Abstract
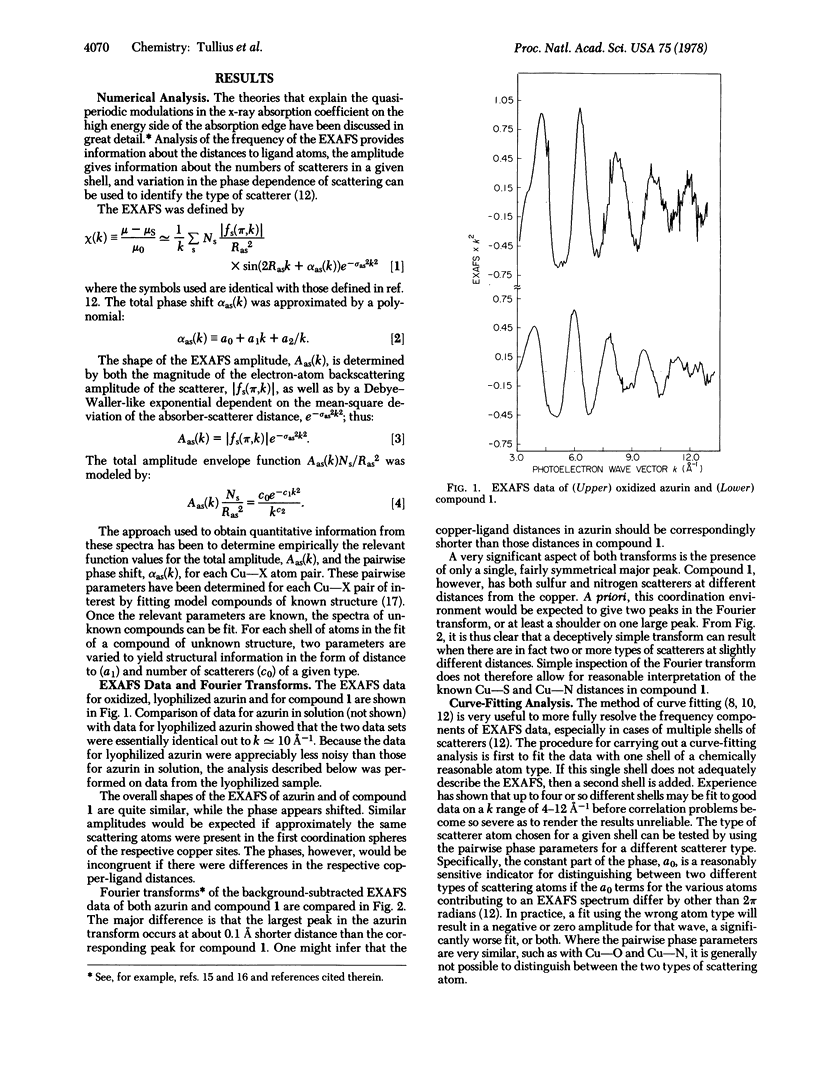
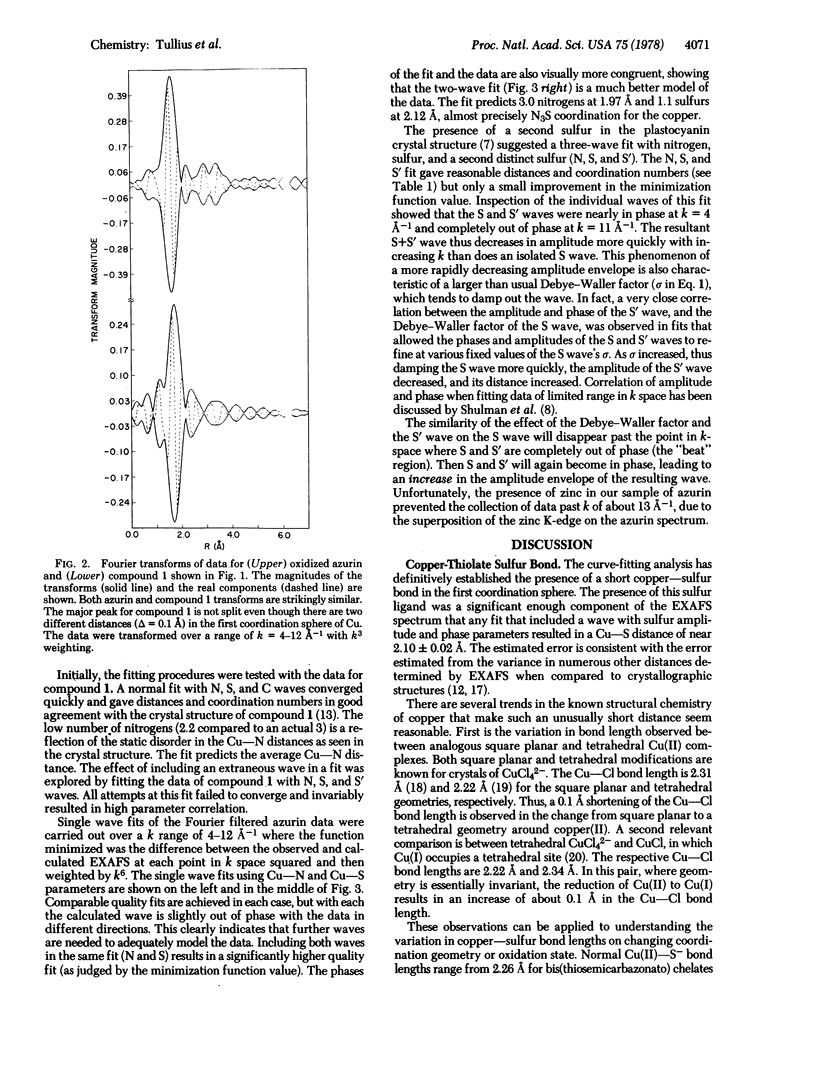
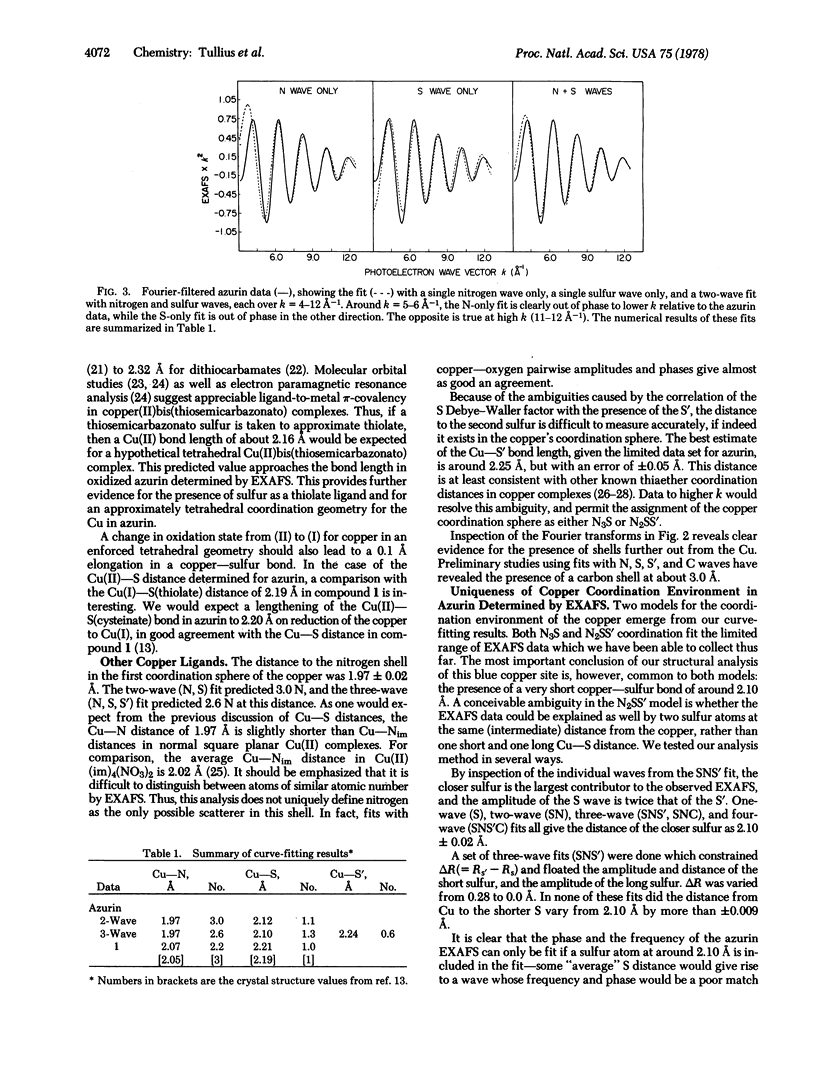
The primary coordination environment of the “blue” copper ion in oxidized azurin has been elucidated by x-ray absorption spectroscopy. The most striking feature is the unambiguous presence of a very short copper-sulfur distance at 2.10 ± 0.02 Å. Nitrogen ligands, presumed to be from imidazoles, are found at 1.97 Å. There is some evidence that the copper coordination sphere may be completed by a second sulfur, the distance of which is determined with much less certainty.
Keywords: x-ray absorption spectroscopy, type 1 copper
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie J. K., Fensom D. J., Freeman H. C., Woodcock E., Hill H. A., Stokes A. M. An NMR investigation of electron transfer in the copper-protein, plastocyanin. Biochim Biophys Acta. 1975 Sep 9;405(1):109–114. doi: 10.1016/0005-2795(75)90320-7. [DOI] [PubMed] [Google Scholar]
- Bunker B., Stern E. A. The iron-sulfur environment in rubredoxin. Biophys J. 1977 Sep;19(3):253–264. doi: 10.1016/S0006-3495(77)85585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger P., Shulman R. G., Kincaid B. M., Brown G. S., Ogawa S. Extended X-ray absorption fine structure determination of iron nitrogen distances in haemoglobin. Nature. 1978 Jul 6;274(5666):30–34. doi: 10.1038/274030a0. [DOI] [PubMed] [Google Scholar]
- Hare J. W., Solomon E. I., Gray H. B. Infrared spectral studies of metal binding effects on the secondary structure of bean plastocyanin. J Am Chem Soc. 1976 May 26;98(11):3205–3209. doi: 10.1021/ja00427a025. [DOI] [PubMed] [Google Scholar]
- Markley J. L., Ulrich E. L., Berg S. P., Krogmann D. W. Nuclear magnetic resonance studies of the copper binding sites of blue copper proteins: oxidized, reduced, and apoplastocyanin. Biochemistry. 1975 Oct 7;14(20):4428–4433. doi: 10.1021/bi00691a014. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Hare J. W., Gray H. B. Spectroscopic studies and a structural model for blue copper centers in proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1389–1393. doi: 10.1073/pnas.73.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. R., Glusker J. P., Gabe E. J., Minkin J. A. The crystal structure of the natitumor agent 3-ethoxy-2-oxobutyraldehyde bis(thiosemicarbazonato) Copper(II). Bioinorg Chem. 1974 Apr;3(3):189–205. doi: 10.1016/s0006-3061(00)80069-1. [DOI] [PubMed] [Google Scholar]
- Thompson J. S., Marks T. J., Ibers J. A. Blue copper proteins: Synthesis, spectra, and structures of CuN(3)(SR) and CuN(3)(SR) active site analogues. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3114–3118. doi: 10.1073/pnas.74.8.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Norton R. S., Allerhand A., Bersohn R. Studies of individual carbon sites of azurin from Pseudomonas aeruginosa by natural-abundance carbon-13 nuclear magnetic resonance spectroscopy. Biochemistry. 1977 Mar 8;16(5):886–894. doi: 10.1021/bi00624a012. [DOI] [PubMed] [Google Scholar]


