiac repair after stem cell implantation. It is important to note that these studies were carried out in immunodeficient mice and that the lack of immune system may influence the paracrine-mediated healing response. This current study further demonstrates that cardiac differentiation of the injected MDSCs is not a prerequisite for improving cardiac function in infarcted myocardium.
In summary, our results suggest that MS-MDSC transplantation significantly improved cardiac contractility, significantly increased the numbers of CD31+ cells in the peri-infarct area, and significantly decreased fibrosis compared with the PBS injected hearts in the injured myocardium in a more effective manner than NS-MDSCs. These findings highlight an important role for cyclic mechanical loading preconditioning to improve the regeneration capacity of MDSCs within the injured myocardium.
Acknowledgments
We would like to thank Joseph Feduska for assistance with CD31 staining and live cell imager operation, James Cummins for editorial assistance, and Marcelle Huard for help with cryosectioning. The authors acknowledge financial support from the Pittsburgh Tissue Engineering Initiative through a postdoctoral fellowship to T.R.C. This work was also supported in part by grants to J. Huard from the NIH (IU54AR050733-01 and HL 069368), the Donaldson Chair and the Hirtzel Foundation at Children's Hospital of Pittsburgh, and the Henry J. Mankin Chair at the University of Pittsburgh, to P. LeDuc from the National Science Foundation, the Office of Naval Research, and the Beckman Young Investigators Program.
Disclosure Statement
During the performance period of this research project, Dr. Huard received remuneration as a consultant to Cook MyoSite, Inc. None of the other authors have any potential conflicts of interest to declare.
References
- 1.Aejaz H. Aleem N. Parveen M. Khaja M. Narusu L. Habiballah C. Stem cell therapy: present status. Transplant Proc. 2007;39:694. doi: 10.1016/j.transproceed.2007.01.069. [DOI] [PubMed] [Google Scholar]
- 2.Cuplice N. Gersh B. Alegria J. Cell therapy for cardiovascular disease: what cells, what diseases and for whom? Nat Clin Pract Cardiovasc Med. 2005;2:37. doi: 10.1038/ncpcardio0073. [DOI] [PubMed] [Google Scholar]
- 3.Laflamme M. Murry C. Regenerating the heart. Nat Biotechnol. 2005;23:845. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 4.Rosamund W. Flegal K. Furie K. Go A. Greenlund K. Haase N., et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 5.Ramos G. Hare J. Cardiac cell-based therapy: cell types and mechanisms of actions. Cell Transplant. 2007;16:951. doi: 10.3727/096368907783338208. [DOI] [PubMed] [Google Scholar]
- 6.Boyle A. Schulman S. Hare J. Oettgen P. Is stem cell therapy ready for patients? Stem cell therapy for cardiac repair. Ready for the next step. Circulation. 2006;114:339. doi: 10.1161/CIRCULATIONAHA.105.590653. [DOI] [PubMed] [Google Scholar]
- 7.Taylor D. Zenovich A. Cell therapy for left ventricular remodeling. Curr Heart Fail Rep. 2007;4:3. doi: 10.1007/s11897-007-0019-0. [DOI] [PubMed] [Google Scholar]
- 8.Gharaibeh B. Lu A. Tebbets J. Zheng B. Feduska J. Crisan M., et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 9.Qu-Petersen Z. Deasy B. Jankowski R. Ikezawa M. Cummins J. Pruchnic R., et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Latif A. Zuba-Surma E. Case J. Tiwari S. Hunt G. Ranjan S., et al. TGF-beta1 enhances cardiomyogenic differentiation of skeletal muscle-derived adult primitive cells. Basic Re Cardiol. 2008;103:514. doi: 10.1007/s00395-008-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arsic N. Mamaeva D. Lamb N. Fernandez A. Muscle-derived stem cells isolated as non-adherent population give rise to cardiac, skeletal muscle and neural lineages. Exp Cell Res. 2008;314:1266. doi: 10.1016/j.yexcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Cao B. Zheng B. Jankowksi R. Kimura S. Ikezawa M. Deasy B., et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- 13.Oshima H. Payne T. Urish K. Sakai T. Ling Y. Gharaibeh B., et al. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12:1130. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 14.Payne T. Oshima H. Sakai T. Ling Y. Gharaibeh B. Cummins J., et al. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12:1264. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M. Methot D. Poppa V. Fujio Y. Walsh K. Murry C. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 16.Qu Z. Balkir L. Deutekom J.V. Robbins P. Pruchnic R. Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki K. Murtuza B. Beauchamp J. Smolenski R. Varela-Carver A. Fukushima S., et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004;18:1153. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 18.Payne T. Oshima H. Okada M. Momoi N. Tobita K. Keller B., et al. A relationship between VEGF, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 19.Niagara M. Haider H. Jiang S. Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 20.Ingber D. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 21.Gillispie P. Walker R. Molecular basis of mechanotransduction. Nature. 2001;413:194. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert P.M. Havenstrite K.L. Magnusson K.E. Sacco A. Leonardi N.A. Kraft P., et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estes B. Gimble J. Guilak F. Mechanical signals as regulators of stem cell fate. Curr Top Dev Biol. 2004;60:91. doi: 10.1016/S0070-2153(04)60004-4. [DOI] [PubMed] [Google Scholar]
- 24.Goldspink G. Mechanical signals, IGF-I gene splicing, and muscle adaptation. J Physiol. 2005;20:232. doi: 10.1152/physiol.00004.2005. [DOI] [PubMed] [Google Scholar]
- 25.Thomas G. Haj A.E. Bone marrow stromal cells are load responsive in vitro. Calcif Tissue Int. 1996;58:101. doi: 10.1007/BF02529731. [DOI] [PubMed] [Google Scholar]
- 26.Ruoslahti E. Stretching is good for a cell. Science. 1997;276:1345. doi: 10.1126/science.276.5317.1345. [DOI] [PubMed] [Google Scholar]
- 27.Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999;194:323. doi: 10.1046/j.1469-7580.1999.19430323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song G. Ju Y. Soyama H. Ohashi T. Sato M. Regulation of cyclic longitudinal mechanical stretch on proliferation of human bone marrow mesenchymal stem cells. Mol Cell Biomech. 2007;4:201. [PubMed] [Google Scholar]
- 29.Kook S. Son Y. Choi K. Lee H. Chung W. Hwang I., et al. Cyclic mechanical stress suppresses myogenic differentiation of adult bovine satellite cells through activation of extracellular signal-regulated kinase. Mol Cell Biochem. 2008;309:133. doi: 10.1007/s11010-007-9651-y. [DOI] [PubMed] [Google Scholar]
- 30.Seko Y. Seko Y. Takahashi N. Shibuya M. Yazaki Y. Pulsatile stretch stimulates vascular endothelial growth factor (VEGF) secretion by cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;254:462. doi: 10.1006/bbrc.1998.9969. [DOI] [PubMed] [Google Scholar]
- 31.Gruden G. Thomas S. Burt D. Zhou W. Chusney G. Gnudi L., et al. Interaction of angiotensin II and mechanical stretch on vascular endothelial growth factor production by human mesangial cells. J Am Soc Nephrol. 1999;10:730. doi: 10.1681/ASN.V104730. [DOI] [PubMed] [Google Scholar]
- 32.Zheng B. Cao B. Crisan M. Sun B. Li G. Logar A., et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- 33.Jankowksi R. Deasy B. Cao B. Gates C. Huard J. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115:4361. doi: 10.1242/jcs.00110. [DOI] [PubMed] [Google Scholar]
- 34.Deasy B. Gharaibeh B. Pollett J. Jones M. Lucas M. Kanda Y., et al. Long-term self-renewal of postnatal muscle-derived stem cells. Mol Cell Biol. 2005;16:3323. doi: 10.1091/mbc.E05-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Payne T. Oshima H. Okada M. Momoi N. Tobita K. Keller B., et al. VEGF, angiogenesis and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 36.Drowley L. Okada M. Beckman S. Vella J. Keller B. Tobita K., et al. Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther. 2010;18:1865. doi: 10.1038/mt.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber K. Janicki J. Shroff S. Pick R. Chen R. Bashey R. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62:757. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 38.Iijima Y. Nagai T. Mizukami M. Ogura T. Wada H. Toko H., et al. Beating is necessary for transdifferentiation of skeletal muscle-derived cells into cardiomyocytes. FASEB J. 2003;17:1361. doi: 10.1096/fj.02-1048fje. [DOI] [PubMed] [Google Scholar]
- 39.Parekh S. Chaudhuri O. Theriot J. Fletcher D. Loading history determines the velocity of actin-network growth. Nat Cell Biol. 2005;7:1219. doi: 10.1038/ncb1336. [DOI] [PubMed] [Google Scholar]
- 40.Tatsumi R. Hattori A. Ikeuchi Y. Anderson J. Allen R. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell. 2002;13:2909. doi: 10.1091/mbc.E02-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai Y. Xu M. Wang Y. Pasha Z. Li T. Ashraf M. HIF-1alpha induced VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol. 2007;42:1036. doi: 10.1016/j.yjmcc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouchentouf M. Benabdallah B. Bigey P. Yau T. Scherman D. Tremblay J. Vascular endothelial growth factor reduced hypoxia-induced death of human myoblasts and improved their engraftment in mouse muscles. Gene Ther. 2007;15:404. doi: 10.1038/sj.gt.3303059. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura N. Yamaguchi M. Ohashi H. Oshima Y. Oka S. Yoshida M., et al. Pulmonary atresia with intact ventricular septum: strategy based on right ventricular morphology. J Thorac Cardiovasc Surg. 2003;126:1417. doi: 10.1016/s0022-5223(03)01035-3. [DOI] [PubMed] [Google Scholar]
- 44.Kasper G. Dankert N. Tuischer J. Hoeft M. Gaber T. Glaeser J.D., et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25:903. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- 45.Boppart M. Kimmel D. Yee J. Cullen D. Time course of osteoblast appearance after in vivo mechanical loading. Bone. 1998;23:409. doi: 10.1016/s8756-3282(98)00119-7. [DOI] [PubMed] [Google Scholar]
- 46.Rai S. Rankin C. Critical and sensitive periods for reversing the effects of mechanosensory deprivation on behavior, nervous system, and developing in Caenorhabditis elegans. Dev Neurobiol. 2007;67:1443. doi: 10.1002/dneu.20522. [DOI] [PubMed] [Google Scholar]
- 47.Niklason L. Gao J. Abbott W. Hirschi K. Houser S. Marini R., et al. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]