Abstract
Objective
Reverse cholesterol transport (RCT) involves the removal of cholesterol from peripheral tissue for excretion in the feces. Here, we determined whether red blood cells (RBCs) can contribute to RCT.
Methods and Results
We performed a series of studies in apoAI-deficient mice where the HDL-mediated pathway of RCT is greatly diminished. RBCs carried a higher fraction of whole blood cholesterol than plasma in apoAI-deficient mice, and as least as much of the labeled cholesterol derived from injected foam cells appeared in RBCs compared to plasma. To determine if RBCs mediate RCT to the fecal compartment, we measured RCT in anemic and control apoAI-deficient mice and found that anemia decreased RCT to the feces by over 35% after correcting for fecal mass. Transfusion of [3H]cholesterol labeled RBCs led to robust delivery of the labeled cholesterol to the feces in apoAI-deficient hosts. In wild type mice, the majority of the blood cholesterol mass, as well as [3H]cholesterol derived from the injected foam cells, was found in plasma, and anemia did not significantly alter RCT to the feces after correction for fecal mass.
Conclusion
The RBC cholesterol pool is dynamic and facilitates RCT of peripheral cholesterol to the feces, particularly in the low HDL state.
It is generally accepted that lipoproteins carry cholesterol in plasma throughout the body. Increasing HDL-C via apoAI gene transfer in mice has been shown to increase reverse cholesterol transport (RCT), as measured by tracing the levels of [3H]cholesterol transferred from injected macrophages to the plasma, liver, and feces.1 Yet, in humans, whole blood is comprised of ~45% red blood cells (RBCs) by volume, and the cholesterol concentration in RBCs is comparable to that found in the plasma, carried by lipoproteins.2 RBC plasma membranes contain free cholesterol that can bidirectionally exchange with plasma lipoprotein cholesterol approaching equilibrium ex vivo in ~6 hours, with the kinetics indicating transfer via aqueous diffusion.3 Despite their significant carrying capacity for cholesterol, the role that RBCs may play in RCT has not been previously addressed. Here we show that apoAI-deficient mice carry most of their cholesterol in the RBC compartment as opposed to the plasma compartment. When we made these apoAI-deficient mice anemic, RCT to the fecal compartment was reproducibly decreased.
Methods
Mice
Wild type, apoAI−/− 4, and apoAI transgenic mice 5, all on the C57BL/6 background, were purchased from The Jackson Laboratory. All experiments were performed in accordance with the Cleveland Clinic Institutional Animal Care and Use Committee.
Reverse Cholesterol Transport Assays
These studies were performed using methods similar to those described previously.6 Murine bone marrow macrophages were cultured from wild type mice for 11 to 14 days in DMEM supplemented with 20% L-cell conditioned medium (as a source of MCSF) and 10% fetal bovine serum. To load and label macrophages with [3H]cholesterol, cells were incubated with DMEM containing 20% L-cell conditioned medium, acetylated LDL (50 μg/ml), and [3H]cholesterol (2 μCi/ml, Perkin Elmer) for one to two days. Foam cells were washed twice with DMEM prior to harvesting for in vivo injection, and ~ 2 million cells containing ~3 million [3H]cholesterol dpm in a volume of 0.25 ml were injected s.c between the shoulder blades of recipient mice. The injected dpm was quantified from an aliquot of the foam cell suspension intended for in vivo injection by extracting [3H]cholesterol with hexane:isopropanol (3:2) and measuring radioactivity by liquid scintillation counting. 75 to 100 μl of blood was collected daily from the tail vein or retro-orbitally and centrifuged to isolate plasma. The plasma radioactivity was determined, and total plasma dpm was calculated by estimating blood volume to be equal to 7% of the body weight and plasma to be 55% of the blood volume, unless a hematocrit value was obtained, in which case the actual % plasma was used in the calculations. RCT to the plasma was calculated as the % of dpm appearing in plasma/total dpm injected. To quantify [3H]cholesterol in RBCs, typically 20 μl of whole blood was centrifuged to isolate cells. The cell pellets were carefully washed twice with phosphate buffered saline (PBS), [3H]cholesterol was extracted with hexane:isopropanol (3:2), and the radioactivity was measured by liquid scintillation counting. The total RBC dpm was calculated by estimating blood volume to be equal to 7% of the body weight and RBC volume to be 45% of the blood volume, or the value based upon the observed hematocrits. RCT to the RBC compartment was quantified as the % of dpm in total red blood cells/injected dpm. Feces were collected daily and allowed to soften in a 50% ethanol solution. In certain experiments, feces collected daily were first dried over night at 55°C, weighed, and then softened in 50% ethanol solution. After hydration, the feces were homogenized and an internal recovery standard of 10,000 dpm of [14C]cholesterol (Perkin Elmer) was added to each sample. The radioactivity in an aliquot of 0.3 ml of the fecal homogenate was measured by liquid scintillation counting after chemiluminescence had decayed. The [14C]cholesterol dpm was used to back calculate the [3H] recovery for the entire fecal homogenate. RCT to the feces was calculated as the % of dpm in feces/injected dpm. We have observed that fecal RCT can be artificially high for some mice if any of the injected labeled cells leak out of the injection site and are consumed orally by the mice. Thus, any mouse with fecal RCT greater than two standard deviations above the mean of the remaining data within each group was excluded from analysis. Fecal neutral sterols were analyzed by modification of a previously described protocol.7 Sterols were saponified in 0.3 ml aliquots of fecal homogenates by adding 0.3 ml of ethanol and 0.4 ml of 1 M NaOH. After heating at 95°C for 2 hrs, neutral sterols were extracted three times with 9 ml hexane, pooled, dried, and counted. The bottom phase was adjusted to neutral pH and counted as the bile acid fraction. Average recovery of the [14C]cholesterol internal standard in the neutral sterol fraction was 96.5 ± 6.2%. Upon sacrifice, each mouse was perfused with PBS by making an incision in the right atrium and injecting the left ventricle with 10 ml of PBS. The liver was harvested, weighed, suspended in PBS, homogenized, and 10,000 dpm of [14C]cholesterol was added as a recovery standard, with aliquot counting and RCT calculation as described above.
Plasma and RBC Cholesterol Assays
Whole blood was centrifuged at 1800 rpm for 5 minutes to separate the plasma from cellular compartments. For simplicity, we refer to the cellular fraction as the RBC compartment, although it also contains leukocytes and platelets. The plasma cholesterol concentration was determined using an enzymatic assay from StanBio Laboratory. The RBC fraction was carefully washed twice in PBS to remove any plasma contamination, and lipids in the cell pellet were extracted with hexane:isopropanal (3:2). After drying the solvent, lipids were dissolved in isopropanol:NP-40 (9:1) and cholesterol was measured as previously described.8 The concentrations of the plasma and cellular cholesterol pools were normalized to one dL of whole blood assuming that the cellular and plasma fractions make up 45% and 55%, respectively, of whole blood volume.
Induction of Anemia
ApoAI−/− or wild type mice were anesthetized with isoflurane and bled retro-orbitally into a heparinzed capillary tube for three consecutive days (approximately 290 μl of blood per day) to induce hemorrhagic anemia. In specified studies, the blood was centrifuged to isolate the plasma, and then the plasma was re-injected retro-orbitally with a 26 G needle into the contra lateral retro-orbital plexus while the mouse was under anesthesia. An additional 60 μl of blood was removed retro-orbitally to determine daily hematocrits. Thus, a total of ~350 μl of blood was bled from each mouse in the anemic group daily. Control mice were anesthetized with isoflurane and then allowed to recover without bleeding. The anemic and control mice were used for RCT studies as described above.
Ex vivo labeling and transfusion of RBCs
A wild type mouse was bled retro-orbitally to collect 1 mL of whole blood. The blood was spun at 400 g for 15 min, and the plasma and buffy coat layers removed. To label RBCs, the RBC pellet was resuspended to 1 mL of PBS and incubated with 10 μCi [3H]cholesterol (1% ethanol final concentration) for 10 minutes at 37°C. The RBCs were washed twice by suspension in PBS and centrifugation at 400g for 15 min, resuspended into 1.5 mL PBS, and injected (100 μl per mouse) retro-orbitally into each recipient mouse while the mouse was anesthetized with isoflurane. At approximately 1 minute after injection, blood was obtained from the tail vein to calculate injected radioactivity at “time zero.” 48 hours later, the recovery of [3H] radioactivity and calculation of the % of the “time zero” dose in the plasma, RBCs, liver, and feces were performed as described above.
Results and Discussion
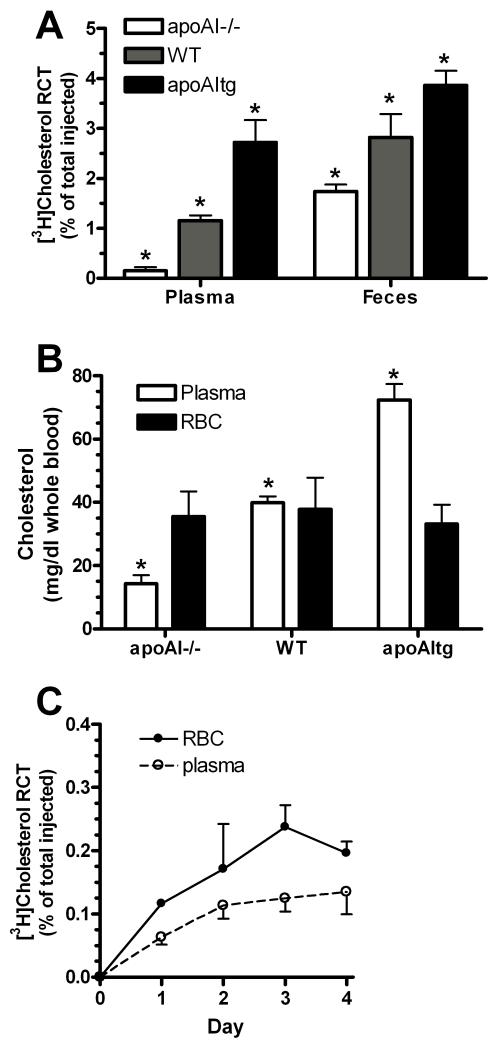
A series of observations led us to hypothesize the existence of a novel non-HDL pathway involving RBCs that contributes to fecal RCT. First, RCT was assessed in murine models by s.c. injection of cholesterol labeled foam cells and following the cholesterol radioactivity into the plasma, hepatic, and fecal compartments over three days. RCT was compared in apoAI-deficient (apoAI−/−), wild type (WT), and human-apoAI transgenic (apoAItg) mice, with varying levels of HDL-cholesterol (12.2 ± 13.5, 92.9 ± 10.5, and 135.7 ± 12.1 mg/dl respectively; p<0.001 by ANOVA posttest). Compared to apoAItg hosts, apoAI−/− hosts had ~18-fold less [3H]cholesterol transfer to the plasma but only 2.2-fold less transfer to the feces (Fig. 1A). Thus, in apoA1−/− mice, where HDL-mediated RCT is greatly diminished, RCT to the feces was relatively preserved. Secondly, we examined the steady state cholesterol content in whole blood and found that the plasma and RBC (including other minor cellular fractions) cholesterol pools were roughly equivalent in WT mice. However, in apoAI−/− mice, the RBC compartment had >2-fold higher cholesterol level than the plasma compartment (Fig. 1B). Thirdly, we followed [3H]cholesterol appearing in both the plasma and RBC compartments in a RCT study of apoAI−/− hosts. Over 4 days, we observed a trend that more of the cholesterol tracer was found in the RBC vs. the plasma pool (Fig 1C), implicating a role for RBCs in RCT. Based on these initial observations, we hypothesized the existence of an alternative non-HDL pathway by which peripheral cholesterol reaches the feces, involving RBCs, the other major reservoir of cholesterol in blood.
Figure 1.
A. RCT to the plasma and fecal compartments three days after s.c injection of [3H]cholesterol labeled foam cells into apoAI−/−, WT, and apoAItg hosts, shown as the % of the injected radioactivity (N=5 ±SD; *, p<0.001 vs. each other by ANOVA Newman-Keuls posttest). B. Cholesterol concentrations in plasma and RBC pools normalized to whole blood volume assuming 45% hematocrit (N=2-5 ± SD; *, p<0.001 from each other by ANOVA posttest) C. RCT to the plasma and RBC compartments over 4 days after s.c injection of [3H]cholesterol labeled foam cells into apoAI−/− hosts (N=3 ± SD).
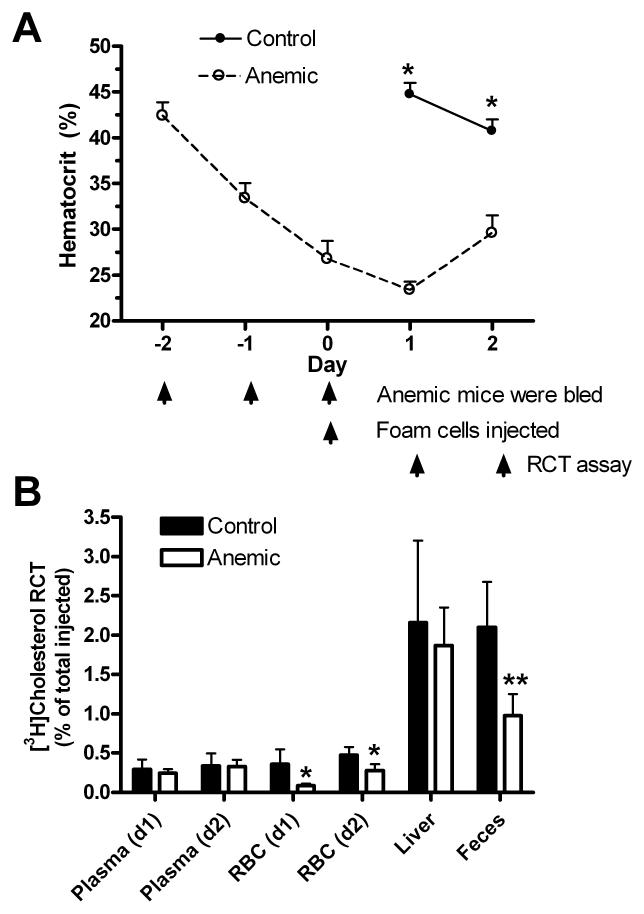
RBCs serving as a non-HDL pathway for RCT would be especially evident in apoAI−/− mice since they carry a greater fraction of cholesterol in the RBC compartment vs. the plasma compartment compared to wild type mice. Thus, decreasing the quantity of RBCs in apoAI−/− mice would be expected to decrease the transfer of [3H]cholesterol from foam cells to the feces. To test this hypothesis, we induced hemorrhagic anemia in apoAI−/− female mice by removing ~0.3 ml of blood from the retro-orbital plexus for three consecutive days, and on the third day (day 0 of the RCT assay in Fig. 2A) cholesterol labeled macrophages were injected s.c. into anemic and control apoAI−/− mice. Blood was drawn from each mouse on two subsequent days to determine hematocrits as well as the RCT to the plasma and RBC compartments. Feces were collected daily, and perfused livers were harvested on day 2. On days 1 and 2 of the RCT study, the hematocrits of the anemic mice were significantly lower than the control mice (Fig. 2A, p<0.001). The decreased hematocrit of the control mice on day 2 vs. day 1 was significant (p<0.01) and reproducible in response to the diagnostic bleeding on day 1. The increase in hematocrits in the anemic mice on day 2 vs. day 1 (p<0.001) was reproducible, and presumably reflects increased erythropoiesis induced by mild hypoxia that can overcome the small blood loss due to diagnostic bleeding on day 1. RCT to the plasma pool was low in both groups and not altered by anemia on either day (Fig. 2B). RCT to the RBCs was higher in the control group than the anemic group on both days (p<0.05), reflecting the smaller RBC pool in anemic mice. The sum of the RCT to the plasma and RBC compartments, namely RCT to whole blood, was reduced by 49% on day 1 and 25% on day 2. Anemia had no statistically significant effect on RCT to the liver, although there was a trend towards lower hepatic RCT in the anemic group. Cumulatively over two days, RCT to the feces was reduced by 54% in the anemic mice (Fig. 2B, p<0.01). We repeated the anemia experiment in male apoAI−/− mice and observed similar effects with a 38% decrease in fecal RCT (p<0.05) and no significant effect on hepatic RCT (data not shown). The experiment was repeated again in female apoAI−/− mice with two modifications: plasma removed during bleeding to induce anemia was reinjected i.v. to restore plasma protein; and, dried fecal weights were determined to ensure that decreased fecal RCT in the anemic group was not due to decreased fecal output. Anemia led to a 58% decrease in RCT to the feces over two days (p<0.01), no significant effect on hepatic RCT, but there was also a 33% decrease in dried fecal weight (p<0.05), which we attribute to the observed lethargy and presumed lower food consumption in the anemic mice. After normalizing RCT to the dried fecal mass, anemia still led to a 35% decrease in RCT/g feces (p<0.05). Thus, the decreased RBC pool size in anemic mice decreased RCT to the feces independent of any effects on decreased fecal output.
Figure 2.
A. Hematocrits of apoAI−/− mice throughout the RCT assay (N=4-5 ±SD; *, p<0.001 vs. controls by two-tailed t-test). B. RCT to various compartments in anemic and control mice (*, p<0.05 vs. control; **, p<0.01 vs. control). RCT to the feces is cumulative over two days.
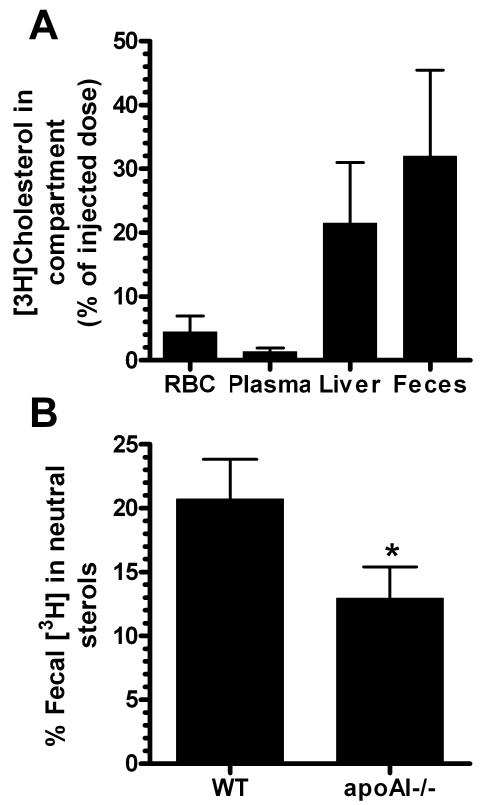
Our observation that anemia greatly impairs RCT to the feces without a significant effect on RCT to the liver suggests that RBCs may deliver their cholesterol to the feces by more than one route, such as the direct trans-intestinal cholesterol efflux pathway9, or that cholesterol delivered to the liver by RBCs vs. HDL is handled differently in the liver. Alternatively, anemia may modestly impair RCT to the liver, but this difference may be too small to be detected without more power. In order to determine whether RBC-cholesterol is efficiently transferred to the feces, we labeled mouse RBCs ex vivo with [3H]cholesterol and transfused them into apoAI−/− mice. Radioactivity in the RBCs, plasma, liver, and feces was measured two days later. Only 4.5% of the injected cholesterol tracer remained in the RBC fraction, demonstrating rapid turnover of this pool, and even less (1.3%) was found in the plasma (Fig. 3A). Most of the radioactivity was recovered in the liver (21.5%) and feces (32%). The observation that the feces and liver contain more of the tracer than the rest of the carcass is remarkable because it demonstrates highly robust transfer of RBC-cholesterol to the liver and feces despite diminished HDL, which is thought to mediate much of the hepatobiliary RCT pathway. To determine whether the trans-intestinal cholesterol efflux pathway might play a prominent role in delivery of RBC cholesterol to the feces, we compared the fraction of fecal [3H]cholesterol in neutral sterols (since direct intestinal cholesterol secretion would increase the fraction in neutral sterols vs. bile acids) between wild type mice (where plasma and RBC cholesterol pools are similar), and apoAI−/− mice (where RBCs constitute the major blood cholesterol pool). We found that the neutral sterol 3H fraction was 20.7 ± 3.1% in wild type mice, but only 13.0 ± 2.4% in apoAI−/− mice (p<0.05, Fig 3B). We observed a compensatory changes for the 3H recovered in the bile acid fraction with 80.2 ± 2.2 % in wild type mice and 88.6 ± 1.9% in apoAI−/− mice (p<0.01). Thus, in apoAI−/− mice where the majority of the RCT [3H]cholesterol pool of whole blood is in RBCs, this cholesterol is more efficiently converted into bile acids compared to wild type mice, suggesting more efficient bile acid synthesis from the RBC-cholesterol pool vs. the HDL-cholesterol pool. Combined with the finding of robust transfer of RBC-derived cholesterol tracer to the liver, our data support the model that RBC-cholesterol traffics through the liver more efficiently than HDL-cholesterol, whose uptake and metabolism is dependent upon SR-BI trafficking. Furthermore, our data suggests that the trans-intestinal pathway does not play a prominent role in RCT mediated through the RBC-cholesterol pool.
Figure 3.
A. Yields of radioactivity in various compartments two days after i.v. transfusion of [3H]cholesterol labeled RBCs into apoAI−/− hosts (N=4 ± SD). B. Percentage of fecal [3H] in neutral sterols from feces collected on day 2 after s.c. injection of [3H]cholesterol labeled foam cells into wild type (WT) and apoA−/− hosts (N=3 WT and 4 apoAI−/−, ± SD, *, p<0.05 vs. WT).
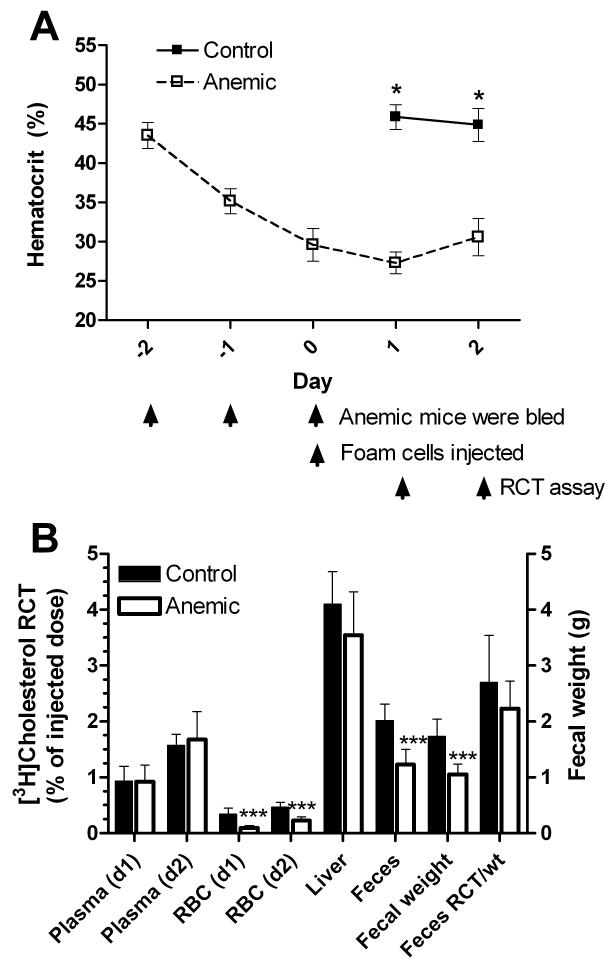
To determine if anemia could modulate RCT in mice with normal HDL-cholesterol levels, we repeated the anemia study previously done on apoA1−/−hosts, but this time in wild type male mice. We induced anemia by bleeding on days −2 to 0 of the anemia time course and the plasma recovered from whole blood was reinjected i.v. (n=7 each in the control and anemic groups), On days 1 and 2 of the RCT study, the hematocrits of the anemic mice were significantly lower than the control mice (Fig. 4A, p<0.001). RCT to the plasma pool in wild type mice was robust, compared to apoAI−/− mice, and not altered by anemia on either day (Fig. 4B). RCT to the RBCs was higher in the control group than the anemic group on both days (p<0.001), reflecting the smaller RBC pool in anemic mice. RCT to whole blood, the sum of RCT to the plasma and RBC compartments, was reduced by 20% on day 1 and only 7% on day 2 (compared to 49% and 25% reductions on days 1 and 2, respectively, in the experiment performed in apoA1−/− deficient hosts shown in Fig. 1B), reflecting the larger proportion of RCT mediated through the plasma (lipoprotein) compartment. Anemia had no statistically significant effect on RCT to the liver. Cumulatively over two days, RCT to the feces was reduced by 39% in the anemic mice (Fig. 4B, p<0.001). However, this effect was due to the significantly lower fecal mass in the anemic group (p<0.001), as after correction for fecal mass, there was only a non-significant trend with 17.4% reduced fecal RCT in the anemic group (Fig. 4B). A power analysis based on this data demonstrated that we would need ~30 mice per group to have 80% power to detect this effect at p<0.05. Since mice are by nature a high HDL species, it is not surprising that anemia did not have a significant impact on total fecal RCT in wild type mice over the two-day time course. On further examination, we found that RBCs accounted for 53.4 and 59.9% of whole blood RCT in apoAI−/− mice (day 1 and day 2, respectively), while only accounting for 9.6 and 12.1% of whole blood RCT in wild type mice (compare Figs. 2B and 4B).
Figure 4.
A. Hematocrits of wild type mice throughout the RCT assay (N=7 ± SD; *, p<0.001 vs. controls by two-tailed t-test). B. RCT to various compartments in anemic and control mice along with fecal weight (right axis) and fecal RCT adjusted to fecal weight (***, p<0.001 vs. control).
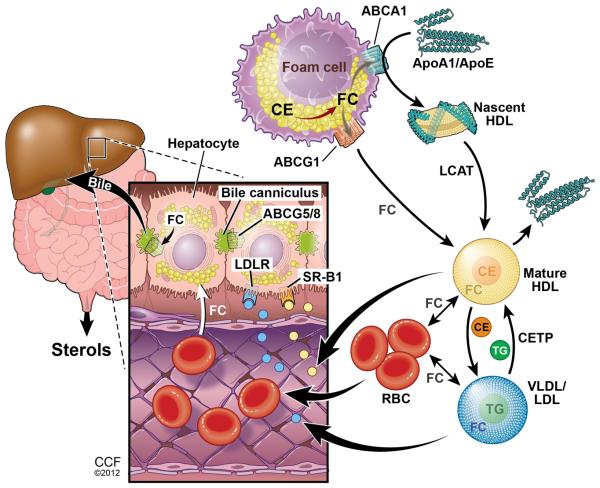
Here we show that the RBC-cholesterol pool may play a previously unknown role in mediating RCT. We propose a model (Fig. 5) in which interstitial apoAI, apoE, HDL and LDL can accept or exchange foam cell cholesterol and deliver it to the blood stream. In our studies in apoAI−/− mice, we suggest that lipid free apoE or other exchangeable apolipoproteins can pick up free cholesterol and phospholipids to form nascent HDL by interacting with ABCA1 on the foam cells. ApoE-containing HDL as well as LDL can also pick up or exchange with [3H]cholesterol in the foam cells and carry it into the blood stream. In whole blood, the free cholesterol pool can passively equilibrate between lipoproteins and cell membranes, with RBCs providing the bulk of cell membranes. Although CETP is not expressed in mice, in humans and other species where it is expressed, CETP may contribute to cholesterol ester exchange from HDL to LDL. When RBCs pass through the liver, they can deliver their cholesterol to sinusoidal endothelial cells which in turn can pass it to hepatocytes. Whether this step is entirely passive or may be facilitated by a transporter is unknown. Alternatively, cholesterol transfer from RBCs to hepatocytes may again be mediated via exchange to lipoproteins that can then migrate into the space of Disse and deliver cholesterol to hepatocytes. However, our finding of a smaller fraction of RCT-derived fecal neutral sterols in apoAI-deficient vs. wild type mice supports the notion that the transfer of RBC cholesterol to hepatocytes may at least partially be independent of lipoproteins. Once in the hepatocyte, most of the RBC-derived free cholesterol is efficiently converted to bile acids, which along with the remaining free cholesterol, is excreted into the bile and then into the intestine.
Figure 5.
Working model for the role of RBCs in RCT. Interstitial apoE, apoAI, HDL, and LDL can accept or exchange free cholesterol from foam cells and enter the blood stream. Lipoprotein free cholesterol can then be transferred to RBC plasma membranes. RBCs transit to the liver where they can donate free cholesterol directly to sinusoidal endothelial cells that are in contact with hepatocyte projections, or can deliver cholesterol to hepatocytes indirectly through cholesterol transfer to lipoproteins that can enter the space of Disse. Illustration by David Schumick, BS, CMI. Reprinted with the permission of the Cleveland Clinic Center for Medical Art & Photography © 2012. All Rights Reserved.
In a longitudinal study of 14,410 human subjects, anemia at baseline was associated with a significant hazard ratio of 1.41 for subsequent coronary vascular disease over a 6-year follow up.10 Although the mechanism for this association is unknown, our findings suggest that diminished RCT in anemic subjects may play a role in this association. Low HDL-cholesterol levels are common in males, and men with 35 mg/dl have HDL levels closer to those seen in apoAI−/− mice (12 mg/dl) than seen in wild type mice (93 mg/dl). Thus, anemia and hematocrits should not be overlooked in ongoing clinical studies of RCT and HDL function.
Acknowledgments
Sources of Funding: This work was supported by NIH grant HL098055 to JDS. KTH was supported by an American Heart Association Predoctoral Fellowship.
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Y, Zanotti I, Reilly MP, Glick JM, Rothblat GH, Rader DJ. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation. 2003;108:661–3. doi: 10.1161/01.CIR.0000086981.09834.E0. [DOI] [PubMed] [Google Scholar]
- 2.Nikolic M, Stanic D, Antonijevic N, Niketic V. Cholesterol bound to hemoglobin in normal human erythrocytes: a new form of cholesterol in circulation? Clin Biochem. 2004;37:22–6. doi: 10.1016/j.clinbiochem.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Lange Y, Molinaro AL, Chauncey TR, Steck TL. On the mechanism of transfer of cholesterol between human erythrocytes and plasma. J Biol Chem. 1983;258:6920–6. [PubMed] [Google Scholar]
- 4.Williamson R, Lee D, Hagaman J, Maeda N. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc Natl Acad Sci U S A. 1992;89:7134–8. doi: 10.1073/pnas.89.15.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin EM, Ishida BY, Clift SM, Krauss RM. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991;88:434–8. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik P, Berisha SZ, Santore J, Agatisa-Boyle C, Brubaker G, Smith JD. Zymosan-mediated inflammation impairs in vivo reverse cholesterol transport. J Lipid Res. 2011;52:951–7. doi: 10.1194/jlr.M011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–7. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 8.Robinet P, Wang Z, Hazen SL, Smith JD. A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells. J Lipid Res. 2010;51:3364–9. doi: 10.1194/jlr.D007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temel RE, Sawyer JK, Yu L, Lord C, Degirolamo C, McDaniel A, Marshall S, Wang N, Shah R, Rudel LL, Brown JM. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarnak MJ, Tighiouart H, Manjunath G, MacLeod B, Griffith J, Salem D, Levey AS. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi: 10.1016/s0735-1097(02)01938-1. [DOI] [PubMed] [Google Scholar]