Abstract
Modulation of host responses is an important strategy by which parasites ensure successful establishment and persistence. Host counteraction against this modulation may be required for the host to develop resistance to infection. In this pilot study, intestinal infection of dogs with E. granulosus induced a strong polarization of the cytokine response towards a Th2 phenotype. Consecutive rounds of infection and cure induced resistance to infection resulting in a dramatically lower parasite burden. Repeatedly-infected resistant dogs also lost immune polarization and developed a balanced Th1/Th2 response. No major differences were observed in the production of regulatory cytokines (IL-10, TGF-β) between dogs with high parasite load and dogs with only few intestinal parasites. These results suggest that E. granulosus-driven immunomodulation contributes to successful infection in the definitive host. This information might be relevant for the development of more effective vaccines against this stage of the parasite.
Keywords: Echinococcus granulosus, dog, cytokines, immunomodulation, cestodes
1. INTRODUCTION
Echinococcus granulosus, the causative agent of cystic echinococcosis, is still endemic in many countries on all continents and continues to be an important cause of morbidity and mortality. Echinococcosis is an emerging zoonotic disease (new areas of infection are frequently reported), and also a re-emerging problem in some regions. (Eckert and Deplazes, 2004, Moro and Schantz, 2006, Schantz, 2006). The life cycle of E. granulosus involves two mammalian hosts. The definitive hosts are primarily dogs which harbor adult worms in their small intestines. Natural intermediate hosts, particularly sheep and cattle, become infected after ingestion of eggs released in the faeces of infected dogs. Humans are accidental intermediate hosts of this parasite (Thompson, 1995). E. granulosus presents different immunological relationships with its hosts. Great effort has been invested to understand the immunobiology of the parasite in the intermediate host (Baz, et al., 2006, Siracusano, et al., 2008, Zhang, et al., 2003). However, there is scarce information about the immunologic response in the definitive host, likely because of the difficulties of working with dogs, the scarcity of tools to evaluate immune responses in them, and the lack of an appropriate alternative experimental model for the establishment of infections with the tapeworm stage.
The immune system of dogs responds to a primary infection with E. granulosus, but the responses appear weak and ineffective suggesting immunomodulation by the parasite. The scoleces of worms successfully installed in dogs’ intestines adhere strongly to crypts and establish intimate contact with the gut mucosae (Howell and Smyth, 1995, Thompson, 1995). Dogs develop serum IgG and local IgA responses to infection and show parasite-specific proliferation of Peyer’s patch cells. There is also preliminary evidence for a role of IgE in the protection against infection (Deplazes, et al., 1994, Jenkins and Rickard, 1986, Moreno, et al., 2004). Moreover, peripheral lymphocytes from dogs experimentally infected with E. granulosus display an enhanced proliferative response to the mitogen Concanavalin A (ConA), and dogs with the highest responses had significantly fewer parasites than less reactive dogs (Al-Khalidi and Barriga, 1986). Notwithstanding, the dynamics of cellular recruitment and the profile of the inflammatory response in the gut of dogs upon infection have not yet been characterized.
Here we report that E. granulosus primary infection modifies the cytokine profile of dog cells in response to a mitogenic (ConA) stimulation. Dogs experimentally infected with E. granulosus showed a strong polarization towards a Th2 phenotype as analyzed by mRNA (messenger RNA) levels of several cytokines in cells isolated from Peyer’s patches and spleen. We also show that the cytokine responses in dogs that undergo successive rounds of infection and cure, resulting in dramatically decreased tapeworm establishment, are similar to the cytokine profile of non-infected dogs.
2. MATERIALS AND METHODS
2.1 Dogs
Eleven dogs of mixed breeds, aged between 3 to 6 months, were purchased from the Animal Control Center in Lima, Peru The dogs were maintained under helminth-free conditions at the facilities of the School of Veterinary Medicine of the Universidad Nacional Mayor de San Marcos, also in Lima, where they were fed with commercial dog food and water ad libitum for at least 3 months before the beginning of experiments. Coprological examinations to screen for intestinal parasites were routinely conducted during the quarantine period. Thirty days before infection all dogs were treated with Drontal Plus (Bayer) at standard doses (praziquantel 5 mg/k, pyrantel 5 mg/k and febantel 50mg/k), and had a further coproparasitological examination to confirm that they were free from parasites before the experiment (Eckert, et al., 2001). The protocol was approved by the Animal Ethic Committee of the School of Veterinary Medicine of the Universidad Nacional Mayor de San Marcos.
2.2 Experimental infections
E. granulosus protoscoleces were aseptically obtained from ovine cysts. For experimental infection purposes, aliquots of a single pool containing 80,000 viable PE in 1ml of PBS were given orally to each dog after a day of fasting as previously described (Casaravilla, et al., 2005, Moreno, et al., 2004, Petavy, et al., 2008). Briefly, protoscoleces were allowed to decant, washed with PBS, and counted under light microscope after confirming their viability by eosin exclusion and flame cell activity (Smyth and Davies, 1974). Protoscolex suspensions were then diluted to the desired concentration and given to the dogs immediately after. Dogs were restrained and the veterinarian on charge verified that the animal swallowed the entire dose.
Nine dogs were used for experimental infections, divided in three groups of three dogs each. Group 1 received a single experimental infection. Groups 2 and 3 received three and six rounds of infection and cure, respectively. All infections were interrupted by treating the dogs with arecoline (4mg/k, 1 or 2 doses) and then with praziquantel 5mg/Kg before the end of the pre-patent period, by day 35 post infection. The results of each round of experimental infections were assessed by evaluating worms after arecoline purgation. Fifteen days later, dogs were re-infected with identical doses of protoscoleces. The control group consisted of two uninfected dogs that received only PBS.
2.3 Sample collection and worm burden
Following the last infection but before the end of the prepatent period (35 dpi after infection) all dogs in each group were euthanized with an intravenous overdose of sodium pentobarbital. The peritoneal cavity was rapidly opened and the Peyer’s patches of the small intestine and spleen were removed for in vitro analysis. To assess parasite burden the rest of the small intestine was opened longitudinally and incubated in saline at 37°C until no more worms were liberated. Most worms were released naturally and the remainders were carefully scraped from intestine. The worms were counted under a magnifying glass.
2.4 Cell cultures
Whole cell suspensions from Peyer’s patches and spleens were obtained by mechanical disruption of the organ (followed by red cell lysis in the case of spleen), and used directly for in vitro stimulation assays as previously reported (Moreno, et al., 2004). Briefly, cells were suspended in RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (Gibco), 2.5 mM L-glutamine (Sigma), 0.05 mM 2-mercaptoethanol, 5,000 units/ml penicillin, 5 mg/ml streptomycin and 10 mg/ml neomycin. The number of cells was adjusted to 5 × 106 cells per ml. Cell suspension was stimulated with 5 μg/ml of ConA (Sigma). Stimulated and non-stimulated cultures were incubated in duplicate for 16 hours at 37°C in 5% CO2-humidified atmosphere Finally cells were lysed in TRizol (Invitrogen), shipped to Uruguay and stored at −80°C until RNA isolation was done.
2.4 Total RNA extraction and reverse transcription
RNA was extracted from cells in TRIzol according to the manufacturer’s protocol. Total RNA was dissolved in nuclease free water and quantified by UV-spectrophotometry. Genomic DNA was removed from RNA before reverse transcription by incubation with 0.35 IU DNase I (Invitrogen) with 1 μg of total RNA. First strand complementary DNA (cDNA) synthesis was carried out immediately by adding 0.5 μg oligo dT primer (Invitrogen) and 200 U M-MLV reverse transcriptase (Invitrogen) in a final volume of 20 μl. cDNA generated was diluted to 200 μl in nuclease free-water and stored at −20°C until use.
2.5 Quantification of relative cytokine mRNA levels
Quantification of canine IL-4, IL-13, IFN-γ, IL-10, and TGF-β mRNA was conducted by real time PCR using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as housekeeping gene for the qRT-PCR. Primers were purchased from Operon (Cologne, Germany) and are all described in Table 1. Real-time PCRs were performed using QuantiTect SYBR Green PCR Kit (QIAGEN), and 2.5 μl of diluted cDNA in the presence of 0.5 μM of each specific primer. Quantitative PCR was performed using the Rotor-Gene 6000 (Corbett Life Science). Samples were initially denatured for 15 minutes at 95 °C, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute during which the fluorescence data were collected (Manna, et al., 2006).
Table 1.
Primers used for quantitative PCR. Citations between brackets indicate the source of primers sequences.
| GenBank Target gene |
Accession Number |
Primer | Sequence (5′ – 3′) | Product length (bp) |
|---|---|---|---|---|
| GAPDH (Manna et al., 2006) | NM_001003142 | Forward Reverse |
TCC TCT AGC CAA AGT CAT CCA TGA GGC ATG GAC GGT GGT CAT |
67 |
| IL-4 (Manna et al.,2006) | NM_001003159 | Forward Reverse |
GCT CCA AAG AAC ACA AGC GAT AAG CTG CCG CAG TAC AGT AGC A |
60 |
| IL-13 (Brachelente et al., 2005) |
AF244915 | Forward Reverse |
TGG TGT GGA GCG TCA ACC T CGG AGA CAT TGA TCA GAG ATT CTA GA |
69 |
| IFN-γ (Manna et al., 2006) | NM_001003174 | Forward Reverse |
GCG GAA AAG GAG TCA GAA TCT GTT CAG GCA GGA TGA CCA TTA TTT CGA |
64 |
| IL-10 (Manna et al., 2006) | NM_001003077 | Forward Reverse |
CCT GGG AGA GAA GCT CAA GAC CAC AGG GAA GAA ATC GGT GAC A |
60 |
| TGF-β (Sauter et al., 2005) | L34956 | Forward Reverse |
CAA GTA GAC ATT AAC GGG TTC AGT TC GGT CGG TTC ATG CCA TGA AT |
70 |
We first compared the average GAPDH expression in stimulated and non-stimulated spleen and Peyer’s patch cells and found that the groups were not statistically different (p = 0.0862). We therefore concluded that the GAPDH gene could be used as a single normalizing gene.
The relative mRNA amount in each sample was calculated using the 2−ΔCt method (Livak and Schmittgen, 2001) were ΔCt = CtCytokine - CtGAPDH, and expressed as the ratio between relative amount to GAPDH of stimulated (SC) and relative amount to GAPDH of non-stimulated (NSC) cells.
2.6 Statistical analysis
One-tailed Mann-Whitney test was used for non-parametric comparisons with a significance level set at P < 0.05. All calculations were performed with GraphPad Prism (GraphPad Software, San Diego, California, USA).
3. RESULTS
3.1 E. granulosus infections and parasite recovery
After each infection round, worms were recovered from the small intestine and counted for each animal. Parasite burden in dogs receiving only one infection (Group 1) ranged from 2,770 to 4,900 worms per dog. Dogs receiving three (Group 2) or six consecutive rounds of cure and re-infection (Group 3) had a marked reduction in parasite burden after the last infection with values ranking from 1 to 91 and 0 to 173 for Groups 2 and 3, respectively (Table 1). Groups 2 and 3 were combined into a single group (re-infected dogs) for further analysis based on their similar parasite burden after last infection. Re-infected dogs had significantly fewer worms after the end of their last infection (P<0.05, Mann-Whitney test). Uninfected control animals were both free of worms as expected.
3.2 Cytokine mRNA expression in dogs infected with E. granulosus
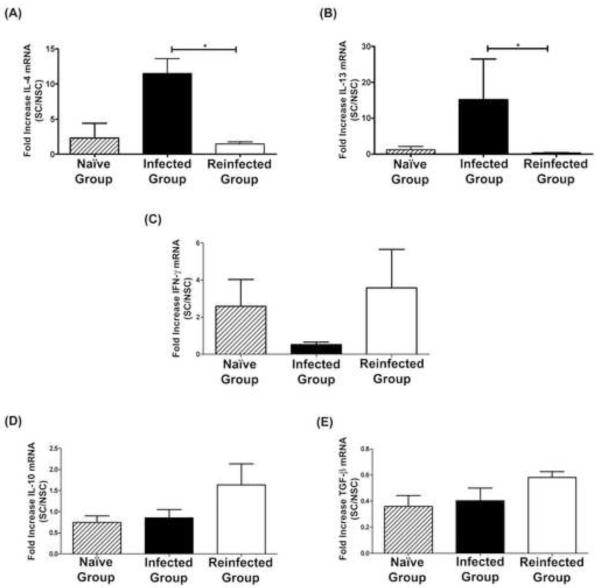
We evaluated the baseline cytokine expression level of non-stimulated cells from Peyer’s patches and spleen obtained from either non-infected, infected or re-infected dogs, and found that there were no statistically significant differences in mRNA cytokine levels (data not shown). We then evaluated the change in the cytokine expression level upon mitogenic stimulation with ConA. Peyer’s patch cells from dogs that received a single infection produced significantly higher amounts of IL-4 and IL-13 in response to ConA than did Peyer’s patch cells from re-infected dogs (P<0.05) (Figures 1A, 1B). The expression levels of both cytokines in uninfected control dogs were similar to those in re-infected dogs. Conversely, the IFN-γ response appeared as suppressed in the group of dogs receiving a single infection compared with the other two groups, although this difference did not reach statistical significance (Figure 1C). The levels of TGF-β and IL-10 were similar in all dog groups (Figures 1D, 1E).
Figure 1.
Cytokine transcriptional response of Peyeŕs patch cells upon stimulation with ConA. Results are shown as means ± SE (Standard Error).
*Significant differences between groups (P < 0.05).
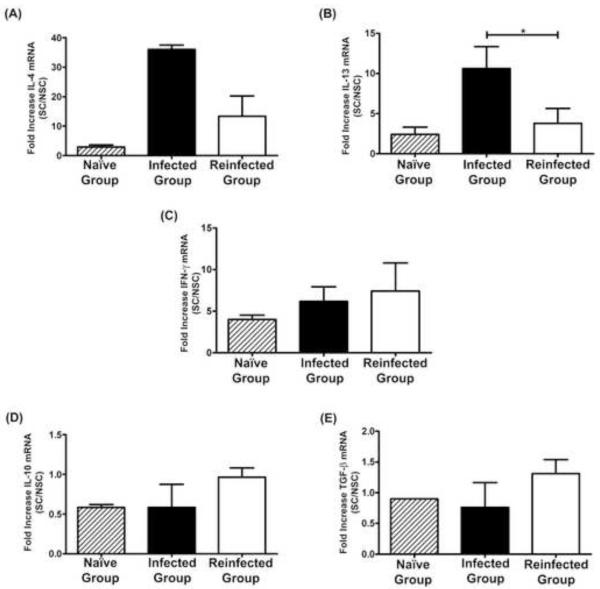
Cytokine expression profiles of spleen cells were similar to those of Peyeŕs patch cells. Spleen cells from single-infected dogs showed a significant increase in the expression level of IL-13 (P<0.05), and a marked tendency for increased IL-4 expression compared with cells from dogs receiving consecutive rounds of infections (Figures 2A, 2B). The response of both Th2 cytokines in non-infected control dogs was lower than in infected dogs, although statistical significance could not be evaluated due to the small numbers of uninfected dogs. In contrast, the IFN-γ response was very similar between groups (Figure 2C). As in Peyer’s patch cells, the expression of regulatory cytokines showed no marked differences between groups (Figures 2D, 2E).
Figure 2.
Cytokine transcriptional response of spleen cells upon stimulation with ConA. Results are shown as means ± SE (Standard Error).
*Significant differences between groups (P < 0.05).
Comparison of cytokine expression levels in Peyer’s patch and spleen cells from dogs that received 3 or 6 rounds of infection showed no differences between them, with the exception of IL-4 and IL-13 in which there was a tendency for decreasing levels from 3 to 6 consecutive infections (results not shown).
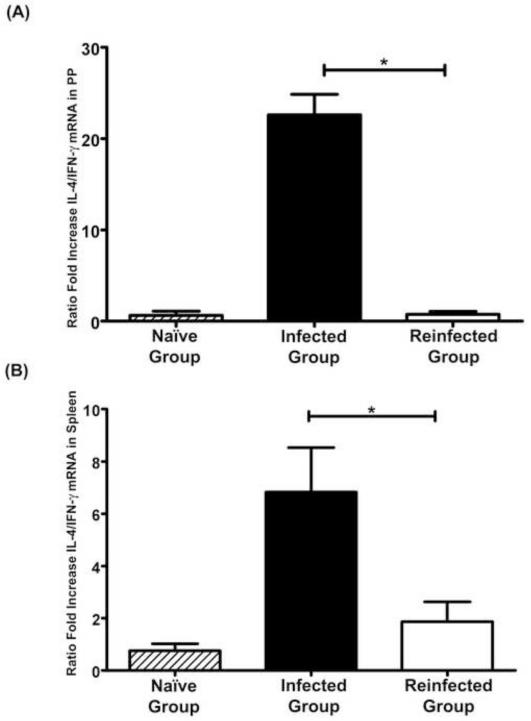
In order to clearly distinguish the variation in Th1/Th2 profile between groups, and to avoid bias due to individual variation, we normalized the data by plotting the ratio between expression levels of IL-4 and IFN-γ mRNA for each dog. Cells from the dogs which received a single infection showed a marked polarization towards a Th2 response (Figure 3). This polarization was observed both at local and systemic levels, with single-infected dogs showing a significantly higher ratio of IL-4/IFN-γ compared with dogs receiving successive rounds of infection and cure (re-infected dogs). Non-infected control dogs showed an IL-4/IFN-γ ratio similar to that observed in re-infected dogs which had very few parasites in the intestine.
Figure 3.
Ratio between fold increase of IL-4 and IFN-γ mRNA (IL-4/IFN-γ). Results are shown as means ± SE (Standard Error) of the response of individual animals in Peyer's patches (A), and in spleen (B) cells.
*Significant differences between groups (P < 0.05).
4. DISCUSSION
Successful establishment of intestinal helminthes is dependent on mechanisms to avoid expulsion by the host. Parasites induce a range of mechanisms to avoid the immune response including inaccessibility of key antigens, production of immunomodulatory factors, and interference with effector mechanisms. Modulation of the host immune response may also be relevant for selection towards commensalism, since it may prevent deleterious effects to the host resulting from exacerbated immune response (Else, 2005, Maizels and Yazdanbakhsh, 2003, Siracusano, et al., 2008).
The limited available data show that establishment of E. granulosus in the gut lumen of the dog induces a parasite-specific immune response (Al-Khalidi and Barriga, 1986, Barriga and Al-Khalidi, 1986, Deplazes, et al., 1994, Heath, 1995, Jenkins and Rickard, 1986, Moreno, et al., 2004), although this response does not seem to interfere with a successful and persistent infection. It has been speculated that E. granulosus depresses mucosal immune-effector mechanisms, thereby prolonging tapeworm survival (Heath, 1995, Wakelin, 1997). Nevertheless, the seminal works by Gemmell and co-workers (1986) showed that dogs confronted to repeated experimental infections with E. granulosus had decreased susceptibility and low parasite burden in the intestine in response to further infections. This suggests that parasite-specific protective mechanisms might be elicited in the gut (Gemmell, et al., 1986). arly reports on canine vaccines (Gemmell, 1962, Herd, et al., 1975, Turner, et al., 1936) have been followed by two recent reports suggesting that the development of protective immunity following vaccination is feasible (Petavy, et al., 2008, Zhang, et al., 2006). On the other hand, epidemiological studies showed that dogs naturally infected with E. granulosus can acquire resistance against this parasite (Budke, et al., 2005, Lahmar, et al., 2001, Moro, et al., 2005). Furthermore, a mathematical model indicated that dogs under high infection pressure develop significant immunity against E. granulosus (Torgerson, 2006). We hypothesized that successful establishment of E. granulosus in dogs requires immunomodulation of the host response, and that the host in-turn develops a response that counteracts this parasite-induced modulation resulting in the development of resistance to infection seen during successive rounds of infections and cure. The results of this pilot study provide initial evidence to support this hypothesis.
We found that cells from dogs experimentally infected with E. granulosus respond to mitogenic stimulation with a cytokine profile significantly polarized towards a Th2 phenotype. The polarization was observed at both local and systemic levels as evidenced by a dramatic increase in the IL-4 /IFN-γ ratio in these animals. Furthermore, in dogs that developed resistance to parasite infection after consecutive rounds of infection and cure, the Th2 polarization disappears and the host develops a more balanced Th1/Th2 response. Susceptible (single-infected) dogs had significantly higher levels of expression of IL-4 at the local level than did resistant (re-infected) dogs, and significantly higher levels of IL-13 both locally and systemically. Although there were no statistically significant differences in IFN-γ response between groups, the differences in the IL-4 /IFN-γ ratios were highly significant.
It could be argued that the weaker Th2 response in re-infected dogs is simply the result of lower parasite burden rather than a marker of resistance. However, there was a trend towards decreased levels of IL-4 and IL-13 when moving from three to six rounds of infection despite both groups of dogs having the same low numbers of worms. This suggests that worm burden is not the only parameter affecting the magnitude of the induced Th2 response in these dogs.
The immune mechanisms involved in resistance to infection and expulsion of intestinal worms have been extensively investigated over many years, mainly through studies of T. spiralis, T. muris, and N. brasiliensis nematodes. From these studies a picture emerged in which resistance to infection depends upon type 2 CD4+ T helper (TH2) cells and type-2 cytokines including IL-4 and IL-13, whereas parasite persistence and host susceptibility is promoted by IFN-γ-expressing CD4+ T helper type-1 (TH1) (Anthony, et al., 2007). Our results differ from these patterns. Several factors could contribute to these differences. Previous studies were conducted in mice or rats, therefore differences could be related to the immunological differences between dogs and these other hosts. Most studies assessed the induction of protective immunity against nematodes whereas E. granulosus is a cestode. Furthermore, E. granulosus is an “exclusively” luminal gut parasite that does not disrupt the intestinal mucosa, whereas the nematodes can invade the intestinal mucosa and many of them have a tissue phase. However, studies in rats involving Hymenolepis diminuta, a cestode with an “exclusively” gastrointestinal phase, showed that infection induces a TH2 response that may result in expulsion or successful establishment depending on the ratio of IL-13/IL-4 (Webb, et al., 2007). Nevertheless, these differences were associated with marked variation in the number of parasites used to challenge the animals, whereas we used a single challenge dose and thus similar differences may not be evident.
The studies presented here were performed in a small number of animals due to the nature of the definitive host (dogs) and logistical limitations. Despite these drawbacks our results suggest that the definitive host may develop at least partial immunity against E. granulosus in the context of a balanced Th1/Th2 response. This information might be relevant for the development of more effective vaccines against this parasite and may foster new studies in larger numbers of animals.
Table 2.
Numbers of worms recovered from uninfected dogs (Naïve Group), dogs that received a single infection (Group 1), and dogs that received 3 (Group 2) or 6 (Group 3) successive rounds of infections with E. granulosus.
| Numbers of worms |
|||
|---|---|---|---|
| Groups/Animal | |||
| Dog N° 1 | Dog N° 2 | Dog N° 3 | |
| Naïve Group | 0 | 0 | - |
| Group 1 | 4,900 | 2,772 | 3,160 |
| Group 2 | 1 | 2 | 91 |
| Group 3 | 39 | 173 | 0 |
5. ACKNOWLEDGEMENTS
This work was supported by a project grant from C.S.I.C., Uruguay and the NIAID NIH TMRC grant P01 AI51976, Peru, and FIC Training grant TW001140. AR was supported by a fellowship from PEDECIBA, Facultad de Ciencias, Uruguay. HG is now a Wellcome Trust Senior International Research Fellow. We thank Dr. Seth O’Neal for his help in editing this manuscript.
6. REFERENCES
- Al-Khalidi NW, Barriga OO. Cell-mediated immunity in the prepatent primary infection of dogs with Echinococcus granulosus. Veterinary Immunology and Immunopathology. 1986;11:73–82. doi: 10.1016/0165-2427(86)90089-9. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature Reviews Immunology. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriga OO, Al-Khalidi NW. Humoral immunity in the prepatent primary infection of dogs with Echinococcus granulosus. Veternary Immunology and Immunopathology. 1986;11:375–389. doi: 10.1016/0165-2427(86)90039-5. [DOI] [PubMed] [Google Scholar]
- Baz A, Ettlin GM, Dematteis S. Complexity and function of cytokine responses in experimental infection by Echinococcus granulosus. Immunobiology. 2006;211:3–9. doi: 10.1016/j.imbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Brachelente C, Muller N, Doherr MG, Sattler U, Welle M. Cutaneous leishmaniasis in naturally infected dogs is associated with a T helper-2-biased immune response. Veterinary Pathology. 2005;42:166–175. doi: 10.1354/vp.42-2-166. [DOI] [PubMed] [Google Scholar]
- Budke CM, Jiamin Q, Craig PS, Torgerson PR. Modeling the transmission of Echinococcus granulosus and Echinococcus multilocularis in dogs for a high endemic region of the Tibetan plateau. International Journal of Parasitology. 2005;35:163–170. doi: 10.1016/j.ijpara.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Casaravilla C, Malgor R, Rossi A, Sakai H, Nonaka N, Kamiya M, Carmona C. Production and characterization of monoclonal antibodies against excretory/secretory products of adult Echinococcus granulosus, and their application to coproantigen detection. Parasitology International. 2005;54:43–49. doi: 10.1016/j.parint.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Thompson RC, Constantine CC, Penhale WJ. Primary infection of dogs with Echinococcus granulosus: systemic and local (Peyer’s patches) immune responses. Veterinary Immunology and Immunopathology. 1994;40:171–184. doi: 10.1016/0165-2427(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clinical Microbiology Reviews. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert J, Deplazes P, Craig PS, Gemmell MA, Gottstein B, Heath D, Jenkins DJ, Kamiya M, Lightowlers MW. Echinococcosis in animals: clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell MA, Meslin F-X, Pawłowski ZS, editors. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. World Organisation for Animal Health (Office International des Epizooties) and World Health Organization; Paris, France: 2001. pp. 73–100. [Google Scholar]
- Else KJ. Have gastrointestinal nematodes outwitted the immune system? Parasite Immunology. 2005;27:407–415. doi: 10.1111/j.1365-3024.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- Gemmell MA. Natural and acquired immunity factors interfering with development during the rapid growth phase of Echinococcus granulosus in dogs. Immunology. 1962;5:496–503. [PMC free article] [PubMed] [Google Scholar]
- Gemmell MA, Lawson JR, Roberts MG. Population dynamics in echinococcosis and cysticercosis: biological parameters of Echinococcus granulosus in dogs and sheep. Parasitology. 1986;92:599–620. doi: 10.1017/s0031182000065483. [DOI] [PubMed] [Google Scholar]
- Heath DD. Immunology of Echinococcus infections. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and Hydatid Disease. CAB; International, UK: 1995. pp. 183–199. [Google Scholar]
- Herd RP, Chappel RJ, Biddell D. Immunization of dogs against Echinococcus granulosus using worm secretory antigens. International Journal of Parasitology. 1975;5:395–399. doi: 10.1016/0020-7519(75)90004-1. [DOI] [PubMed] [Google Scholar]
- Howell MJ, Smyth JD. Maintenance and cultivation of Echinococcus species in vitro and in vivo. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and Hydatid Disease. CAB International; UK: 1995. pp. 201–232. [Google Scholar]
- Jenkins DJ, Rickard MD. Specific antibody responses in dogs experimentally infected with Echinococcus granulosus. American Journal of Tropical Medicine and Hygiene. 1986;35:345–349. doi: 10.4269/ajtmh.1986.35.345. [DOI] [PubMed] [Google Scholar]
- Lahmar S, Kilani M, Torgerson PR. Frequency distributions of Echinococcus granulosus and other helminths in stray dogs in Tunisia. Annals of Tropical Medicine and Parasitology. 2001;95:69–76. doi: 10.1080/00034980020035933. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Reviews Immunology. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- Manna L, Reale S, Viola E, Vitale F, Foglia Manzillo V, Michele PL, Caracappa S, Gravino AE. Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs. Veterinary Parasitology. 2006;142:271–280. doi: 10.1016/j.vetpar.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Moreno M, Benavidez U, Carol H, Rosenkranz C, Welle M, Carmona C, Nieto A, Chabalgoity JA. Local and systemic immune responses to Echinococcus granulosus in experimentally infected dogs. Veterinary Parasitology. 2004;119:37–50. doi: 10.1016/j.vetpar.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Moro P, Schantz PM. Cystic echinococcosis in the Americas. Parasitology International. 2006;55:S181–S186. doi: 10.1016/j.parint.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Moro PL, Lopera L, Bonifacio N, Gonzales A, Gilman RH, Moro MH. Risk factors for canine echinococcosis in an endemic area of Peru. Veterinary Parasitology. 2005;130:99–104. doi: 10.1016/j.vetpar.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Petavy AF, Hormaeche C, Lahmar S, Ouhelli H, Chabalgoity A, Marchal T, Azzouz S, Schreiber F, Alvite G, Sarciron ME, Maskell D, Esteves A, Bosquet G. An oral recombinant vaccine in dogs against Echinococcus granulosus, the causative agent of human hydatid disease: a pilot study. PLoS Neglected Tropical DIseases. 2008;2:e125. doi: 10.1371/journal.pntd.0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter SN, Allenspach K, Gaschen F, Grone A, Ontsouka E, Blum JW. Cytokine expression in an ex vivo culture system of duodenal samples from dogs with chronic enteropathies: modulation by probiotic bacteria. Domestic Animal Endocrinology. 2005;29:605–622. doi: 10.1016/j.domaniend.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Schantz PM. Progress in diagnosis, treatment and elimination of echinococcosis and cysticercosis. Parasitology International 55. 2006;55:S7–S13. doi: 10.1016/j.parint.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Siracusano A, Rigano R, Ortona E, Profumo E, Margutti P, Buttari B, Delunardo F, Teggi A. Immunomodulatory mechanisms during Echinococcus granulosus infection. Experimental Parasitology. 2008;119:483–489. doi: 10.1016/j.exppara.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Smyth JD, Davies Z. In vitro culture of the strobilar state of Echinococcus granulosus (sheep strain): a review of basic problems and results. International Journal of Parasitology. 1974;4:631–644. doi: 10.1016/0020-7519(74)90028-9. [DOI] [PubMed] [Google Scholar]
- Thompson RCA. Biology and systematics of Echinococcus. In: Thompson RCA, Lymbery AJ, editors. Echinococcus and Hydatid Disease. CAB International; UK: 1995. pp. 1–50. [Google Scholar]
- Torgerson PR. Canid immunity to Echinococcus spp.: impact on transmission. Parasite Immunology. 2006;28:295–303. doi: 10.1111/j.1365-3024.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- Turner EL, Berberian DA, Dennis EW. The production of artificial immunity in dogs against Echinococcus granulosus. Journal of Parasitology. 1936;22:14–28. [Google Scholar]
- Wakelin D. Immune response to Echinococcus infection: parasite avoidance and host protection. Parassitologia. 1997;39:355–358. [PubMed] [Google Scholar]
- Webb RA, Hoque T, Dimas S. Expulsion of the gastrointestinal cestode, Hymenolepis diminuta by tolerant rats: evidence for mediation by a Th2 type immune enhanced goblet cell hyperplasia, increased mucin production and secretion. Parasite Immunology. 2007;29:11–21. doi: 10.1111/j.1365-3024.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clinical Microbiology Reviews. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang Z, Shi B, Li J, You H, Tulson G, Dang X, Song Y, Yimiti T, Wang J, Jones MK, McManus DP. Vaccination of dogs against Echinococcus granulosus, the cause of cystic hydatid disease in humans. Journal of Infectious Diseases. 2006;194:966–974. doi: 10.1086/506622. [DOI] [PubMed] [Google Scholar]