Abstract
Amniotic fluid holds great promise as a stem cell source, especially in neonatal applications where autologous cells can be isolated and used. This study examined chemical-mediated differentiation of amniotic fluid-derived stem cells (AFSC) into endothelial cells and verified the function of AFSC-derived endothelial cells (AFSC-EC). AFSC were isolated from amniotic fluid obtained from second trimester amnioreduction as part of therapeutic intervention from pregnancies affected with twin-twin transfusion syndrome. Undifferentiated AFSC were of normal karyotype with a subpopulation of cells positive for the embryonic stem cell marker SSEA4, hematopoietic stem cell marker c-kit, and mesenchymal stem cell markers CD29, CD44, CD73, CD90, and CD105. Additionally, these cells were negative for the endothelial marker CD31 and hematopoietic differentiation marker CD45. AFSC were cultured in endothelial growth media with concentrations of vascular endothelial growth factor (VEGF) ranging from 1 to 100 ng/mL. After 2 weeks, AFSC-EC expressed von Willebrand factor, endothelial nitric oxide synthase, CD31, VE-cadherin, and VEGF receptor 2. Additionally, the percentage of cells expressing CD31 was positively correlated with VEGF concentration up to 50 ng/mL, with no increase at higher concentrations. AFSC-EC showed a decrease in stem cells markers c-kit and SSEA4 and were morphologically similar to human umbilical vein endothelial cells (HUVEC). In functional assays, AFSC-EC formed networks and metabolized acetylated low-density lipoprotein, also characteristic of HUVEC. Nitrate levels for AFSC-EC, an indirect measure of nitric oxide synthesis, were significantly higher than undifferentiated controls and significantly lower than HUVEC. These results indicate that AFSC can differentiate into functional endothelial-like cells and may have the potential to provide vascularization for constructs used in regenerative medicine strategies.
Introduction
Human amniotic fluid-derived stem cells (AFSC) offer distinct advantages for use in the field of regenerative medicine when compared with other stem and progenitor cell types. AFSC have been shown to express markers characteristic of both embryonic stem cells (ESC) and mesenchymal stem cells (MSC)1,2 and have the ability to differentiate across all three germ layers, including cardiovascular lineages,2–4 while maintaining the nontumor forming properties of adult stem cells.5,6 Additionally, AFSC are capable of maintaining prolonged undifferentiated proliferation at rates similar to ESC.2,7
AFSC lack a unique surface antigen that can be used for positive selection; one strategy for the enrichment of AFSC is based on the adherence of cells to plastic dishes within 24 to 48 h.1,8 This subpopulation can be further enriched by immunological selection based on expression of the membrane receptor c-kit, which is present in 1.0% to 5.0% of total cells in amniotic fluid (AF) and placenta.1,2,7 In bone marrow-derived MSC, c-kit+ selection has been shown to be a marker of cardiovascular progenitor cells and also serves to remove mature cells from the adherent population.9 The use of growth factors, such as vascular endothelial growth factor (VEGF), to induce chemical-mediated endothelial differentiation of ESC and MSC is well characterized.10–12 In these stem cell populations, incubation with VEGF at concentrations of 50 ng/mL or higher has been shown to trigger production of proteins constitutively expressed in endothelial cells, such as von Willebrand factor (vWF),12–15 endothelial nitric oxide synthase (eNOS),14,15 PECAM-1/CD31,14,16,17 vascular endothelial cadherin (VE-cadherin),12,14,16 and VEGF receptor 2 (VEGFR2/KDR/Flk-1)12,16 and acquisition of functional markers characteristic of endothelial cells, such as uptake of acetylated low density lipoproteins (ac-LDL)16,17 and network formation when plated on Matrigel.12,14–16 While several groups have reported that AFSC exposure to similar VEGF concentrations results in certain endothelial phenotypes,18,19 the functionality of endothelial cells differentiated from human AFSC compared to a primary mature cell source, such as human umbilical vein endothelial cells (HUVEC), has yet to be thoroughly documented.
To determine whether AFSC were capable of differentiation into functional endothelial cells, we cultured c-kit+ human AFSC in an endothelial growth medium with supplemental VEGF. We then quantified expression of the endothelial specific proteins vWF, eNOS, CD31, VE-cadherin, and VEGFR2, assessed morphological changes, evaluated the loss of stem cell specific markers postdifferentiation, and evaluated cell function through network formation, acetylated-LDL uptake, VEGF basal levels, and nitric oxide production.
Materials and Methods
Isolation of human AFSC
Primary human AF was obtained from patients in their second trimester undergoing planned amnioreduction as part of a therapeutic treatment for twin-twin transfusion syndrome (TTTS). Collection from TTTS cases provides at least an eightfold increase in AF per patient compared with routine amniocentesis while maintaining a cell population with a normal karyotype. The experimental protocol and informed consent forms were approved by the Institutional Review Boards of Baylor College of Medicine and Rice University. Isolation of AFSC was modified based on previously published studies.1,8,20 AF was centrifuged at 1200 rpm for 10 min, and collected cells were plated at 2500 cells/cm2 on standard plastic Petri dishes and cultured in a modified α-Minimum Essential Media: 63% αMEM (Invitrogen, Carlsbad, CA), 18% Chang Basal Medium (Irvine Scientific, Santa Ana, CA), 2% Chang C supplement (Irvine Scientific), 15% fetal bovine serum (PAA Laboratories, Dartmouth, MA), and GlutaMAX (Invitrogen) at 37°C and 5% CO2 in a humidified environment. Media was changed every 2–3 days, and cells were passaged at 60%–70% confluence. At the first passage, a subpopulation of progenitor cells was isolated through fluorescence-activated cell sorting for expression of the membrane receptor CD117/c-kit (BD Biosciences, Bedford, MA). Cell colonies were detached into single cells (Accutase; Sigma-ALdrich, St. Louis, MO; 37°C, 10 min), and c-kit+ cells were collected using a Dako MoFlo sterile cell sorter.
Analysis of undifferentiated, C-kit+ AF Cells
Standard G-banding karyotyping was performed on undifferentiated AFSC at passage 5. Additionally, flow cytometry using a BD LSR II Flow Cyotmeter was performed to evaluate the expression of the embryonic stem cell marker SSEA4, hematopoietic stem cell marker c-kit, mesenchymal stem cell markers CD29, CD44, CD73, CD90, and CD105, endothelial marker CD31, and hematopoietic differentiation marker CD45. All flow cytometry antibodies and corresponding isotype controls were purchased from BD Biosciences, specific to human proteins, and used at manufacturer recommended concentrations. FACSDiva software (BD Biosciences) was used for all flow cytometry data collection. FlowJo software (Tree Star, Inc., Ashland, OR) was used for data analysis.
Endothelial differentiation of AFSC
C-kit+ AFSC at passage 4 were plated on gelatin coated 12-well plates at a density of 3000 cells/cm2, allowed to attach for 24 h in the modified αMEM, then cultured in Endothelial Growth Media 2 (EGM-2; Lonza, Walkersville, MD) with a total concentration of either 1, 5, 10, 25, 50, or 100 ng/mL VEGF (VEGF165, Pierce Biotechnology, Rockford, IL). EGM-2 contained epidermal growth factor, hydrocortisone, GA-1000 (gentamicin, amphotericin-b), fetal bovine serum, basic fibroblast growth factor, insulin-like growth factor, ascorbic acid, and heparin at manufacturer concentrations. All VEGF concentrations are final concentrations and not in addition to the 2 ng/mL supplied by Lonza.
Media was changed every 2–3 days, and the degree of differentiation was assessed after 14 days. HUVEC (courtesy of Nancy Turner, Rice University) cultured in EGM-2 without supplemental VEGF were used as a positive control while c-kit+ AFSC in modified αMEM were used as a negative control. Both controls were analyzed at passage 4.
Immunostaining
To observe the presence and localization of endothelial-like cells, the differentiated AFSC population was fixed with 4% paraformaldehyde (Alfa Aesar, Ward Hill, MA), permeabilized with Triton ×100 (CalBioChem, San Diego, CA), blocked with 1% bovine serum albumin (BSA; EMD Chemicals, Gibbstown, NJ) for 1 hr at 25°C, and stained with antibodies against human vWF, (V8 Protease Fragment II; QED Bioscience Inc., San Diego, CA), eNOS (Abcam Inc., Cambridge, MA), VEGF receptor 2 (VEGFR2, Sigma-Aldrich), and VE-cadherin (BD Bioscience). Primary antibodies were used at a concentration of 1:100 overnight at 4°C, with secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted to 1:800 used for 30 min at 25°C. Cells were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) with Vecta Shield (Vector, Burlingame, CA). Undifferentiated AFSC and differentiated AFSC incubated with secondary antibodies alone served as negative controls, while HUVEC served as positive controls. Images were obtained using a DMI 6000B (Lieca Microsystems, Bannockburn, IL) fluorescence microscope.
Morphology
ImageJ was used to process phase contrast images through thresholding and calculate the circularity index, computed as 4π*area/(perimeter)2, of each in-frame cell.
Postdifferentiation flow cytometry
Using the flow cytometry protocol and equipment previously described, differentiated AFSC were detached into single cells and stained with fluorescently conjugated antibodies. Targets and their respective isotype controls were as follows: CD31 (FITC IgG1κ), VE-cadherin (PE IgG1κ), VEGFR2 (AF647 IgG1), SSEA4 (PE IgG3κ), c-kit (PE IgG1κ). All flow cytometry antibodies and corresponding isotype controls were purchased from BD Biosciences, specific to human proteins, and used at manufacturer recommended concentrations.
Western blotting
Western blot antibodies were purchased from Abcam Inc., electrophoresis and transfer materials were purchased from Bio-Rad (Hercules, CA), and developing materials were purchased from Thermo Scientific. After 14 days of differentiation, total protein lysates were isolated from AFSC and analyzed using a bicinchoninic acid kit (BCA; Thermo Scientific, Rockford, IL). Extracts were denatured using β-mercaptoethanol and boiling for 5 min, then diluted to equal concentrations of total protein. The samples were electrophoresed by 0.1% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose membranes at 100 V for 1.5 and 1.0 h, respectively. Membranes were washed in tris-buffered saline with 0.05% Tween-20 (TBST), then blocked with 5% nonfat milk in TBST to reduced nonspecific binding. Membranes were incubated overnight at 4°C with rabbit polyclonal antibodies against eNOS (1:300 dilution in TBST) and mouse monoclonal antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000 dilution in TBST). Membranes were washed and incubated for 30 min at 25°C with secondary antibodies conjugated to horseradish peroxidase (HRP) at a dilution of 1:1000. A 1 min Luminol reagent exposure was used to provide chemiluminescence, and images were developed using high-sensitivity x-ray film. Western blots were normalized to GAPDH expression. Western blot analyze was performed using Image J (NIH, Bethesda, MD).
Functional analysis of differentiated AFSC
Network formation on Matrigel
The potential for differentiated AFSC to form networks was assessed using Growth Factor Reduced Matrigel Matrix (BD Biosciences) according to the manufacturer's instructions. Briefly, Matrigel was allowed to thaw on ice overnight, and then added to culture plates at a concentration of 50 μL/cm2. The coated plates were then incubated at 37°C for at least 30 min to allow for solidification. AFSC cultured for 14 days in EGM-2 supplemented with VEGF were dissociated with trypsin (Thermo Scientific), replated at 7500 cells/cm2 on the Matrigel substrate, and incubated at 37°C for 24 h. Network formation was assessed using standard light microscopy and the percentage of cells connected was determined by thresholding using ImageJ, then manually counting cells that were constituents of networks. In all functional assays, samples were compared to undifferentiated AFSC and HUVEC under the same protocols.
Ac-LDL uptake
Endothelial function was measured in terms of uptake of acetylated low density lipoprotein (ac-LDL). AFSC cultured for 14 days in EGM-2 supplemented with VEGF were incubated with 10 μg/mL Alexa Fluor 488 conjugated ac-LDL (Invitrogen) for 4 h, then fixed with 4% paraformaldehyde and counterstained with DAPI. For statistical analysis, in-frame cells were manually scored in terms of degree of metabolized ac-LDL and categorized as either negligible fluorescence, marginal to moderate fluorescence, or strong fluorescence (similar to HUVEC). The percentage of cells in each category was averaged across multiple frames, and then compared by cell type.
VEGF production
Basal levels of VEGF production were assessed using a human VEGF-specific enzyme-linked immunosorbent assay (Quantikine kit, R&D Systems, Inc., Minneapolis, MN). Cells were rinsed and incubated in EGM-2 without supplemental VEGF for 48 h. According to manufacturer protocols, cell culture supernatants were collected and centrifuged, then incubated with HRP-conjugated antibody against VEGF for 2 h. A substrate solution was added to the wells and color developed in proportion to the amount of bound VEGF. The optical density of each well was determined at 450 nm, with a correction wavelength of 540 nm, using an Infinite M1000 PRO microplate reader (Tecan, Männedorf, Switzerland).
Nitric oxide production
The Measure-iT High-Sensitivity Nitrite Assay Kit (Invitrogen) was used according to manufacturer recommendations to provide a surrogate for detection of nitric oxide. Briefly, the total byproduct of nitric oxide was measured in the form of nitrites after conversion of nitrates via nitrate reductase. Total nitrite levels for differentiated AFSC were calculated based on a nitrite calibration curve, then normalized to total protein levels for each sample.
Statistical analysis
Data are expressed as mean±standard deviation. The sample numbers for each experiment are represented in their respective figures. Analysis of variance analysis followed by a post-hoc student t-test with a Dunn–Bonferroni correction for multiple comparisons was performed for all comparisons. A value of p<0.05 was considered significant in all tests.
Results
Isolation and characterization of AFSC
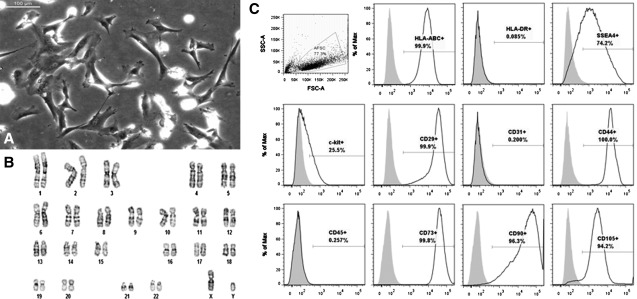
Adherent AFSC were successfully isolated from 100 mL samples of AF and had a fibroblast-like, spindle-shaped morphology similar to that of MSC (Fig. 1A). Standard G-banding karyotyping revealed a normal diploid male karyotype in 18/20 metaphase spreads. The remaining 2 spreads showed a tetraploid karyotype (Fig. 1B).
FIG. 1.
Characterization of undifferentiated AFSC. AFSC were sorted for c-kit+ cells at passage 2, cultured to passage 5, and analyzed. (A) Light microscopy reveals a spindle-shaped morphology similar to MSC. Scale bar is 100 μm. (B) Standard G-banding karyotyping of AFSC shows a normal diploid male karyotype. (C) FACS analysis reveals expression of immunological marker HLA-ABC, but not HLA-DR, expression of embryonic and mesenchymal stem cell markers SSEA4, c-kit, CD29, CD44, CD73, CD90, and CD105, and no significant expression of endothelial and hematopoietic differentiation markers. AFSC, amniotic fluid-derived stem cells; MSC, mesenchymal stem cells; FACS, fluorescence activated cell sorting.
Flow cytometry on undifferentiated AFSC, sorted for c-kit+ at passage 2 and then cultured through passage 5, showed the cells were strongly positive for the ESC marker SSEA4, MSC markers CD29, CD44, CD73, CD90, and CD105, and the immunological marker HLA-ABC, while a subpopulation (25.5%) of these cells expressed c-kit. Additionally, AFSC were negative for the immunological marker HLA-DR, the hematopoietic differentiation marker CD45, and the endothelial marker PECAM-1/CD31 (Fig. 1C). These results are comparable to those of other groups.1,2,18,19
Differentiation of AFSC into endothelial-like cells
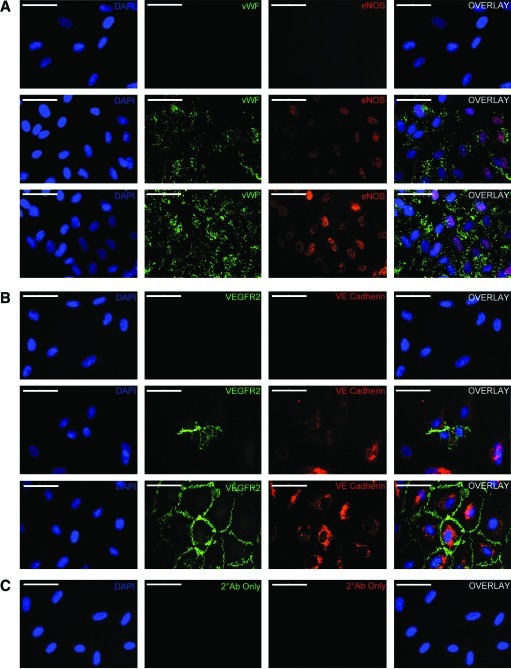
Differentiation of AFSC into endothelial-like cells (AFSC-EC) was induced by incubating AFSC in the presence of VEGF concentrations of 1, 5, 10, 25, 50, or 100 ng/mL. Immunofluorescence showed protein staining and localization characteristic of endothelial cells. Undifferentiated AFSC showed no specific staining for vWF, eNOS, VEGRF2, or VE-cadherin, while AFSC after incubation in 50 ng/mL VEGF for 2 weeks had significantly enhanced expression of each of these proteins (Fig. 2A, B). Staining localization in differentiated AFSC was similar to that of HUVEC, though the percentage of cells expressing these markers, and therefore overall intensity, was reduced. Incubation of AFSC-EC with secondary antibodies alone resulted in negligible fluorescence (Fig. 2C).
FIG. 2.
Protein localization within AFSC-derived endothelial cells. (A) Immunostaining for von Willebrand factor (green) and endothelial nitric oxide synthase (red) and (B) VEGFR2 (green) and VE-cadherin (red) in (from top to bottom) undifferentiated AFSC, AFSC-EC after 2 weeks of culture at 50 ng/mL VEGF, and HUVEC controls. (C) Images taken of AFSC-EC at identical exposures without primary antibodies, as negative controls. Nuclei counterstained with DAPI (blue), overlap shown at right. Scale bars are 100 μm. AFSC-EC, AFSC-derived endothelial cells; VEGF, vascular endothelial growth factor; HUVEC, human umbilical vein endothelial cells; DAPI, 4′,6-diamidino-2-phenylindole; VE-Cadherin, vascular endothelial cadherin; VEGFR2, vascular endothelial growth factor receptor 2. Color images available online at www.liebertonline.com/tea
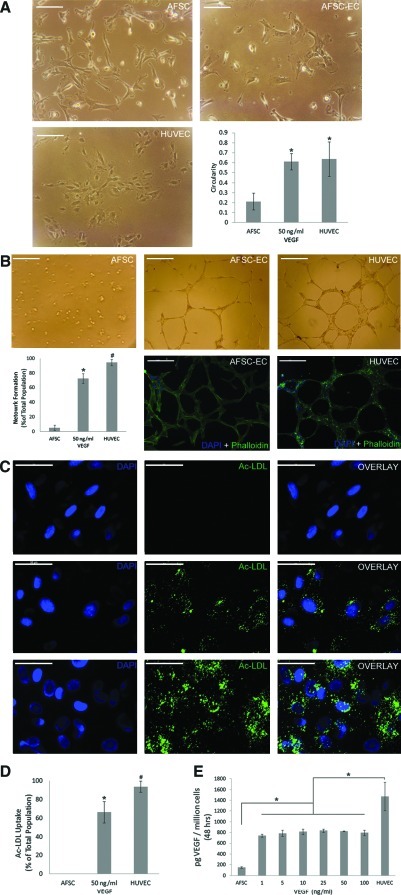
Based on a circularity index between 0 and 1, AFSC-EC morphology after 14 days was significantly different when compared with undifferentiated AFSC (n=10 frames; 0.61±0.08 vs 0.21±0.08, respectively; p<0.001) and similar to that of HUVEC (0.64±0.17) (Fig. 3A).
FIG. 3.
Functional analysis of AFSC-derived endothelial cells. (A) Morphological analysis of AFSC, AFSC-EC differentiated at 50 ng/mL VEGF for 14 days, and HUVEC based on a circularity index between 0 and 1 (n=10 frames; 0.21±0.08, 0.61±0.08, 0.64±0.17, respectively). *p<0.001 compared with AFSC. Scale bars are 100μm. (B) Assessment of network formation by AFSC, AFSC-EC, and HUVEC on Matrigel (n=10 frames; 5.2%±3.6%, 72.6%±6.8%, 94.5%±4.3%, respectively; *p<0.001 vs AFSC, #p<0.001 vs AFSC-EC). Confocal fluorescence imaging of phalloidin (green) counterstained with DAPI (blue). Scale bars are 200 μm. (C, D) Fluorescence imaging of acetylated low-density lipoprotein (green) and percentage of AFSC, AFSC-EC, and HUVEC metabolizing ac-LDL (n=10 frames; 0.02%±0.04%, 66.5%±11.4%, 93.7±6.0, respectively; *p<0.001 vs AFSC, #p<0.001 vs AFSC-EC). Nuclei counterstained with DAPI (blue), overlap at right. Scale bars are 50 μm. (E) Basal VEGF expression (pg/106 cells) over 48 h by AFSC (n=5; 151±14), AFSC-EC across all VEGF incubation concentrations (742±28, 787±58, 815±52, 836±33, 828±7, 796±51), and HUVEC (1470±267). *p<0.005 compared with AFSC-EC across all concentrations. Error bars are±SD. Color images available online at www.liebertonline.com/tea
The ability to form interconnected networks was assessed by plating undifferentiated AFSC, differentiated AFSC cultured in 50 ng/mL VEGF, and HUVEC on Matrigel thin films. Few undifferentiated AFSC showed network formation (n=10 frames; 5.2%±3.6%) and the vast majority of the cells maintained a rounded morphology. After differentiation, significantly more AFSC-EC formed networks (72.6%±6.8%; p<0.001), though less than HUVEC controls (94.5%±4.3%; p<0.001) (Fig. 3B).
Functional characterization was also performed by assessing uptake of AF488-conjugated ac-LDL. Undifferentiated AFSC showed little to no phagocytosis of ac-LDL (n=10 frames; 0.02%±0.04%), while AFSC after incubation in 50 ng/mL VEGF after 2 weeks showed a varying degree of ac-LDL uptake. A majority of the total population of AFSC-EC showed some degree of ac-LDL uptake (64.5%±10.9%; p<0.001 vs AFSC), whereas a smaller, yet still significant population (43.9%±7.1%; p<0.001 vs AFSC) showed similar ac-LDL uptake patterns to those of the HUVEC positive control (93.7±6.0)(Fig. 3C, D).
The levels of basal VEGF expression (pg/106 cells) over 48 h by AFSC-EC across all VEGF incubation concentrations (n=5; 742±28, 787±58, 815±52, 836±33, 828±7, 796±51) was significantly greater than undifferentiated AFSC (151±14; p<0.005 for all conditions) and significantly lower than HUVEC controls (1470±267; p<0.005 for all conditions) (Fig. 3E).
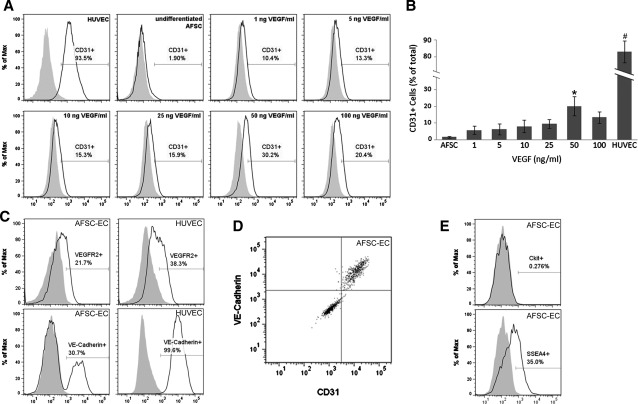
Quantitative flow cytometry of CD31 expression compared to VEGF concentration in culture conditions showed a positive correlation. Undifferentiated AFSC had a minimal CD31 expressing cells (n=5 with 5×105 cells/run; 1.6%±0.6%), whereas AFSC-EC showed increased CD31 expression at VEGF incubation concentrations of 1, 5, 10, 25, 50, and 100 ng/mL after 2 weeks (5.8%±2.5%, 6.2%±3.3%, 8.1%±3.8%, 9.6%±2.7%, 22.6%±7.4%, 13.5%±3.4%, respectively) (Fig. 4A, B). AFSC-EC incubated with 50 ng/mL VEGF displayed significantly increased CD31 expression compared with AFSC (p<0.05), and decreased compared with HUVEC (85.8%±6.7%; p<0.001 vs AFSC-EC). Similarly, the percentage of VE-cadherin and VEGFR2 positive AFSC-EC at 50 ng/mL VEGF (n=1 run with 5×105 cells; 21.7% and 30.7%, respectively), were higher than those of AFSC (0.9% and 1.2%) and lower than those of HUVEC (38.3% and 99.6%) (Fig. 4C), and a subpopulation of AFSC-EC that was triple positive for CD31, VE-cadherin, and VEGFR2 expression were identified (8.9% of total population) (Fig. 4D). AFSC-EC also showed a decrease in expression of the stem cell markers c-kit and SSEA4 compared with undifferentiated AFSC (0.3% and 35.0%, respectively, down from 25.5% and 74.2%) (Fig. 4E).
FIG. 4.
Flow cytometry analysis of AFSC-derived endothelial cells. (A, B) CD31 expression in AFSC, AFSC-EC across all VEGF concentrations, and HUVEC shown as percentage increase versus isotype controls (n=5 with 5×105 cells/run; 1.6%±0.6%; 5.8%±2.5%, 6.2%±3.3%, 8.1%±3.8%, 9.6%±2.7%, 22.6%±7.4%, 13.5%±3.4%; 85.8%±6.7%; *p<0.05 vs AFSC, #p<0.001 vs AFSC-EC). (C) VE-cadherin and VEGFR2 expression in AFSC-EC at 50 ng/mL VEGF (n=1 run with 5×105 cells; 21.7% and 30.7%, respectively) and HUVEC (38.3% and 99.6%). (D) Subpopulation of AFSC-EC at 50 ng/mL VEGF triple positive for CD31, VE-cadherin, and VEGFR2 expression (8.94% of total population). (E) Reduced expression of stem cell markers c-kit and SSEA4 postdifferentiation in AFSC-EC (0.3% and 35.0%, respectively). Error bars are±SD. CD31, cluster of differentiation 31.
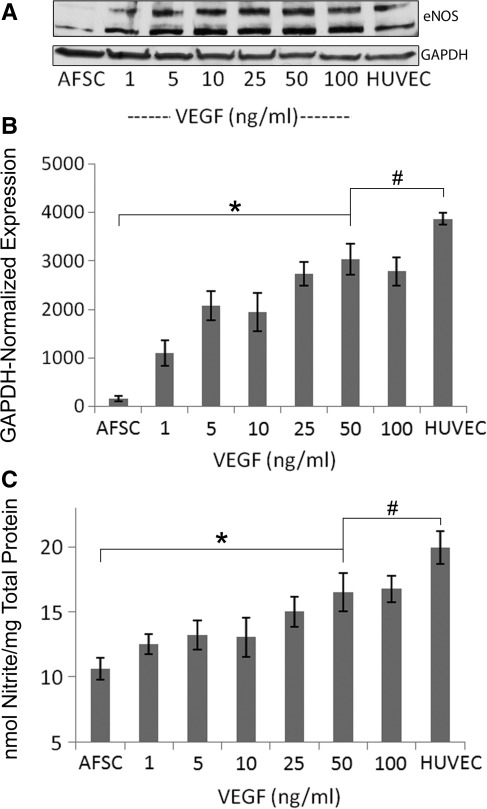
ENOS expression in AFSC-EC at 50 ng/mL VEGF was significantly greater than in undifferentiated AFSC controls (n=5; fluorescence intensities of 3000±320 and 157±61, respectively, normalize to GAPDH; p<0.001), although significantly less than HUVEC controls (3870±130; p<0.05). The expression of eNOS significantly correlates with VEGF concentration (Fig. 5A, B). Note also the double-banding pattern present in HUVEC controls and AFSC-EC (∼130 kDa and 110 kDa). This pattern has been previously observed in other studies21,22 and possibly results from alternative splicing or degradation. Correspondingly, eNOS activity of AFSC-EC at 50 ng/mL VEGF, analyzed through measurement of nitrite levels normalized to total protein concentration, was significantly greater than in undifferentiated AFSC controls (17.33±1.44 vs 9.88±0.82 nmol/mg; p<001), and significantly less than HUVEC controls (19.81±1.25 nmol/mg; p<0.05) (Fig. 5C). Agreement between eNOS production, the formation of nitric oxide, and CD31 expression confirms a correlation between VEGF concentration and acquisition of endothelial-like properties by differentiated AFSC.
FIG. 5.
Analysis of nitric oxide synthesis in AFSC-derived endothelial cells. (A) Western blot analysis of endothelial nitric oxide synthase expression in AFSC, AFSC-EC, and HUVEC (B) GAPDH-normalized expression of eNOS for AFSC, AFSC-EC at 50 ng/mL, and HUVEC (n=5; 157±61, 3000±320, 3870±130; *p<0.001, #p<0.05) (C) Nitrate levels of AFSC, AFSC-EC at 50 ng/mL, and HUVEC normalized to total protein concentration (n=5; 9.88±0.82 nmol nitrite/mg, 17.33±1.44 nmol nitrite/mg, and 19.81±1.25 nmol/mg; *p<0.001, #p<0.05). Error bars are±SD. GAPDH - glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Broad potential for differentiation, a high rate of proliferation, and the ease of isolation make AFSC well suited for use in autologous or allogeneic regenerative medicine therapies.23–25 This study illustrates the capability of c-kit+ AFSC to differentiate into endothelial-like cells on both the protein expression and cell function levels. Growth factor induced-differentiation of AFSC was augmented by supplementation of VEGF in EGM-2, and a plateau in the acquisition of endothelial-like characteristics was determined to occur between 25 and 50 ng/mL VEGF over the course of 2 weeks. These findings are consistent with recent literature that has examined VEGF-induced differentiation of embryonic and MSC.10–15
Several distinct subpopulations of AF-derived cells express mulitpotency markers, but c-kit+ AFSC have been consistently shown to have the potential to differentiate into endothelial-like cells.1,2,7 This immunoselection method is effective at deriving stem cell-like AFSC from the initial heterogeneous cell population. However, this technique is limited in its clinical application due to the use of xenogenic antibodies. While the method used in this paper is appropriate for the current investigation, isolation without xeno-immunogenic substances would be necessary to make differentiated cells safe for human use.26 C-kit+ endothelial progenitor cells such as these could be utilized for the development of engineered blood vessels, vascularization of engineered tissues, and promotion of vessel growth in ischemic tissue.
Previous studies have observed endothelial differentiation of AFSC when cells were cultured in EGM-2 supplement with 2 ng/mL recombinant human basic fibroblast growth factor for 8 days, as determined by CD31 expression alone, or either 10 or 50 ng/mL VEGF for 14 days, with results quantified by combined CD31 and vWF expression.18–20 Although the molecular mechanisms responsible for vasculogenesis and angiogenesis are currently not fully understood, the importance of the VEGF signaling pathway for both processes is evident.27 Therefore, VEGF is often used as the primary stimulus for chemical-mediated differentiation of AF-derived, mesenchymal, and ESC into endothelial cells in vitro. Additionally, the effect of shear force and hypoxia on the stimulation of vWF, eNOS, and VEGF production has been examined in ESC, MSC, and AFSC.20,28,29 Across these studies, including our own, endothelial marker expression was seen as early as 1 week, though expression was significantly increased within the second week of VEGF exposure.
The enzyme eNOS plays an essential role downstream of VEGF signaling pathway, stimulating migration, proliferation, and vasodilation in vivo through nitric oxide production. Genetic upregulation of eNOS has been shown to increase VEGF-dependent neovascularization, suggesting eNOS expression in our differentiated cells can further promote the endothelial phenotype.30
When AFSC were cultured in media without VEGF, immunofluorescent staining did not reveal vWF, eNOS, VE-cadhern, or VEGFR2 expressions, which are all constitutively expressed in mature endothelial cells. However, significant expression of these markers was present with the addition of VEGF at 50 ng/mL to EGM-2 for 2 weeks, and localization of vWF in Weibel-Palade bodies, and eNOS, VE-cadherinin, and VEGFR2 in cell membranes was observed in these samples. Incubation of differentiated AFSC in secondary antibodies alone did not reveal significant fluorescence, confirming the specificity of the primary antibodies used. Analysis using Western blotting revealed increased expression of eNOS as a function of VEGF concentrations ranging from 1 to 50 ng/mL with no significant increase in marker expression at higher concentrations. To our knowledge, this study shows for the first time that AFSC-EC are capable of producing functional eNOS, as determined by nitric oxide production, at physiologically relevant concentrations.
Further examination revealed that CD31 expression by AFSC-EC was also dependent on VEGF. Over a range of VEGF concentrations from 1–100 ng/mL, 50 ng/mL resulted in maximal expressions of eNOS and CD31 expression, with little to no added acquisition of endothelial properties at higher VEGF concentrations. In some cases, concentrations of VEGF as high as 100 ng/mL had the potential for reduced efficacy, as shown in this study through CD31 expression and prior studies with ESC.11 Additionally, the basal level of VEGF production by AFSC-EC across all VEGF concentrations was negligible compared with supplemental concentrations. At the optimal VEGF concentration, postdifferentiation analysis showed a decrease in the stem cell markers SSEA4 and c-kit and the presence of a subpopulation of AFSC-EC that were triple positive for CD31, VE-cadherin, and VEGFR2, which supports the hypothesis of endothelial differentiation.
AFSC-EC were morphologically similar to HUEVC and, in addition to nitric oxide production, displayed other functional characteristics of fully mature endothelial cells. Vasculogenic potential of these cells was shown by assessing the formation of networks when plated on a semi-solid substrate, and internalization of ac-LDL distinctly identified endothelial cells based on metabolic activity. The primary advantages of using ac-LDL metabolism as a marker are that it allows for labeling of live endothelial cells without the need for fixation or permeabilization to optimize staining, does not affect cell viability, and is not removed during trypsinization due to incorporation into lysosomal membranes; therefore, the use of fluorescently labeled ac-LDL will allow for isolation of AFSC-EC from the undifferentiated AFSC population.
AFSC isolated through adherence and c-kit+ immunoselection, then exposed to 50 ng/mL VEGF for 14 days, express key endothelial molecular markers (vWF, eNOS, CD31, VE-cadherin, and VEGFR2), were morphologically similar to HUVEC, and display functional phenotypes associated with endothelial cells (nitric oxide production, network formation, and ac-LDL uptake). These results suggest that AFSC are able to differentiate into functional endothelial cells in vitro and are suited for evaluation for vasculogenic potential within a tissue-engineered construct in vivo.
Acknowledgments
We thank Dr. Joel Moake for expertise and critical reading of the manuscript and Luis Juarez and Sarah Mason for assistance with experiments. HUVEC were isolated and generously provided by the Moake Lab. This work was supported by the NIH (OMB), NSF GRFP (JJP), Houston-Rice Alliance for Graduate Education and the Professoriate (OMB), HHMI Med into Grad Program (OMB), NSF CAREER (JGJ), and the Virginia and L.E. Simmons Family Foundation (JGJ).
Disclosure Statement
There are no competing financial interests.
References
- 1.Chiavegato A. Bollini S. Pozzobon M. Callegari A. Gasparotto L. Taiani J., et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol. 2007;42:746. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 2.De Coppi P. Bartsch G., Jr. Siddiqui M.M. Xu T. Santos C.C. Perin L., et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 3.Tsai M.S. Lee J.L. Chang Y.J. Hwang S.M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum Reprod. 2004;19:1450. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- 4.Prusa A.R. Marton E. Rosner M. Bettelheim D. Lubec G. Pollack A., et al. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol. 2004;191:309. doi: 10.1016/j.ajog.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 5.In ’t Anker P.S. Scherjon S.A. Kleijburg-van der Keur C. Noort W.A. Claas F.H. Willemze R., et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 6.Bailo M. Soncini M. Vertua E. Signoroni P.B. Sanzone S. Lombardi G., et al. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- 7.Sidiqqui M.M. Atala A. Amniotic fluid-derived pluripotential cells: adult and fetal. In: Lanza R., editor; Blau H., editor; Melton D., editor; Moore M., editor; Donnall Thomas E., editor; Verfaillie C., et al., editors. Handbook of Stem Cells. Amsterdam: Elsevier Academic Press; 2004. pp. 175–180. [Google Scholar]
- 8.Sartore S. Lenzi M. Angelini A. Chiavegato A. Gasparotto L. De Coppi P., et al. Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes. Eur J Cardiothorac Surg. 2005;28:677. doi: 10.1016/j.ejcts.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Gambini E. Pompilio G. Biondi A. Alamanni F. Capogrossi M.C. Agrifoglio M., et al. C-kit+ cardiac progenitors exhibit mesenchymal markers and preferential cardiovascular commitment. Cardiovasc Res. 2011;89:362. doi: 10.1093/cvr/cvq292. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y. Amende I. Hampton T.G. Yang Y. Ke Q. Min J.Y., et al. Vascular endothelial growth factor promotes cardiomyocyte differentiation of embryonic stem cells. Am J Physiol Heart Circ Physiol. 2006;291:H1653. doi: 10.1152/ajpheart.00363.2005. [DOI] [PubMed] [Google Scholar]
- 11.Nourse M.B. Halpin D.E. Scatena M. Mortisen D.J. Tulloch N.L. Hauch K.D., et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol. 2009;30:80. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oswald J. Boxberger S. Jorgensen B. Feldmann S. Ehninger G. Bornhauser M., et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 13.Tokalov S.V. Gruner S. Schindler S. Abolmaali N.D. Endothelial Differentiation of Rat Mesenchymal Stem Cells. Res J Biol Sci. 2007;2:307. [Google Scholar]
- 14.Zhang P. Moudgill N. Hager E. Tarola N. Dimatteo C. McIlhenny S., et al. Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev. 2011;20:977. doi: 10.1089/scd.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z. Jiang Y. Hao H. Gupta K. Xu J. Chu L., et al. Endothelial nitric oxide synthase is dynamically expressed during bone marrow stem cell differentiation into endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1760. doi: 10.1152/ajpheart.01408.2006. [DOI] [PubMed] [Google Scholar]
- 16.Gerecht-Nir S. Ziskind A. Cohen S. Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811. doi: 10.1097/01.lab.0000106502.41391.f0. [DOI] [PubMed] [Google Scholar]
- 17.Wu K.H. Zhou B. Lu S.H. Feng B. Yang S.G. Du W.T., et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 18.Yeh Y.C. Wei H.J. Lee W.Y. Yu C.L. Chang Y. Hsu L.W., et al. Cellular cardiomyoplasty with human amniotic fluid stem cells: in vitro and in vivo studies. Tissue Eng Part A. 2010;16:1925. doi: 10.1089/ten.TEA.2009.0728. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P. Baxter J. Vinod K. Tulenko T.N. Di Muzio P.J. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 2009;18:1299. doi: 10.1089/scd.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delo D.M. De Coppi P. Bartsch G., Jr. Atala A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006;419:426. doi: 10.1016/S0076-6879(06)19017-5. [DOI] [PubMed] [Google Scholar]
- 21.Fleming I. Fisslthaler B. Dimmeler S. Kemp B.E. Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 22.Golderer G. Werner E.R. Leitner S. Grobner P. Werner-Felmayer G. Nitric oxide synthase is induced in sporulation of Physarum polycephalum. Genes Dev. 2001;15:1299. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perin L. Sedrakyan S. Da Sacco S. De Filippo R. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol. 2008;86:85. doi: 10.1016/S0091-679X(08)00005-8. [DOI] [PubMed] [Google Scholar]
- 24.Walther G. Gekas J. Bertrand O.F. Amniotic stem cells for cellular cardiomyoplasty: promises and premises. Catheter Cardiovasc Interv. 2009;73:917. doi: 10.1002/ccd.22016. [DOI] [PubMed] [Google Scholar]
- 25.Lovati A.B. Corradetti B. Lange Consiglio A. Recordati C. Bonacina E. Bizzaro D., et al. Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluid-derived progenitor cells. Vet Res Commun. 2011;35:103. doi: 10.1007/s11259-010-9457-3. [DOI] [PubMed] [Google Scholar]
- 26.Vemuri M.C. Schimmel T. Colls P. Munne S. Cohen J. Derivation of human embryonic stem cells in xeno-free conditions. Methods Mol Biol. 2007;407:1. doi: 10.1007/978-1-59745-536-7_1. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt A. Brixius K. Bloch W. Endothelial precursor cell migration during vasculogenesis. Circ Res. 2007;101:125. doi: 10.1161/CIRCRESAHA.107.148932. [DOI] [PubMed] [Google Scholar]
- 28.Wang H. Riha G.M. Yan S. Li M. Chai H. Yang H., et al. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto K. Sokabe T. Watabe T. Miyazono K. Yamashita J.K. Obi S., et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 30.Duda D.G. Fukumura D. Jain R.K. Role of eNOS in neovascularization: no for endothelial progenitor cells. Trends Mol Med. 2004;10:143. doi: 10.1016/j.molmed.2004.02.001. [DOI] [PubMed] [Google Scholar]