Abstract
Joint-derived stem cells are a promising alternative cell source for cartilage repair therapies that may overcome many of the problems associated with the use of primary chondrocytes (CCs). The objective of this study was to compare the in vitro functionality and in vivo phenotypic stability of cartilaginous tissues engineered using bone marrow-derived stem cells (BMSCs) and joint tissue-derived stem cells following encapsulation in agarose hydrogels. Culture-expanded BMSCs, fat pad-derived stem cells (FPSCs), and synovial membrane-derived stem cells (SDSCs) were encapsulated in agarose and maintained in a chondrogenic medium supplemented with transforming growth factor-β3. After 21 days of culture, constructs were either implanted subcutaneously into the back of nude mice for an additional 28 days or maintained for a similar period in vitro in either chondrogenic or hypertrophic media formulations. After 49 days of in vitro culture in chondrogenic media, SDSC constructs accumulated the highest levels of sulfated glycosaminoglycan (sGAG) (∼2.8% w/w) and collagen (∼1.8% w/w) and were mechanically stiffer than constructs engineered using other cell types. After subcutaneous implantation in nude mice, sGAG content significantly decreased for all stem cell-seeded constructs, while no significant change was observed in the control constructs engineered using primary CCs, indicating that the in vitro chondrocyte-like phenotype generated in all stem cell-seeded agarose constructs was transient. FPSCs and SDSCs appeared to undergo fibrous dedifferentiation or resorption, as evident from increased collagen type I staining and a dramatic loss in sGAG content. BMSCs followed a more endochondral pathway with increased type X collagen expression and mineralization of the engineered tissue. In conclusion, while joint tissue-derived stem cells possess a strong intrinsic chondrogenic capacity, further studies are needed to identify the factors that will lead to the generation of a more stable chondrogenic phenotype.
Introduction
The long-term outcome in cartilage repair studies in vivo is often disappointing,1 which may be at least partially attributable to the known limitations of current treatment options. Cell-based therapies, such as autologous chondrocyte (CC) implantation, involve the creation of an additional cartilage injury to biopsy tissue, and dedifferentiation of CCs is known to occur during monolayer expansion.2–4 For optimum cartilage repair, a CC-like phenotype needs to be re-established by the transplanted cells and maintained in the long term.5 Joint-derived stem cells, including infrapatellar fat pad (fat pad-derived stem cells [FPSCs]) and synovial membrane-derived stem cells (SDSCs) are a promising alternative cell source for cartilage repair therapies that may overcome many of the problems associated with the use of primary CCs.6–11 When compared to mesenchymal stem cells (MSCs) derived from other tissues, joint tissue-derived stem cells have demonstrated superior capacity for chondrogenesis.12,13 This has led to increased interest in the field of regenerative medicine to develop novel stem cell-based therapies using SDSCs for the treatment of damaged and diseased cartilage.14
A central concern associated with the use of MSCs is their inability to form stable cartilage resistant to hypertrophy or fibrous dedifferentiation. To test the phenotypic stability and the capacity to form stable cartilage an ectopic model is commonly used, where the cells alone, or seeded into scaffolds, are implanted under the skin or into the muscle of transgenic mice.5,15 CCs are known to form stable ectopic cartilage16,17; however, cartilaginous tissues derived from SDSCs have been shown to undergo fibrous dedifferentiation or complete degeneration when implanted in an ectopic model.18–21 Bone marrow-derived stem cells (BMSCs) are known to rapidly expand in culture while retaining their capacity to differentiate, making them ideally suitable for a wide spectrum of clinical applications for repair of damaged or defective tissue,22,23 but previous studies have observed that BMSCs express type X collagen (a marker of hypertrophy) on induction of chondrogenesis and tend to undergo endochondral ossification after subcutaneous implantation.24,25 This might therefore hinder the potential of BMSCs as a candidate for replacing culture-expanded CCs in cell-based repair of cartilage lesions.5,26
The hydrogel or scaffold in which MSCs are encapsulated may play a key role in determining their in vivo phenotypic stability. For example, adipose-derived stem cells maintained as spheroids have been shown to undergo hypertrophy and calcification after ectopic transplantation27; however, when encapsulated in Matrigel™, a gelatinous protein mixture, chondrogenesis and suppression of the calcification was observed.28 In vivo, agarose has been shown to stabilize the CC phenotype29 and to promote the formation of ectopic cartilage after injection of the hydrogel into the subperiosteal space.24 Further, when joint-derived stem cells are encapsulated in agarose hydrogels, they generate cartilaginous tissues whose mechanical functionality increases with time in culture.30–32 Therefore, agarose would appear to be a promising material to facilitate both the development of a functional engineered cartilaginous graft in vitro and maintenance of a chondrogenic phenotype in vivo.
The objective of this study was to compare the in vitro functionality and in vivo phenotypic stability of cartilaginous tissues engineered using BMSCs and joint tissue-derived stem cells that are encapsulated into agarose hydrogels. Our initial hypothesis was that joint tissue-derived stem cells seeded in agarose hydrogels would form a stable cartilaginous tissue in vivo. In the first phase of the study, we compared the in vitro functionality of cartilaginous tissues engineered using different joint-derived stem cells to BMSCs. The influence of exposing these engineered tissues to factors known to promote hypertrophy in vitro was also assessed. In the second phase of the study, the in vivo stability of these engineered cartilaginous tissues was compared in a subcutaneous nude mouse model.
Materials and Methods
Cell isolation and expansion
CCs and BMSCs were harvested from immature (4 months old) porcine tissue, isolated, and expanded as previously described.32 Synovial membrane (SM) and fat pad (FP) tissue was harvested from the femoropatellar joint, sliced, and rinsed with phosphate-buffered saline (PBS; Sigma-Aldrich) containing penicillin/streptomycin (200 U/mL; Gibco, Biosciences) and amphotericin B (2.5 μg/mL; Sigma-Aldrich). SM and FP pieces were incubated with Dulbecco's modified Eagle medium (DMEM) GlutaMAX (Gibco, Biosciences) containing collagenase type II (315 U/mg; Worthington, Langanbach Services) for 3–4 h under constant rotation at 37°C. The resulting cell suspension was then filtered through a 40-μm pore-size cell sieve (Falcon Ltd.) and the filtrate centrifuged and rinsed with PBS twice.
CCs and stem cells were seeded at a density of 5×103 cells/cm2 in 175 cm2 T flasks and expanded to passage two (∼11 population doublings for CCs, 14 for BMSCs, and 16 population doublings for joint-derived stem cells) in a humidified atmosphere at 37°C and 5% CO2. Viable cells were counted using a hemacytometer and 0.4% trypan blue staining. Isolated cells from different tissues were maintained in DMEM GlutaMAX supplemented with 10% v/v fetal bovine serum (FBS; Gibco, Biosciences), 5 ng/mL of human fibroblast growth factor-2 (Prospec), and 100 U/mL penicillin/streptomycin during the expansion phase (14 days, passage 2). At the first passage, colonies were stained with crystal violet (Sigma-Aldrich) and counted to obtain the colony-forming cell fraction.
Agarose gel constructs formation
After isolation and expansion stem cells were suspended in DMEM and mixed with 4% agarose (Type VII; Sigma-Aldrich) in PBS at a 1:1 ratio. This produced a 2% agarose gel containing 20×106 cells/mL. The gel was cast in a stainless steel mold to produce a 3-mm-thick agarose sheet, from which cylindrical constructs (Ø5×3 mm) were created using a biopsy punch. The gels were cultured in a chemically defined chondrogenic medium consisting of DMEM GlutaMAX supplemented with 100 U/mL penicillin/streptomycin, 100 μg/mL sodium pyruvate, 40 μg/mL L-proline, 50 μg/mL L-ascorbic acid-2-phosphate, 4.7 μg/mL linoleic acid, 1.5 mg/mL bovine serum albumin, 1×insulin–transferrin–selenium, 100 nM dexamethasone (all from Sigma-Aldrich), and 10 ng/mL of human transforming growth factor-β3 (TGF-β3; Prospec). On day 21 an additional group was exposed to hypertrophic medium for 28 days, by changing the following parameters: TGF-β3 withdrawal, addition of 1 nM L-thyroxine (Sigma-Aldrich), and reduction of the level of dexamethasone to 1 nM.33 The medium was exchanged every 3 or 4 days.
In vivo subcutaneous transplantation
After 21 days of in vitro culture under chondrogenic conditions, stem cell-seeded hydrogels were subcutaneously transplanted into the back of transgenic mice and CC-seeded agarose hydrogels (20×106 cells/mL) were also implanted as a control group. Nude mice (Balb/c; Harlan) were utilized in this study. Animals were weighed and anesthetized with an intraperitoneal injection of 10 mg/kg xylazine (Chanazine® 2%; Chanelle) and 100 mg/kg ketamine (Narketan®; Vetoquinol). Under sterile conditions, two skin incisions (1.5 cm long) were made along the central line of the spine, one at the shoulders and one at the hips. A subcutaneous dissection was made, and a subcutaneous pocket was created on both sides of the wound. Two to three constructs were inserted in each subcutaneous pocket. Incisions were closed with 4-0 Vicryl plus (Ethicon, Johnson&Johnson) sutures and tissue glue (Vetloc xcel). Animals were allowed to recover in a warm environment. Mice were euthanized 4 weeks after the surgery by CO2 inhalation and the constructs were analyzed for gross appearance, histological, immunohistochemical, and biochemical properties. The animal protocol was reviewed and approved by the ethics committee of Trinity College Dublin.
Mechanical, biochemical, and histological analysis
Agarose constructs were mechanically evaluated using a standard materials testing machine (Zwick Roell Z005; Herefordshire) with a 5N load cell, as previously described.32,34 The equilibrium compressive modulus was determined from the equilibrium force following unconfined compression testing to 10% strain. The dynamic modulus was determined by applying an additional 1% dynamic strain at 1 Hz. Construct dimensions were measured using a toolmaker's microscope (Mitutoyo UK Ltd.). Biochemistry was performed to determine DNA, collagen, and sulfated glycosaminoglycan (sGAG) content. DNA content was quantified using the Hoechst Bisbenzimide 33258 dye assay. The proteoglycan content was determined using the dimethylmethylene blue dye-binding assay (Blyscan; Biocolor Ltd.), and total collagen content was determined by measuring the hydroxyproline content.35,36 Alkaline phosphatase activity was analyzed in the media, determined using the SensoLyte® pNPP Alkaline Phosphatase Assay Colorimetric kit (AnaSpec, Inc.) and the absorbance was measured at 405 nm. For histological evaluation, gels were fixed in 4% paraformaldehyde and embedded in paraffin, cut into 5-μm-thick sections, and stained with 1% alcian blue 8GX, pH=1 (Sigma-Aldrich) in 0.1 M HCl to assess sGAG content and alizarin red to detect calcium deposition. Type, I, II, and X collagen content were evaluated with a standard immunohistochemical technique as previously described.32 Positive and negative controls were included in the immunohistochemistry staining protocol for each batch.
Microcomputed tomography
Microcomputed tomography (μCT) scans were carried out on the in vivo specimens to provide a quantitative measure of mineralization within the engineered constructs. A Scanco Medical 40 μCT system (Scanco Medical) was used for the evaluation with a 70 kVp X-ray source (114 μA).
Statistical analysis
Five to nine samples were analyzed per group at each time point, with three to seven samples used for biochemical/mechanical analyses and two samples were used for histology. Differences in mechanical and biochemical properties with cell type and/or time-in-culture were determined by analysis of variance with time and cell source as independent factors, followed by Tukey's post hoc test (95% confidence Interval). Statistics were preformed with MINITAB 15.1 (Minitab) and GraphPad Prism 4 software package. Biochemical and mechanical properties of constructs are expressed in the form of mean±standard error of the mean. Statistical significance was declared at p≤0.05.
Results
In vitro development of engineered tissues maintained in either chondrogenic or hypertrophic media
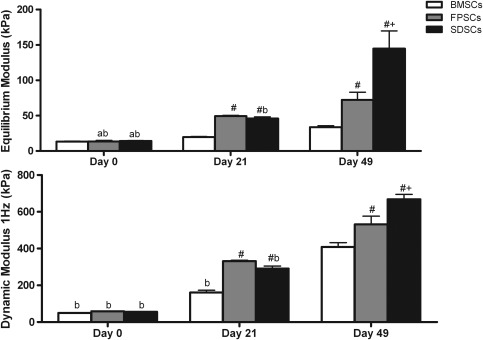
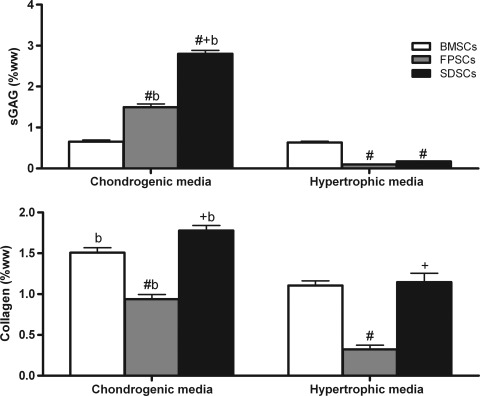
The mechanical properties of all stem cell-seeded hydrogels significantly increased with time in culture, with SDSC-seeded constructs exhibiting the highest equilibrium (144.98±12.49 kPa) and dynamic modulus (668.4±13.28 kPa) after 49 days of in vitro culture (Fig. 1). sGAG accumulation was significantly higher (p<0.0001) in SDSC constructs (2.8%±0.1% w/w) when compared to either FPSC- (1.49%±0.3% w/w) or BMSC-seeded (0.65%±0.1% w/w) constructs (Fig. 2). Collagen accumulation was also highest in SDSC constructs, reaching 1.77%±0.1% w/w compared with 0.93%±0.1% w/w for FPSCs or 1.5%±0.1% w/w for BMSCs. To assess the tendency of cartilaginous tissues engineered using different sources of stem cells to proceed along an endochondral pathway, hypertrophic factors were additionally added to the culture media of a subset of constructs from day 21 of culture. This was observed to significantly reduce sGAG accumulation in joint tissue-derived constructs and significantly reduce collagen accumulation in all groups (Fig. 2).
FIG. 1.
Equilibrium and 1 Hz dynamic modulus of stem cell-seeded agarose gels cultured in vitro with chondrogenic media at days 0, 21, and 49. Data represent the mean±standard error of the mean (SEM). Significance: a versus day 21; b versus day 49; # versus bone marrow-derived stem cells (BMSCs),+ versus fat pad-derived stem cells (FPSCs) (p<0.05).
FIG. 2.
Sulfated glycosaminoglycan (sGAG) and collagen content measured in agarose gel-seeded with stem cells cultured with chondrogenic media for 49 days or with hypertrophic media (chondrogenic media for 21 days followed by 28 days of hypertrophic media). Data are expressed in percentages of wet weight and represent the mean±SEM. Significance: b versus hypertrophic media; # versus BMSCs, + versus FPSCs (p<0.05).
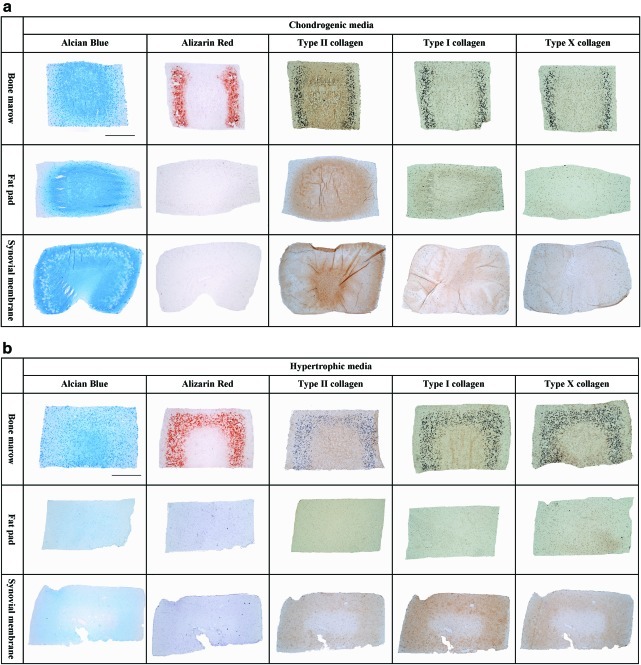
Histological analysis revealed an intense staining for alcian blue in all stem cell-seeded constructs continuously maintained in chondrogenic media (Fig. 3a). Alcian blue staining was more intense in the core of BMSC and FPSC constructs, with more homogenous staining observed in SDSC constructs. Some cell clustering was also observed in SDSC constructs. Calcium deposition was detected toward the periphery of the BMSC group as evidenced by positive staining for alizarin red. Immunohistochemical staining also indicated the presence of type I and X collagen staining in the BMSC hydrogels; in contrast, joint-derived stem cells showed an increased intensity for type II collagen in comparison with the other collagen types (Fig. 3a). With the addition of hypertrophic factors to the media, more intense staining for alizarin red and weaker staining for type II collagen was observed in BMSC-seeded gels (Fig. 3b). Only weak staining for type X collagen was observed in the SDSC and FPSC constructs. Alcian blue staining was weaker in all groups when supplemented with hypertrophic factors, particularly the SDSC and FPSC constructs (Fig. 3b).
FIG. 3.
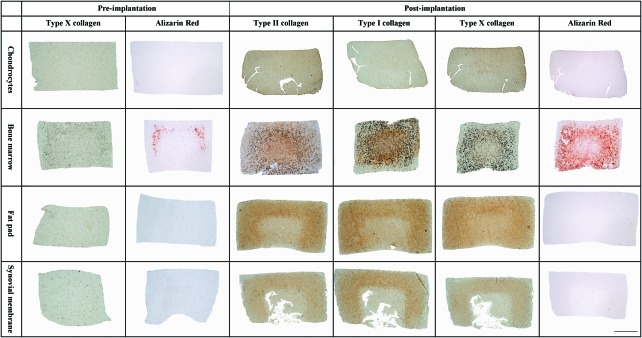
(a) Agarose gels seeded with stem cells from bone marrow, infrapatellar fat pad (FP), and synovial membrane (SM) cultured with chondrogenic medium for 49 days. (b) Agarose gels seeded with stem cells from bone marrow, FP, and SM cultured with hypertrophic media (chondrogenic media for 21 days followed by 28 days of hypertrophic media). Gels were stained for alcian blue (stains sGAG), alizarin red (stains calcium deposits), type II collagen, type I collagen, and type X collagen. Scale bar: 1 mm. Color images available online at www.liebertonline.com/tea
Analysis of the media from all groups revealed negligible levels of alkaline phosphatase activity except for the BMSC group in both chondrogenic- and hypertrophic media (data not shown).
In vivo phenotypic stability of cartilaginous tissues engineered using different sources of stem cells
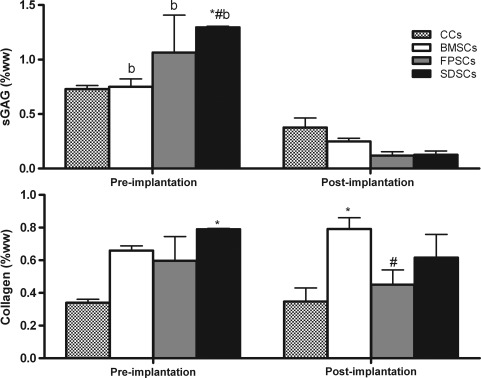
At the time of implantation (21 days of in vitro culture), all constructs contained sGAG and collagen, with sGAG accumulation highest in SDSC constructs at 1.3% w/w (±0.01; Fig. 4). (Staining for alcian blue, type I collagen, and type II collagen at the time of implantation was similar to that provided in Fig. 3a.) Postimplantation (21 days of chondrogenic medium followed by 28 days in vivo), sGAG content was significantly lower for all stem cell-seeded constructs (p<0.005), while no significant decrease was observed in the CC-seeded constructs (Fig. 4). Total collagen content did not significantly change for any group during the in vivo cultivation phase (Fig. 4). A reduction in DNA content was observed after subcutaneous implantation (see Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/tea, which also provides details of sGAG and collagen content normalized to DNA pre- and postimplantation).
FIG. 4.
sGAG and collagen content measured in the chondrocytes (CCs) and stem cells gels at the in vitro preimplantation time point (21 days of chondrogenic media) and postimplantation (21 days of chondrogenic media followed by 28 days of in vivo culture). Data are expressed in percentages of wet weight and represent the mean±SEM. Significance: b versus postimplantation; # versus BMSCs, * versus CCs (p<0.05).
Prior to implantation, BMSC constructs stained positive for the hypertrophic marker type X collagen, particularly toward the periphery of the construct with evidence of early calcium production also observed. None of the other cell types (FPSCs, SDSCs, or CCs) stained positive for calcium deposition and only stained weakly for type X collagen (Fig. 5). Postimplantation, type X collagen- and alizarin red staining were observed throughout the construct in the BMSC group. All other cell types also stained positive for type X collagen to varying degrees, but they did not stain for calcium deposits. Type II and I collagen were also present in joint tissue-derived stem cell constructs postimplantation, while CCs also stained positive for type II but weakly for type I collagen (Fig. 5).
FIG. 5.
Agarose gels seeded with either CCs or bone marrow, infrapatellar fat pad (FP), and synovial membrane (SM) derived stem cells stained for type X collagen and alizarin red (stains calcium deposits) at the in vitro preimplantation time point (21 days of chondrogenic media). Postimplantation gels (21 days of chondrogenic media followed by 28 days of in vivo culture) stained for type II collagen, type I collagen, type X collagen, and alizarin red (stains calcium deposition). Scale bar: 1 mm. Color images available online at www.liebertonline.com/tea
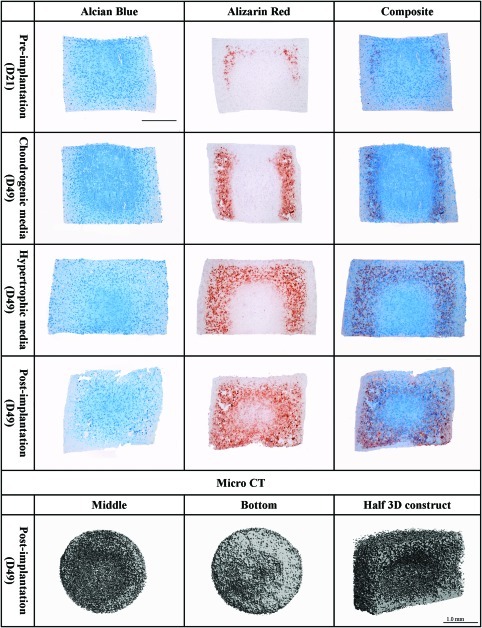
As evident by the spatially distinct regions of BMSC-seeded constructs staining positive for either alcian blue or alizarin red, it would appear that either a chondrogenic or an osteogenic/endochondral phenotype is being supported depending on the spatial location within the engineered tissue. To further investigate this, the temporal and spatial patterns of matrix staining were compared during chondrogenic differentiation (Fig. 6). During in vitro differentiation, BMSC constructs contained varying amounts of sGAG (as evident by the alcian blue staining) and matrix calcification deposits (as detected with alizarin red staining). The calcium deposits were evident after 21 days, intensified with further time in culture, and became more evident after implantation. When the alcian blue and the alizarin red staining were superposed in a composite image (right column), regions of calcium deposits appeared to correlate with regions that stained weakly for alcian blue. After in vivo implantation, more homogeneous staining was observed (Fig. 6). μCT analysis confirmed the findings from the alizarin red staining, with more mineral deposition detected around the construct periphery. Nine percent of the volumetric composition of the BM gels was classified as mineral, while negligible mineral deposition was detected in all other constructs. The cross-sectional μCT image from the bottom of the construct demonstrates more dense mineral deposition in comparison with the middle of the engineered tissue (Fig. 6).
FIG. 6.
Agarose gels seeded with bone marrow mesenchymal stem cells stained for alcian blue (stains sGAG), alizarin red (stains calcium deposits), and a composite image of both. Different time points of the study are shown in the figure: preimplantation (21 days of chondrogenic media supplementation in vitro), supplemented with chondrogenic media for 49 days in vitro, supplemented with hypertrophic media in vitro (chondrogenic media for 21 days followed by 28 days of hypertrophic media), and postimplantation (21 days of chondrogenic media followed by 28 days of in vivo culture). Bottom of the image: microcomputed tomography scans of the middle (left image), bottom (middle image), and half section (right image) of BMSC-seeded hydrogel harvested postimplantation. Scale bar: 1 mm. Color images available online at www.liebertonline.com/tea
Discussion
The aim of this study was to compare the in vitro chondrogenic potential of SDSCs, FPSCs, and BMSCs after encapsulation in agarose hydrogels and their subsequent in vivo phenotypic stability after subcutaneous implantation in nude mice. We demonstrated that SDSCs encapsulated in these hydrogels accumulated sGAG at levels approaching that of native articular cartilage, although the mechanical properties and collagen content are still lower than healthy tissue. Despite previous findings that agarose hydrogels can support chondrogenesis in the subperiosteal space,24 it was observed that the in vitro CC-like phenotype generated in all stem cell-seeded constructs was transient and lost once implanted in the back of nude mice. FPSCs and SDSCs appeared to undergo fibrous dedifferentiation or resorption with a dramatic loss in sGAG content, while BMSCs followed a more endochondral pathway as evident by type X collagen staining, calcium deposition, and type I collagen production. Therefore, we were unable to corroborate our initial hypothesis that joint tissue-derived stem cells seeded in agarose hydrogels would form a stable cartilaginous tissue in vivo after subcutaneous implantation.
In agreement with previous studies from our lab, FPSCs were able to generate cartilaginous constructs at least as mechanically functional as BMSCs.32 Further, here it has been demonstrated that SDSCs generated the most mechanically functional tissue. Comparable or even greater levels of matrix accumulation have been observed in similar studies using immature bovine SDSCs.31 In their study, removal of TGF-β from the culture media after 21 days was found to enhance subsequent chondrogenesis. After 56 days in culture both the equilibrium modulus and sGAG content approached native cartilage values, although the collagen content was still low.31 We have previously shown that porcine FPSC-seeded agarose gels need continuous TGF-β3 supplementation to maintain high levels of extracellular matrix synthesis,30 although it has been demonstrated that continuous exposure to TGF-β3 is not needed for robust chondrogenesis of BMSCs and that its addition in the first week of culture is the most critical.37 It may be that the optimal TGF-β supplementation conditions for robust chondrogenesis are cell-type and/or scaffold-type dependant, highlighting the importance of making comparisons between these different cell types with caution. Therefore, the results of this study cannot necessarily be interpreted to imply that SDSCs have a greater in vitro chondrogenic potential than other stem cell types such as BMSCs, as it may be that we have unintentionally used culture conditions (e.g., expansion protocols, media formulations, and cell seeding densities) that are more suited to inducing chondrogenesis of SDSCs over other cell types.
BMSCs and joint tissue-derived stem cells appeared to initiate along unique differentiation pathways in response to TGF-β3 stimulation, as clearly observed in the histology sections stained for alizarin red. BMSCs have been shown to be phenotypically different from other stem cell sources, producing markers of hypertrophy, mineralization, and catabolic enzymes.27,38,39 In this study, mineral deposits were observed near the periphery of the BMSC constructs after 3 weeks, which may suggest that aspects of bone development are being recapitulated in this in vitro culture system. Given that these mineral deposits do not appear in the more chondrogenic core-regions of the constructs, where cartilage-matrix-specific gene expression has been shown to be higher,40 suggests that a population of stem cells located toward the periphery of the hydrogel are proceeding along either an intramembranous or an endochondral ossification pathway. This may be due to the oxygen tension in this region of the construct, which is higher than in the core of the engineered tissue.41 A low oxygen tension has been recently shown to suppress the endochondral phenotype in chondrogenically primed BMSCs.42 Further, due to the developing chondrogenic core becoming stiffer, it may provide a more appropriate substrate stiffness for osteogenic differentiation, as stiffer substrates have been shown to support the osteogenic phenotype.43,44 Alternatively, or perhaps in conjunction, stem cells that have undergone chondrogenic differentiation in the core of the construct may be releasing factors that support mineralization of the periphery, mimicking aspects of bone development, such as intracartilaginous ossification, where a bone collar forms around a cartilaginous template.45 BMSCs would appear predetermined toward progressing along this route regardless of the fact that they are encapsulated in a hydrogel known to support chondrogenesis.2,24,46
This characteristic pattern of differentiation was only observed for BMSCs. Joint-derived stem cells showed no sign of mineral deposition, similar to what is observed for CC-seeded hydrogels. Regardless, joint-derived stem cells failed to form stable ectopic cartilage in vivo. Previous studies have shown that SDSCs chondrogenically primed in vitro fail to form stable cartilage in an ectopic environment.18–21 In contrast, CCs maintained a stable ectopic phenotype as previously described by other authors.16,17 We initially hypothesized that the encapsulation of stem cells within agarose might provide them with the necessary environment to support a more stable chondrogenic phenotype in vivo, but this approach was not successful.
The results of this study further highlight the need for novel approaches to promote a more stable chondrogenic phenotype in vivo. For example, coculture of BMSCs with CCs may create a chondrogenic niche to direct chondrogenesis of MSCs.47 The codelivery of parathyroid hormone-related protein with TGF-β3 in alginate microspheres has been shown to promote chondrogenesis and at least partially suppress hypertrophy.48 Alternatively, a longer in vitro culture period might support a more stable cartilaginous phenotype.28,49 Encapsulation in an appropriate biomaterial might also suppress endochondral ossification.28 It is interesting to note that certain aspects of the development of engineered cartilaginous grafts after subcutaneous implantation could be recapitulated by the addition of hypertrophic factors to the culture media, pointing to the utility of this type of in vitro analysis in the screening of strategies aimed at suppressing hypertrophy of chondrogenically primed MSCs. Of course, it may be that this differentiation pathway may not proceed in an orthotopic environment. Physiological joint loading, absent in the subcutaneous model, and the specifics of joint biochemical environment may suppress fibrous dedifferentiation and/or endochondral ossification and support a more stable chondrogenic phenotype.50–55 Further, the subcutaneous environment provides intimate contact with blood vessels and hence oxygen, which will differ from the avascular environment of articular cartilage. As previously discussed, a low oxygen tension has been recently shown to suppress the endochondral phenotype of chondrogenically primed BMSCs.42
In conclusion, while the results of this study demonstrate that SDSCs generate a more mechanically functional tissue in vitro, cartilaginous constructs generated by encapsulating different sources of MSCs in agarose hydrogels failed to form stable cartilaginous tissue in vivo after subcutaneous implantation. Further in vivo studies are needed to assess the ability of joint-derived stem cells seeded in agarose hydrogels to regenerate articular cartilage within critically sized defects of the joint surface.
Supplementary Material
Acknowledgments
This work was supported by the Science Foundation Ireland under the President of Ireland Young Researcher Award (Grant No: SFI/08/YI5/B1336) and a starter grant from the European Research Council (StemRepair–Project No. 258463).
Disclosure Statement
No competing financial interests exist.
References
- 1.Jakobsen R.B. Engebretsen L. Slauterbeck J.R. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232. doi: 10.2106/JBJS.D.02904. [DOI] [PubMed] [Google Scholar]
- 2.Benya P.D. Padilla S.R. Nimni M.E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 3.Diaz-Romero J. Gaillard J.P. Grogan S.P. Nesic D. Trub T. Mainil-Varlet P. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol. 2005;202:731. doi: 10.1002/jcp.20164. [DOI] [PubMed] [Google Scholar]
- 4.Barbero A. Grogan S. Schäfer D. Heberer M. Mainil-Varlet P. Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Pelttari K. Winter A. Steck E. Goetzke K. Hennig T. Ochs B.G., et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 6.De Bari C. Dell'Accio F. Tylzanowski P. Luyten F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.English A. Jones E.A. Corscadden D. Henshaw K. Chapman T. Emery P., et al. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology (Oxford) 2007;46:1676. doi: 10.1093/rheumatology/kem217. [DOI] [PubMed] [Google Scholar]
- 8.Hermida-Gomez T. Fuentes-Boquete I. Gimeno-Longas M.J. Muinos-Lopez E. Diaz-Prado S. de Toro F.J., et al. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. J Rheumatol. 2011;38:339. doi: 10.3899/jrheum.100614. [DOI] [PubMed] [Google Scholar]
- 9.Jones E. McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 2008;47:126. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 10.Khan W.S. Tew S.R. Adesida A.B. Hardingham T.E. Human infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res Ther. 2008;10:R74. doi: 10.1186/ar2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pei M. He F. Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76:1044. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi Y. Sekiya I. Yagishita K. Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 13.Shirasawa S. Sekiya I. Sakaguchi Y. Yagishita K. Ichinose S. Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 14.Pascual M. The chondrocelect experience: durable and effective treatments for cartilage damage in the knee. In: TiGenix C.G., editor. Fifth European Chapter of the Tissue Engineering and Regenerative Medicine International Society; Granada, Spain: 2011. [Google Scholar]
- 15.Cui J.H. Park S.R. Park K. Choi B.H. Min B.H. Preconditioning of mesenchymal stem cells with low-intensity ultrasound for cartilage formation in vivo. Tissue Eng. 2007;13:351. doi: 10.1089/ten.2006.0080. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Accio F. De Bari C. Luyten F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Sato T. Chenb G. Ushida T. Ishiia T. Ochiaia N. Tateishib T. Tissue-engineered cartilage by in vivo culturing of chondrocytes in PLGA–collagen hybrid sponge. Mater Sci Eng C. 2001;17:83. [Google Scholar]
- 18.De Bari C. Dell'Accio F. Luyten F.P. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 19.Dickhut A. Pelttari K. Janicki P. Wagner W. Eckstein V. Egermann M., et al. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 20.Marsano A. Millward-Sadler S.J. Salter D.M. Adesida A. Hardingham T. Tognana E., et al. Differential cartilaginous tissue formation by human synovial membrane, fat pad, meniscus cells and articular chondrocytes. Osteoarthritis Cartilage. 2007;15:48. doi: 10.1016/j.joca.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Park Y. Sugimoto M. Watrin A. Chiquet M. Hunziker E.B. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthritis Cartilage. 2005;13:527. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Emans P.J. van Rhijn L.W. Welting T.J. Cremers A. Wijnands N. Spaapen F., et al. Autologous engineering of cartilage. Proc Natl Acad Sci U S A. 2010;107:3418. doi: 10.1073/pnas.0907774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell E. Both S.K. Odorfer K.I. Koevoet W. Kops N. O'Brien F.J., et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord. 2011;12:31. doi: 10.1186/1471-2474-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson C. Brantsing C. Svensson T. Brisby H. Asp J. Tallheden T., et al. Differentiation of human mesenchymal stem cells and articular chondrocytes: analysis of chondrogenic potential and expression pattern of differentiation-related transcription factors. J Orthop Res. 2007;25:152. doi: 10.1002/jor.20287. [DOI] [PubMed] [Google Scholar]
- 27.Hennig T. Lorenz H. Thiel A. Goetzke K. Dickhut A. Geiger F., et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 28.Dickhut A. Gottwald E. Steck E. Heisel C. Richter W. Chondrogenesis of mesenchymal stem cells in gel-like biomaterials in vitro and in vivo. Front Biosci. 2008;13:4517. doi: 10.2741/3020. [DOI] [PubMed] [Google Scholar]
- 29.Selmi T.A.S. Verdonk P. Chambat P. Dubrana F. Potel J.F. Barnouin L., et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: Outcome at two years. J Bone Joint Surg Series B. 2008;90:597. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 30.Buckley C.T. Vinardell T. Thorpe S.D. Haugh M.G. Jones E. McGonagle D., et al. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J Biomech. 2010;43:920. doi: 10.1016/j.jbiomech.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Sampat S.R. O'Connell G.D. Fong J.V. Alegre-Aguaron E. Ateshian G.A. Hung C.T. Growth factor priming of synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17:2259. doi: 10.1089/ten.tea.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinardell T. Buckley C.T. Thorpe S.D. Kelly D.J. Composition-function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J Tissue Eng Regen Med. 2011;5:673. doi: 10.1002/term.357. [DOI] [PubMed] [Google Scholar]
- 33.Mueller M.B. Tuan R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58:1377. doi: 10.1002/art.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley C.T. Thorpe S.D. O'Brien F.J. Robinson A.J. Kelly D.J. The effect of concentration, thermal history and cell seeding density on the initial mechanical properties of agarose hydrogels. J Mech Behav Biomed Mater. 2009;2:512. doi: 10.1016/j.jmbbm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Ignatéva N.Y. Danilov N.A. Averkiev S.V. Obrezkova M.V. Lunin V.V. Sobol E.N. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem. 2007;62:51. [Google Scholar]
- 36.Kafienah W. Sims T.J. Biochemical methods for the analysis of tissue-engineered cartilage. Methods Mol Biol. 2004;238:217. doi: 10.1385/1-59259-428-x:217. [DOI] [PubMed] [Google Scholar]
- 37.Buxton A.N. Bahney C.S. Yoo J.U. Johnstone B. Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A. 2011;17:371. doi: 10.1089/ten.tea.2009.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segawa Y. Muneta T. Makino H. Nimura A. Mochizuki T. Ju Y.J., et al. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 39.Winter A. Breit S. Parsch D. Benz K. Steck E. Hauner H., et al. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 40.Haugh M.G. Meyer E.G. Thorpe S.D. Vinardell T. Duffy G.P. Kelly D.J. Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A. 2011;17:3085. doi: 10.1089/ten.tea.2011.0198. [DOI] [PubMed] [Google Scholar]
- 41.Buckley C.T. Meyer E. Kelly D.J. The influence of construct scale on the composition and functional properties of cartilaginous tissues engineered using bone-marrow derived mesenchymal stem cells. Tissue Eng Part A. 2012;18:382. doi: 10.1089/ten.TEA.2011.0145. [DOI] [PubMed] [Google Scholar]
- 42.Sheehy S. Buckley C.T. Kelly D.J. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2012;417:305. doi: 10.1016/j.bbrc.2011.11.105. [DOI] [PubMed] [Google Scholar]
- 43.Park J.S. Chu J.S. Tsou A.D. Diop R. Tang Z. Wang A., et al. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials. 2011;32:3921. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 45.Gray H. Pickering Pick T. The bone. In: Howden R., editor. Gray's Anatomy. Philadelphia: Courage Books; 1974. pp. 1096–1106. [Google Scholar]
- 46.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 47.Liu X. Sun H. Yan D. Zhang L. Lv X. Liu T., et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31:9406. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 48.Bian L. Zhai D.Y. Tous E. Rai R. Mauck R.L. Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu K. Zhou G.D. Liu W. Zhang W.J. Cui L. Liu X., et al. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183. doi: 10.1016/j.biomaterials.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Carter D.R. Beaupre G.S. Giori N.J. Helms J.A. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355(Suppl):S41–S55. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- 51.O'Driscoll S.W. Keeley F.W. Salter R.B. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am. 1988;70:595. [PubMed] [Google Scholar]
- 52.Salisbury Palomares K.T. Gerstenfeld L.C. Wigner N.A. Lenburg M.E. Einhorn T.A. Morgan E.F. Transcriptional profiling and biochemical analysis of mechanically induced cartilaginous tissues in a rat model. Arthritis Rheum. 2010;62:1108. doi: 10.1002/art.27343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakitani S. Goto T. Pineda S.J. Young R.G. Mansour J.M. Caplan A.I., et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Vinardell T. Rolfe R.A. Buckley C.T. Meyer E.G. Ahearne M. Murphy P. Kelly D.J. Hydrostatic pressure acts to stabilise a chondrogenic phenotype in porcine joint tissue derived stem cells. E cells and Materials. 2012;23:121. doi: 10.22203/ecm.v023a09. [DOI] [PubMed] [Google Scholar]
- 55.Steward A.J. Thorpe S.D. Buckley C.T. Wagner D.R. Kelly D.J. Cell-matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomaterialia. 2012. http://dx.doi.org/10.1016/j.actbio.2012.03.016. http://dx.doi.org/10.1016/j.actbio.2012.03.016 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.