Abstract
Hydrogel-encapsulating culture systems support the consistent growth of ovarian follicles from various species, such as mouse, non-human primate, and human; however, further innovations are required for the efficient production of quality oocytes from early-stage follicles. In this report, we investigated the coculture of mouse ovarian follicles with mouse embryonic fibroblasts (MEFs), commonly used as feeder cells to promote the undifferentiated growth of embryonic stem (ES) cells, as a means to provide the critical paracrine factors necessary for follicle survival and growth. Follicles were encapsulated within alginate hydrogels and cocultured with MEFs for 14 days. Coculture enabled the survival and growth of early secondary (average diameter of 90–100 μm) and primary (average diameter of 70–80 μm) follicles, which developed antral cavities and increased in diameter to 251–347 μm. After 14 days, follicle survival ranged from 70% for 100-μm follicles to 23% for 70-μm follicles. Without MEF coculture, all follicles degenerated within 6–10 days. Furthermore, 72%–80% of the oocytes from surviving follicles underwent germinal vesicle breakdown (GVBD), and the percentage of metaphase II (MII) eggs was 41%–69%. Medium conditioned by MEFs had similar effects on survival, growth, and meiotic competence, suggesting a unidirectional paracrine signaling mechanism. This advancement may facilitate the identification of critical factors responsible for promoting the growth of early-stage follicles and lead to novel strategies for fertility preservation.
Introduction
Lifesaving cancer treatments, such as chemotherapy and radiation, threaten the fertility of young girls and women by diminishing the follicle pool and triggering premature ovarian failure. Current treatments include autotransplantation of cryopreserved ovarian tissue post-treatment, which risks the reintroduction of cancer cells.1 Alternatively, in vitro follicle maturation and fertilization techniques could be used to produce meiotically competent eggs, and subsequently embryos, from cryopreserved ovarian tissue. Thus, in vitro culture systems for ovarian follicles are enabling tools for the advancement of fertility preservation technology for cancer patients.2 The efficient growth and maturation of isolated preantral follicles is supported by various culture systems.3–6 Moreover, considerable progress has been made to support the partial development of isolated early-stage (primordial and primary) follicles,7–10 which are more abundant in the ovary and survive cryopreservation.11
Culture systems based on hydrogel encapsulation of follicles have been engineered using biomaterials, such as alginate, to maintain the spherical structure and cell–cell interactions of the follicle. Alginate, which has been used to support tissue growth for numerous applications,12 is an algae-derived polysaccharide polymer. Follicles cultured within hydrogels develop antral cavities and morphologically resemble in vivo grown follicles. To date, alginate-based culture systems support the consistent growth of mouse follicles,13–16 which has yielded healthy, fertile offspring.17 The growth of follicles from higher-order species, such as non-human primates and humans, is also supported.18–20 Furthermore, these culture systems have enabled mechanistic studies of follicle development, such as the influence of hormones, mechanics, and extracellular matrix proteins.14,15,21,22 While effective for secondary follicles, alginate-based culture systems have not translated to the culture of individual primordial and primary follicles.
The growth of primordial and primary follicles has been successful using in situ organ culture.23–25 Ovarian stromal cells provide structural support and participate in bidirectional paracrine signaling with the follicle,26–30 which has been linked to theca cell differentiation and primary follicle development. Initial coculture studies employing stromal and granulosa cells demonstrated enhanced follicle survival and growth.8,31,32 Recently, theca-interstitial cells (TICs)33 have been utilized to improve the survival and growth of follicles within alginate hydrogels.34 However, the isolation of these cells results in a heterogeneous population that may change throughout culture.
In this report, we investigated the coculture of ovarian follicles with mouse embryonic fibroblasts (MEFs) to provide factors that promote follicle survival and growth. MEFs, which are isolated from developing mouse embryos, are commonly used as feeder cells to promote the undifferentiated growth of embryonic stem (ES) or induced pluripotent stem cells.35 The use of MEFs overcomes the isolation and purification challenges associated with ovarian stromal cells. These cells are homogenous, easy to produce, and commercially available. Specifically, this study examined the ability of MEFs to support the survival and growth of early secondary (average diameter of 90–100 μm) and primary (average diameter of 70–80 μm) follicles in alginate hydrogels. Conditioned medium (CM) was employed as a control to distinguish between bidirectional and unidirectional paracrine signaling. In addition to survival and growth, the oocytes were analyzed for meiotic competence. These studies provide the foundation for identifying the factors that promote early follicle development, and may ultimately provide a mechanism to promote follicle growth in fertility preservation applications.
Materials and Methods
Animals and materials
Follicles were isolated from the ovaries of 7–10-day-old CD1 female mice. Mice were maintained in accordance with the policies of the National Institutes of Health and Northwestern University's Animal Care and Use Committee. A temperature-, humidity-, and light (12 h light/12 h dark)-controlled barrier facility within Northwestern University's Center of Comparative Medicine was used to house and breed the mice. Mice were provided with food and water ad libitum. The mice were fed Teklad Global irradiated chow (2919 or 2916) that does not contain soybean or alfalfa meal but does contain minimal phytoestrogens. Unless otherwise specified, all chemicals were purchased from Sigma-Aldrich and medium formulations from Invitrogen. Sodium alginate (55%–65% guluronic acid) was provided by FMC BioPolymers.
Follicle isolation, alginate encapsulation, and medium formulations
Previously established procedures14,15,22 for follicle isolation and alginate encapsulation were followed with slight modifications. Briefly, individual follicles (65–105 μm) were mechanically isolated using insulin gauge needles in dissection medium (Leibovitz's L-15 medium supplemented with 1% fetal bovine serum and 0.5% penicillin–streptomycin). Follicles were separated into four size classes based on average initial diameter (70, 80, 90, and 100 μm). After isolation, the follicles were washed and stored in maintenance medium (minimum essential medium Eagle–alpha modification [α-MEM] supplemented with 1% fetal bovine serum and 0.5% penicillin–streptomycin). Follicles were then transferred into 5–6 μL solutions of 0.25% alginate and immersed into a 50 mM CaCl2 and 140 mM NaCl solution for 1–2 min to crosslink. Beads were then placed into separate wells of a 96-well flat-bottom tissue culture plate (Corning Costar #3596) containing 100 μL growth medium (α-MEM supplemented with 3 mg/mL bovine serum albumin [MP Biomedicals], 1 mg/mL bovine fetuin, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, and 10 mIU/mL recombinant human follicle-stimulating hormone [FSH] [A.F. Parlow, NHPP and NIDDK]).
Mouse embryonic fibroblasts
Inactivated MEFs (Invitrogen, Gibco #S1520-100) were thawed and resuspended according to the company's protocol with minor modifications. In place of the suggested MEF culture medium (Dulbecco's Modified Eagle Medium [DMEM] with 10% FBS), follicle growth medium was used. A hemocytometer was used to determine cell concentration, and viability was determined to be ∼90% via trypan blue staining.
MEF coculture seeding concentration
Early secondary (average diameter of 90 and 100 μm) and primary (average diameter of 80 μm) follicles were initially cocultured with MEFs at various seeding concentrations (1.0–4.0×104 cells/well) (Supplementary Fig S1 and Supplementary Tables S1–S2; Supplementary Data are available online at www.liebertonline.com/tea). No trend in follicle survival and growth was observed; all concentrations were equally effective at enhancing the survival and growth of all follicles sizes. As a result, subsequent studies employed a concentration of 2.0×104 cells/well, which is the recommended seeding density).
Follicle and MEF coculture
MEFs were seeded at 2.0×104 viable cells/well in a 96-well flat-bottom culture plate (Corning Costar #3596). After 20 h of culture (overnight), the cells were washed once in 100 μL of PBS for 5 min and 100 μL of fresh growth medium was added to each well. After follicle isolation and encapsulation, individual alginate beads were placed into each well. The coculture was then conducted at 37°C in 5% CO2 for 14 days. Every 2 days, half of the medium (50 μL) was replaced with fresh growth medium.
MEF-conditioned medium
The MEF-CM was produced following previously established procedures for ES cell coculture with minor modifications.36 Briefly, MEFs were seeded at a concentration of 6.3×104 cells/cm2 in either 6- or 12-well plates. This concentration is equivalent to coculture (2.0×104 cells/well in a 96-well plate). After 20 h of culture (overnight), the cells were washed once in PBS for 5 min and fresh growth medium was added. The cells were then incubated at 37°C in 5% CO2. Every 2 days, the medium was collected and replaced. CM was collected for up to 1 week after initial plating of the cells. The CM was stored at −20°C for up to 1 month. Before use, CM was thawed at 4°C overnight and re-supplemented with 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, and 10 mIU/mL recombinant human FSH. MEF-CM was used in place of growth medium with the standard follicle culture procedures. Half the CM (50 μL) was replaced every other day.
Follicle survival and growth
Images of follicles were collected every other day using an inverted Leica DM light microscope (Leica). Follicle diameters were measured using ImageJ software (National Institutes of Health). An average of two perpendicular diameter measurements was used for each follicle at each time point. Survival was determined via follicle morphology. Follicles with fragmented, shrunken, degenerated (DG), or detached oocytes were considered not viable.
Oocyte meiotic competence
Follicles were removed from the alginate beads by incubating with 50 μL of α-MEM containing 10 IU/mL alginate lyase for 30 min. Next, follicles were incubated in α-MEM supplemented 10% fetal bovine serum, 5 ng/mL epidermal growth factor, and 1.5 IU/mL human chorionic gonadotropin for 14–16 h.37 Oocytes were then removed from the follicles using 0.3% hyaluronidase. Images were captured via light microscope (inverted Leica DM) to assess oocyte meiotic competence. Oocytes were classified as follows: germinal vesicle (GV) if the GV was present, GV breakdown (GVBD) if the GV was not present, metaphase II (MII) if there was GVBD and a polar body in the perivitelline space, or DG if the oocyte was fragmented or shrunken.
Initial coculture of primary follicles
Primary follicles with an initial average diameter of 80 μm were cultured with MEFs for 6 days and then removed from coculture. After 6 days of coculture, follicles encapsulated in alginate were transferred with forceps to culture wells containing 50 μL CM and 50 μL fresh growth medium. Every other day, half of the medium was replaced with fresh growth medium.
Statistical analysis
Coculture survival, growth, and meiotic competence measurements were conducted using 13 independent cultures of ∼40–80 follicles each. Similarly, the CM and control measurements were conducted using five and three independent cultures, respectively. Analysis was performed using Microsoft Excel and GraphPad Prism. Survival data were analyzed using the Kaplan–Meier log-rank test, and growth/meiotic competence data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey for single time point comparisons. A p-value of less than 0.05 was considered statistically significant.
Results
Survival and growth of cocultured follicles
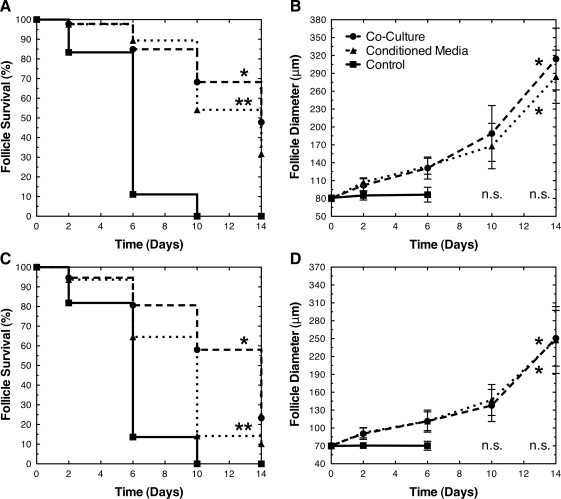
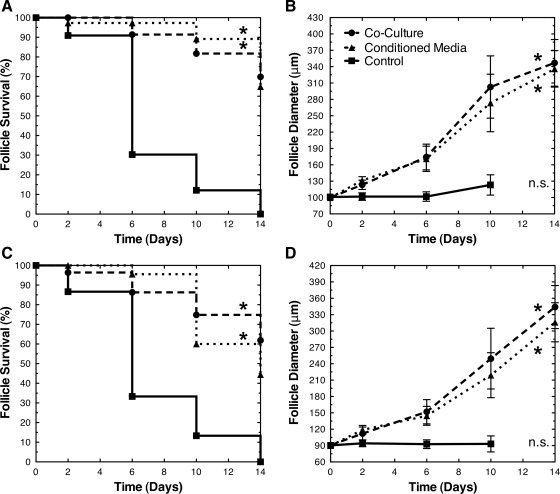
Coculture with MEFs enhanced the survival of early secondary (average diameter of 90 and 100 μm) and primary (average diameter of 70 and 80 μm) follicles (Figs. 1–3 and Table 1). The initial diameters (mean±standard deviation) of the cocultured follicles were 100±3, 91±3, 81±3, and 71±3 μm. The initial diameters of the cocultured and control follicle groups were not significantly different (p>0.05). After 14 days of coculture, 70% of 100 μm, 62% of 90 μm, 48% of 80 μm, and 23% of 70 μm follicles survived. None of the control early secondary follicles survived past day 10, and none of the control primary follicles survived past day 6. For all size classes, the survival of cocultured follicles was significantly greater than the control (p<0.001). In the control group, the oocytes were extruded from nonsurviving follicles; conversely, in coculture, the structure of growing follicles remained intact.
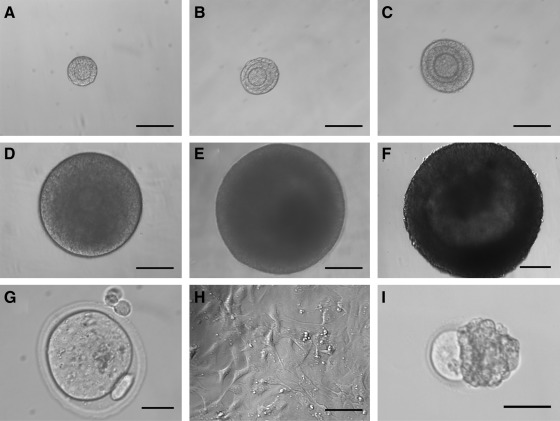
FIG. 1.
Follicle, oocyte, and mouse embryonic fibroblast (MEF) morphology. Representative images of encapsulated follicles in alginate hydrogels on (A) day 0, (B) day 2, (C) day 6, (D) day 10, and (E) day 14 of culture. Images of an (F) antral follicle after removal from alginate bead, (G) metaphase II (MII) egg, (H) MEFs at day 2 of culture, and (I) nonsurviving follicle with extruded oocyte from control group. Scale bars are (A–F, H) 100 μm, (G) 25 μm, and (I) 50 μm.
FIG. 3.
Primary follicle survival and growth. MEF coculture and CM enabled the survival and growth (diameter mean±standard deviation) of primary follicles: (A, B) 80 μm and (C, D) 70 μm. In the control group, there were no surviving (n.s.) follicles on days 10 and 14. Significant differences between survival curves and growth trajectories relative to control were indicated by asterisks (* and **, p<0.05). Survival curves labeled with different numbers of asterisks were significantly different (p<0.05). For panels (B) and (D), the diameters of follicles in coculture and CM were significantly greater than control on days 2 and 6 (p<0.05). The diameters of follicles in coculture and CM were not significantly different on days 6, 10, and 14 (p>0.05).
Table 1.
Follicle Number and Survival Versus Culture Time
| Follicle size (μm) | Method | Day 0, n | Day 2, n (%) | Day 6, n (%) | Day 10, n (%) | Day 14, n (%) |
|---|---|---|---|---|---|---|
| 100 | Coculture | 93 | 93 (100) | 85 (91) | 76 (82) | 65 (70) |
| CM | 37 | 36 (97) | 36 (97) | 33 (89) | 24 (65) | |
| Control | 33 | 30 (91) | 10 (30) | 4 (12) | 0 (0) | |
| 90 | Coculture | 139 | 134 (96) | 120 (86) | 104 (75) | 86 (62) |
| CM | 45 | 45 (100) | 43 (96) | 27 (60) | 20 (44) | |
| Control | 30 | 26 (87) | 10 (33) | 4 (13) | 0 (0) | |
| 80 | Coculture | 186 | 182 (98) | 158 (85) | 127 (68) | 89 (48) |
| CM | 85 | 83 (98) | 76 (89) | 46 (54) | 27 (32) | |
| Control | 36 | 30 (83) | 4 (11) | 0 (0) | 0 (0) | |
| 70 | Coculture | 150 | 142 (95) | 121 (81) | 87 (58) | 35 (23) |
| CM | 127 | 119 (94) | 82 (65) | 18 (14) | 13 (10) | |
| Control | 22 | 18 (82) | 3 (14) | 0 (0) | 0 (0) |
CM, conditioned medium.
The growth and development of early secondary and primary follicles was substantially enhanced by coculture with MEFs (Figs. 1–3 and Table 2). After 14 days of coculture, the 100±3, 91±3, 81±3, and 71±3 μm follicles increased in diameter to 347±43, 344±39, 314±52, and 251±47 μm, respectively. Surviving follicles in all size classes developed antral cavities by day 14. The growth of cocultured follicles was significantly greater than the control follicles on days 2, 6, and 10 (p<0.05). The control follicles reached diameters of only 123±19, 93±15, 86±12, and 70±8 μm, respectively, by days 6–10.
Table 2.
Growth of Surviving Follicles Versus Culture Time
| Follicle size (μm) | Method | Day 0 (μm) | Day 2 (μm) | Day 6 (μm) | Day 10 (μm) | Day 14 (μm) |
|---|---|---|---|---|---|---|
| 100 | Coculture | 100±3 | 123±9* | 174±24* | 303±57* | 347±43 |
| CM | 100±3 | 132±7** | 171±23* | 273±53* | 336±33 | |
| Control | 101±3 | 102±7 | 102±9 | 123±19 | n.s. | |
| 90 | Coculture | 91±3 | 112±12* | 152±22* | 249±56* | 344±39 |
| CM | 90±3 | 119±8** | 145±17* | 219±41* | 316±36 | |
| Control | 90±3 | 94±6 | 93±8 | 93±15 | n.s. | |
| 80 | Coculture | 81±3 | 102±11* | 131±19* | 189±47 | 314±52 |
| CM | 80±3 | 107±7** | 134±13* | 168±38 | 284±45 | |
| Control | 81±3 | 85±8 | 86±12 | n.s. | n.s. | |
| 70 | Coculture | 71±3 | 91±10* | 111±16* | 138±27 | 251±47 |
| CM | 70±3 | 92±9* | 112±19* | 147±26 | 248±56 | |
| Control | 70±4 | 71±4 | 70±8 | n.s. | n.s. |
Diameter presented as mean±standard deviation.
Significant differences for each follicle size and time point relative to control were indicated by asterisks (* and **, p<0.05). Diameters labeled with different numbers of asterisks were significantly different (p<0.05).
n.s., no surviving follicles.
Oocyte meiotic competence of cocultured follicles
MII eggs were obtained from early secondary and primary follicles after coculture with MEFs for 14 days (Table 3). The oocytes from surviving follicles with initial diameters in the range of 80–100 μm had significantly greater (p<0.01) MII rates (58%–69%) than the 70 μm follicles (41%). GVBD, GV, and DG rates ranged from 72% to 80%, 6% to 15%, and 9% to 17%, respectively. No significant difference was observed in GVBD, GV, or DG rates (p>0.05). GVBD and MII rates were consistent with previous studies, which reported 78%–88% GVBD and 56%–67% MII rates with secondary follicles (120 μm) cultured in alginate hydrogels.14
Table 3.
Oocyte Meiotic Competence
| Follicle size (μm) | Method | Follicles, n | Oocytes, n | MII, n (%)a | GVBD, n (%) | GV, n (%) | DG, n (%) |
|---|---|---|---|---|---|---|---|
| 100 | Coculture | 67 | 47 | 21 (58) | 36 (77) | 3 (6) | 8 (17) |
| CM | 35 | 23 | 8 (57) | 14 (61) | 3 (13) | 6 (26) | |
| 90 | Coculture | 91 | 56 | 29 (64) | 45 (80) | 6 (11) | 5 (9) |
| CM | 45 | 20 | 8 (57) | 14 (70) | 2 (10) | 4 (20) | |
| 80 | Coculture | 113 | 54 | 27 (69) | 39 (72) | 8 (15) | 7 (13) |
| CM | 69 | 22 | 7 (54) | 13 (59) | 4 (18) | 5 (23) | |
| 70 | Coculture | 94 | 22 | 7 (41) | 17 (77) | 3 (14) | 2 (9) |
| CM | 127 | 13 | 5 (63) | 8 (62) | 1 (8) | 4 (31) |
The percentage of MII eggs was calculated relative to GVBD oocytes.
DG, degenerated; GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II.
Survival, growth, and meiotic competence of follicles in CM
MEF-CM enabled the survival and growth of early secondary and primary follicles (Figs. 2 and 3; Tables 1 and 2). After 14 days of culture in CM, 65% of 100 μm, 44% of 90 μm, 32% of 80 μm, and 10% of 70 μm follicles survived. Across all follicle size classes, the survival of follicles in CM was significantly greater than the control (p<0.001). The initial diameters of the follicles in CM were 100±3, 90±3, 80±3, and 70±3 μm and were not significantly different (p>0.05) from the control or cocultured follicles. After 14 days of culture in CM, the follicles increased to 336±33, 316±36, 284±45, and 248±56 μm, respectively. The growth of follicles in CM was significantly greater than the control follicles on days 2, 6, and 10 (p<0.05). Furthermore, CM supported the production of MII eggs (Table 3). MII, GVBD, GV, and DG rates ranged from 54% to 63%, 59% to 70%, 8% to 18%, and 20% to 31%, respectively. No significant difference was observed in MII, GVBD, GV, or DG rates (p>0.05).
FIG. 2.
Early secondary follicle survival and growth. MEF coculture and conditioned media (CM) enabled the survival and growth (diameter mean±standard deviation) of early secondary follicles: (A, B) 100 μm and (C, D) 90 μm. In the control group, there were no surviving (n.s.) follicles on day 14. Significant differences between survival curves and growth trajectories relative to control were indicated by asterisks (*, p<0.05). For panels (B) and (D), the diameters of follicles in coculture and CM were significantly greater than control on days 2, 6, and 10 (p<0.001). The diameters of follicles in coculture and CM were not significantly different on days 6, 10, and 14 (p>0.05).
A comparison of coculture and CM indicated significant differences in the survival of primary follicles with an initial diameter of 80 μm (p<0.05) and 70 μm (p<0.001). No significant differences were observed in the survival of early secondary follicles (p>0.05). Furthermore, for all follicle size classes, growth was not significantly different between coculture and CM (p>0.05) on days 6, 10, and 14. Similarly, the meiotic competence rates (MII, GVBD, GV, and DG) between coculture and CM were not significantly different (p>0.05).
Growth medium supplemented with leukemia inhibitory factor (LIF) and/or basic-fibroblast growth factor (bFGF) was tested in an attempt to reproduce the effects of MEF-CM. These factors have been used to replace MEF feeder layers in ES cell culture.38–40 LIF and bFGF were tested individually and in combination at 100 ng/mL. The survival and growth of follicles receiving this treatment were not significantly different (p>0.05) from the control (data not shown).
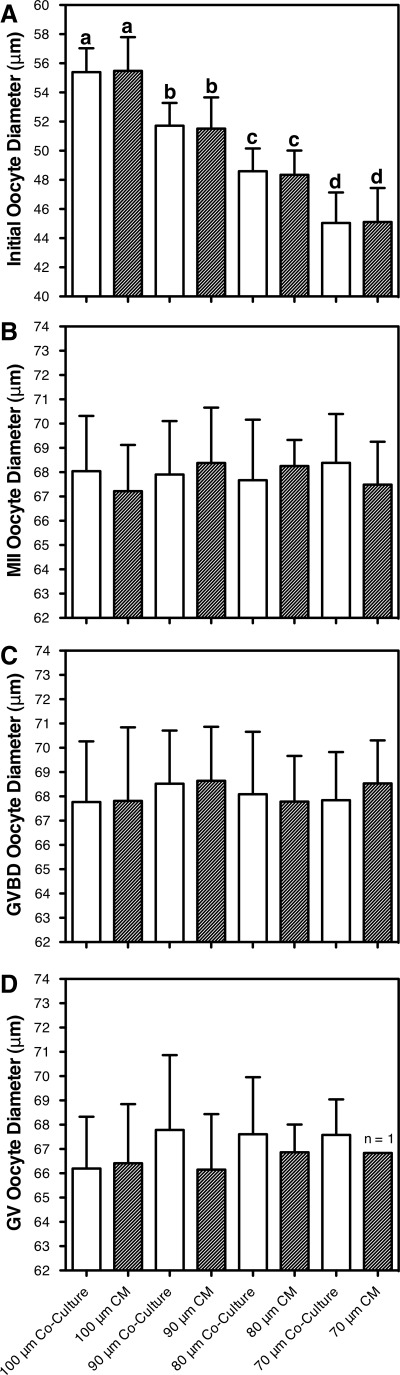
Oocyte growth
MEF coculture and CM promoted oocyte growth in early secondary and primary follicles (Fig. 4). The initial oocyte diameter (excluding zona pellucida) of follicles in MEF coculture ranged from 55±2 μm for 100 μm follicles to 45±2 μm for 70 μm follicles. Oocyte diameter was significantly different across follicle size classes (p<0.001). Within each follicle size class, no significant difference was observed between coculture and CM (p>0.05). Furthermore, terminal oocyte (MII, GVBD, and GV) diameter was measured (excluding polar body and zona pellucida) after in vitro maturation (day 15). For all follicle size classes, the terminal oocyte diameter (MII, GVBD, and GV) was significantly greater than the initial oocyte diameter (p<0.001). The terminal GV oocyte diameter ranged from 64 to 72 μm, which is consistent with previous reports that produced embryos and live births.14,17 No significant differences in the diameter of MII, GVBD, and GV oocytes were observed across follicle size classes (p>0.05). Furthermore, no significant differences in the diameter of MII, GVBD, and GV oocytes were observed between coculture and CM (p>0.05).
FIG. 4.
Initial and terminal oocyte size. MEF coculture and CM promoted oocyte growth in early secondary (90–100 μm) and primary (70–80 μm) follicles. (A) The initial (day 0) oocyte diameter (mean±standard deviation, excluding zona pellucida) was plotted for each follicle size class in coculture (white bars) and CM (gray bars). Each follicle size class had significantly different initial oocyte diameters (p<0.001). Bars labeled with different lower case letters (a–d) are significantly different. Within each follicle size class, no significant difference was observed between coculture and CM (p>0.05). After in vitro maturation (day 15), the diameter of (B) MII, (C) germinal vesicle breakdown (GVBD), and (D) GV oocytes was measured (excluding zona pellucida and polar bodies). For each follicle size class, the terminal oocyte diameter (MII, GVBD, and GV) was significantly greater than the initial oocyte diameter (p<0.001).
Initial coculture of primary follicles
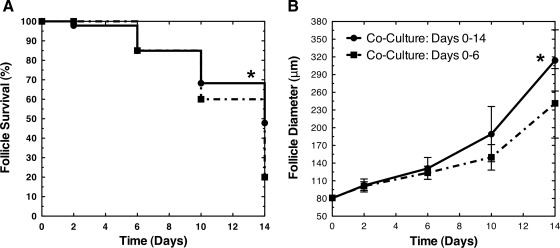
The effect of MEFs after the primary stage was investigated to assess the stage-specific influence of coculture. Previous reports demonstrated the growth of two-layer secondary follicles (120 μm in diameter) within alginate hydrogels without coculture.14,17 Primary follicles with an initial average diameter of 81±5 μm were cultured with MEFs for 6 days, removed from coculture, and then cultured without MEFs for an additional 8 days (Fig. 5). By day 6 of coculture, these follicles had a diameter of 124±11 μm. After 14 total days of culture (6 days of coculture and then 8 days without coculture), the follicles increased in diameter to 241±59 μm. On days 10 and 14, the growth of the initially cocultured follicles was significantly less (p<0.05) than the follicles that remained in coculture (314±52 μm). Moreover, the survival of the initially cocultured follicles (20%) was significantly reduced (p<0.05) relative to the follicles cocultured for 14 days (48%).
FIG. 5.
Initial coculture of primary follicles. Primary follicles have decreased (A) survival and (B) growth (diameter mean±standard deviation) if cocultured with MEFs for only 6 days compared to 14 days. Primary follicles with an average diameter of 80 μm were cultured with MEFs for 6 days, removed from coculture, and then cultured without MEFs for an additional 8 days. For panel (B), the follicle diameters of full (days 0–14) and initial (days 0–6) coculture were not significantly different on days 0, 2, and 6 (p>0.05). The diameters of full and initial coculture were significantly different on days 10 and 14 (p<0.05). Significant differences between survival curves and growth trajectories relative to control were indicated by asterisks (*, p<0.05).
Discussion
This study has demonstrated that MEFs, which have been traditionally used to promote the undifferentiated growth of ES cells, enable the culture of early secondary and primary mouse ovarian follicles within alginate hydrogels. These results build upon our previous study utilizing ovarian stromal or TICs,34 which enabled the culture of early secondary and late primary follicles with an initial average diameter of 90 μm. Therefore, nonovarian feeder cells, such as MEFs, can also be used to enhance the survival and growth of ovarian follicles. Moreover, the use of MEFs avoided the challenge of isolating and purifying ovarian stromal cells, which were inhomogeneous and variable between isolations. Conversely, MEFs are well characterized, homogenous, and widely used throughout the biotechnology industry.
The mechanism underlying MEF-stimulated follicle growth appears to be unidirectional paracrine signaling from the MEFs to the follicle, as both MEF coculture and CM enabled the culture of all follicle size classes. It remains to be determined if the MEF factors are the same produced in vivo by ovarian stromal cells. Surprisingly, various factors that influence ES cell differentiation are also critical in early follicle development. These factors include LIF,38,41 bFGF,29,39,40 kit ligand or stem cell factor,27,29 insulin-like growth factor,28,42 bone morphogenetic proteins,43,44 and activin A.45,46 While the mechanisms remain unclear, these factors have been implicated in primordial/primary follicle development, theca cell recruitment/differentiation, and granulosa cell proliferation. The supplement of these factors has not yet proven to be an effective means for the culture of isolated early-stage follicles. Nevertheless, in this study, LIF and bFGF were tested in an attempt to identify critical factors. These factors are widely used to replace MEF feeder layers and prevent ES cell differentiation.38–40 As reported, the addition of these factors did not improve the survival or growth of early secondary or primary follicles, which suggests that different or additional factors are necessary to support and stimulate follicle growth.
For primary follicles, the use of CM resulted in significantly lower survival than direct coculture. Decreased effectiveness of CM is also observed in ES cell culture.36 This difference may be related to the handling of the CM. The CM was frozen, thawed, and stored for up to 1 month, which could attenuate its effects. CM provides an initial concentration of factors that decline over time. In contrast, MEFs in direct coculture continuously secrete factors into the medium throughout the culture period.
Coculture promoted faster follicle growth than isolated follicle culture. Cocultured follicles grow from 120 to 300 μm in ∼8 days. Without coculture, 120 μm follicles require 12 days to reach 300 μm.14 Moreover, a decreased growth rate was observed after removing two-layer secondary follicles from coculture. The decreased growth rate of the follicles removed from coculture was similar to the growth rate of follicles without coculture. Therefore, in addition to promoting the development of primary follicles, coculture enhanced the growth rate of secondary follicles.
Follicle culture systems that utilize flat surfaces can support the growth of mouse primary follicles (80–90 μm) without coculture or CM.6 To culture these follicles, repeated physical manipulations are required to prevent cell attachment and migration away from the oocyte. In contrast, alginate-based culture systems must employ coculture to support the growth and survival of these follicles. Furthermore, primary follicle culture varies depending on species. For example, non-human primate follicles do not require coculture. These follicles survive and develop in alginate-fibrin hydrogels,16 whereas mouse primary follicles require additional factors. While the mechanisms remain unclear, the MEF paracrine factors stimulate mouse follicle growth, allowing the follicle to survive, transition to the secondary stage, and produce oocytes responsive to in vitro maturation.
In conclusion, we have demonstrated that MEFs enable the culture of early secondary and primary mouse ovarian follicles within alginate hydrogels. By employing MEF coculture or CM, primary follicles as small as 70 μm in average diameter can be cultured to the antral stage and matured to produce MII eggs. Hence, paracrine signaling between the MEFs and follicle was a major contributor to follicle survival and growth. This culture system may facilitate the identification of factors that promote early-stage follicle growth and lead to novel strategies for fertility preservation.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Ariella Shikanov and Dr. Min Xu for training, guidance, and discussion. This research was supported by grants from the National Institutes of Health (NIH) (Grant No. PL1EB008542) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) as part of the Specialized Co-operative Centers Program in Reproduction and Infertility Research (SCCPIR) (Grant No. U54HD41857). The content of this research is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NICHD.
Disclosure Statement
No competing financial interests exist.
References
- 1.Meirow D. Hardan I. Dor J. Fridman E. Elizur S. Ra'anani H. Slyusarevsky E. Amariglio N. Schiff E. Rechavi G. Nagler A. Ben Yehuda D. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 2.Jeruss J.S. Woodruff T.K. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eppig J.J. Mouse oocyte development in vitro with various culture systems. Dev Biol. 1977;60:371. doi: 10.1016/0012-1606(77)90135-x. [DOI] [PubMed] [Google Scholar]
- 4.Nayudu P.L. Osborn S.M. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 5.Boland N.I. Humpherson P.G. Leese H.J. Gosden R.G. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798. doi: 10.1095/biolreprod48.4.798. [DOI] [PubMed] [Google Scholar]
- 6.Lenie S. Cortvrindt R. Adriaenssens T. Smitz J. A reproducible two-step culture system for isolated primary mouse ovarian follicles as single functional units. Biol Reprod. 2004;71:1730. doi: 10.1095/biolreprod.104.028415. [DOI] [PubMed] [Google Scholar]
- 7.Abir R. Roizman P. Fisch B. Nitke S. Okon E. Orvieto R. Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod. 1999;14:1299. doi: 10.1093/humrep/14.5.1299. [DOI] [PubMed] [Google Scholar]
- 8.Itoh T. Hoshi H. Efficient isolation and long-term viability of bovine small preantral follicles in vitro. In Vitro Cell Dev Biol Anim. 2000;36:235. doi: 10.1290/1071-2690(2000)036<0235:EIALTV>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Saha S. Shimizu M. Geshi M. Izaike Y. In vitro culture of bovine preantral follicles. Anim Reprod Sci. 2000;63:27. doi: 10.1016/s0378-4320(00)00162-7. [DOI] [PubMed] [Google Scholar]
- 10.Muruvi W. Picton H.M. Rodway R.G. Joyce I.M. In vitro growth and differentiation of primary follicles isolated from cryopreserved sheep ovarian tissue. Anim Reprod Sci. 2009;112:36. doi: 10.1016/j.anireprosci.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Ting A.Y. Yeoman R.R. Lawson M.S. Zelinski M.B. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Augst A.D. Kong H.J. Mooney D.J. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 13.Pangas S.A. Saudye H. Shea L.D. Woodruff T.K. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 14.Xu M. West E. Shea L.D. Woodruff T.K. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 15.West E.R. Xu M. Woodruff T.K. Shea L.D. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shikanov A. Xu M. Woodruff T.K. Shea L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu M. Kreeger P.K. Shea L.D. Woodruff T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu M. West-Farrell E.R. Stouffer R.L. Shea L.D. Woodruff T.K. Zelinski M.B. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu M. Barrett S.L. West-Farrell E. Kondapalli L.A. Kiesewetter S.E. Shea L.D. Woodruff T.K. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J. Lawson M.S. Yeoman R.R. Pau K.Y. Barrett S.L. Zelinski M.B. Stouffer R.L. Secondary follicle growth and oocyte maturation during encapsulated three-dimensional culture in rhesus monkeys: effects of gonadotrophins, oxygen and fetuin. Hum Reprod. 2011;26:1061. doi: 10.1093/humrep/der049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreeger P.K. Fernandes N.N. Woodruff T.K. Shea L.D. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942. doi: 10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreeger P.K. Deck J.W. Woodruff T.K. Shea L.D. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eppig J.J. O'Brien M.J. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien M.J. Pendola J.K. Eppig J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682. doi: 10.1095/biolreprod.102.013029. [DOI] [PubMed] [Google Scholar]
- 25.Jin S.Y. Lei L. Shikanov A. Shea L.D. Woodruff T.K. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magoffin D.A. Magarelli P.C. Preantral follicles stimulate luteinizing hormone independent differentiation of ovarian theca-interstitial cells by an intrafollicular paracrine mechanism. Endocrine. 1995;3:107. doi: 10.1007/BF02990061. [DOI] [PubMed] [Google Scholar]
- 27.Parrott J.A. Skinner M.K. Kit ligand actions on ovarian stromal cells: effects on theca cell recruitment and steroid production. Mol Reprod Dev. 2000;55:55. doi: 10.1002/(SICI)1098-2795(200001)55:1<55::AID-MRD8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Huang C.T. Weitsman S.R. Dykes B.N. Magoffin D.A. Stem cell factor and insulin-like growth factor-I stimulate luteinizing hormone-independent differentiation of rat ovarian theca cells. Biol Reprod. 2001;64:451. doi: 10.1095/biolreprod64.2.451. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson E.E. Skinner M.K. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol. 2004;214:19. doi: 10.1016/j.mce.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Orisaka M. Tajima K. Mizutani T. Miyamoto K. Tsang B.K. Fukuda S. Yoshida Y. Kotsuji F. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol Reprod. 2006;75:734. doi: 10.1095/biolreprod.105.050344. [DOI] [PubMed] [Google Scholar]
- 31.Osborn S. Gook D. Stern K. Speirs A. The isolation and culture of human primordial follicles from fresh ovarian tissue. Hum Reprod. 1997;12-1:153. [Google Scholar]
- 32.Wu M.F. Huang W.T. Tsay C. Hsu H.F. Liu B.T. Chiou C.M. Yen S.C. Cheng S.P. Ju J.C. The stage-dependent inhibitory effect of porcine follicular cells on the development of preantral follicles. Anim Reprod Sci. 2002;73:73. doi: 10.1016/s0378-4320(02)00119-7. [DOI] [PubMed] [Google Scholar]
- 33.Magoffin D.A. Erickson G.F. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology. 1988;122:2345. doi: 10.1210/endo-122-5-2345. [DOI] [PubMed] [Google Scholar]
- 34.Tingen C.M. Kiesewetter S.E. Jozefik J. Thomas C. Tagler D. Shea L. Woodruff T.K. A macrophage and theca cell enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 36.Xu C. Inokuma M.S. Denham J. Golds K. Kundu P. Gold J.D. Carpenter M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 37.Smitz J. Cortvrindt R. Hu Y. Epidermal growth factor combined with recombinant human chorionic gonadotrophin improves meiotic progression in mouse follicle-enclosed oocyte culture. Hum Reprod. 1998;13:664. doi: 10.1093/humrep/13.3.664. [DOI] [PubMed] [Google Scholar]
- 38.Williams R.L. Hilton D.J. Pease S. Willson T.A. Stewart C.L. Gearing D.P. Wagner E.F. Metcalf D. Nicola N.A. Gough N.M. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 39.Xu C. Rosler E. Jiang J. Lebkowski J.S. Gold J.D. O'sullivan C. Delavan-Boorsma K. Mok M. Bronstein A. Carpenter M.K. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 40.Xu R.-H. Peck R.M. Li D.S. Feng X. Ludwig T. Thomson J.A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2:185. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson E.E. Kezele P. Skinner M.K. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188:65. doi: 10.1016/s0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 42.Bendall S.C. Stewart M.H. Menendez P. George D. Vijayaragavan K. Werbowetski-Ogilvie T. Ramos-Mejia V. Rouleau A. Yang J. Bossé M. Lajoie G. Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 43.Lee W.S. Otsuka F. Moore R.K. Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- 44.Ying Q.L. Nichols J. Chambers I. Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 45.Li R. Phillips D.M. Mather J.P. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136:849. doi: 10.1210/endo.136.3.7867593. [DOI] [PubMed] [Google Scholar]
- 46.Beattie G.M. Lopez A.D. Bucay N. Hinton A. Firpo M.T. King C.C. Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.