Abstract
The enzyme-linked immunosorbent spot (ELISpot) assay is one of the most commonly used methods to measure antigen-specific T cells in both mice and humans. Some of the primary reasons for the popularity of the method are that ELISpot is highly quantitative, can measure a broad range of magnitudes of response and is capable of assessing critical cellular immune-related activities such as IFN-γ secretion and granzyme B release. Furthermore, ELISpot is adaptable not only to the evaluation of a variety of T-cell functions, but also to B cells and innate immune cells. It is no wonder that ELISpot has evolved from a research tool to a clinical assay. Recent Phase I and II studies of cancer vaccines, tested in a variety of malignancies, have suggested that ELISpot may be a useful biomarker assay to predict clinical benefit after therapeutic immune modulation. This article will discuss the most common applications of ELISpot, overview the efforts that have been undertaken to standardize the assay and apply the method in the analysis of human clinical trials, and describe some important steps in the process of developing a clinical-grade ELISpot.
Keywords: clinical trials, ELISpot, immune biomarker, quantitative, standardize
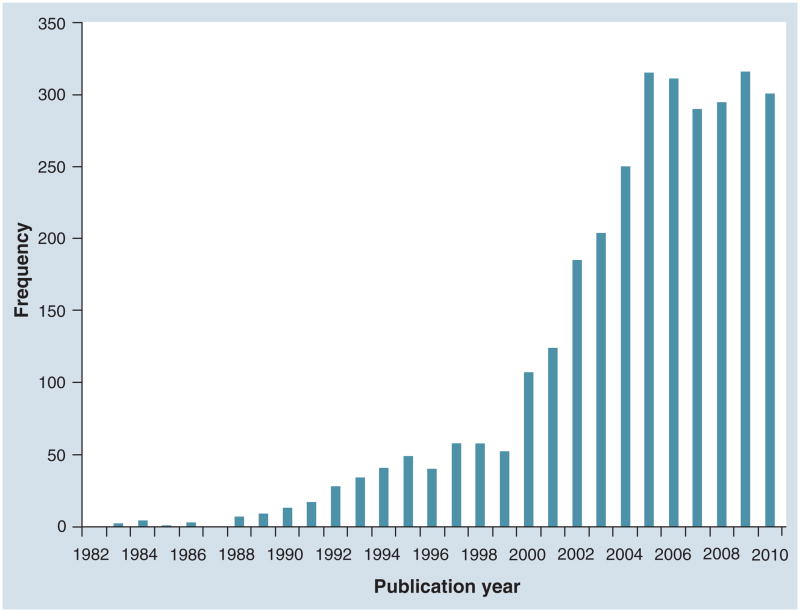
The first description of the enzyme-linked immunosorbent spot (ELISpot) assay was published over 25 years ago [1]. Since then, ELISpot has become one of the most commonly used immunoassays in the evaluation of human clinical trials of vaccines and other forms of immunotherapy. Indeed, the number of publications using ELISpot methodology has increased exponentially since that first report. We searched PubMed online for the keyword ‘ELISpot’ and downloaded all available citations as of early December 2010 (Figure 1). The first year with a publication citing ‘ELISpot’ per se was 1983, and since then the number of publications has gone up dramatically to 288 publications in 2005 and remains high. Originally, ELISpot was developed as an alternative to the hemolytic plaque [2] and the protein A plaque assay [3]. The ELISpot assay differed from the older techniques by omitting the use of conjugated red blood cells and replacing them with a stable plastic surface on which antigens were adhered. The spatial separation of the antigen-specific cells and the use of a visible spot to enumerate the cells allowed for improved quantitation of antibody-secreting cells.
Figure 1. Frequency of publications citing ELISpot.
Data were collected from a PubMed search for the keyword ‘ELISpot’. Publication dates were sorted by year and a histogram showing frequency of publications per year was generated using the XML code for date of publication. The figure was last updated in early December 2010.
Over the last two decades, a number of protocol enhancements have been published, beginning with the ‘reverse’ ELISpot technique (RELISpot). Adhering antibodies instead of antigens to the solid-phase surface reversed the protocol to detect secreted antigens instead of secreted antibodies. The introduction of nitrocellulose membranes and epitope-specific monoclonal antibodies allowed the ‘reverse’ detection of secreted IFN-γ, which resulted in the ELISpot assay being increasingly applied to the study of vaccine-induced responses [4,5]. Commercially available antibodies, 96-well nitrocellulose plates and, later, polyvinylidene fluoride plates, further enhanced the performance and high-throughput capabilities of the ELISpot assay. Indeed the adaptability of the assay to multiple platforms is a major reason for its popularity today (Box 1).
Box 1. Advantages of enzyme-linked immunosorbent spot assay.
Platform is adaptable to multiple cell types (e.g., T cells and B cells) as well as to multiple secreted products (e.g., cytokines and granzyme B)
Can be standardized across multiple laboratories
Quantitative
Measures cell function, which may be a predictor of outcome after immunotherapy
Lower limit of detection than flow-based immunoassays
Inexpensive
There are additional reasons for the rapid adoption of the assay as a workhorse in clinical immunologic monitoring (Box 1). Since the technique is similar to ELISA and western blotting, troubleshooting assay development uses methods familiar to most laboratory personnel. The assay has a lower limit of detection than immunoassays dependent on flow cytometric techniques. Moreover, the ELISpot provides not only a quantitative but also a functional readout for therapy-induced immune responses. Since ELISpot has been shown to be standardizable across laboratories and the method can be performed without expensive instrumentation, ELISpot has been used as a primary assay in clinical trials performed in developing countries. It should be noted, however, that the advent of the ELISpot reader has added to the standardization of the assay for clinical use as well as to the cost of the assay.
This article will outline the most common research and clinical applications of ELISpot, overview the efforts that have been ongoing in the standardization of the assay and, finally, provide information concerning some of the elements needed within a laboratory to execute ELISpot at a level suitable for clinical trials.
Common ELISpot adaptions for immune monitoring
IFN-γ ELISpot
Although the ELISpot assay was initially developed to detect antibody-secreting cells [1], it has been widely used to evaluate both CD4+ and CD8+ T cells responding to an antigenic or mitogenic stimulus [6,7]. Among the various types of ELISpot, the IFN-γ-based assay is the most common application of the technique [8,9].
IFN-γ is an abundant cytokine produced by Th1 cells and is frequently used to track specific CD8+ responses. As many different cytokines, including other Th1 cytokines (such as IL-2 and TNF-α) and also Th2 cytokines (such as IL-15 and IL-13) are produced by a smaller proportion of antigen-specific T cells, the use of ELISpot for these cytokines is often limited [10]. While IFN-γ-secreting antigen-specific T cells can be detected directly from the peripheral blood, detection of other types of cytokine-secreting cells may require an in vitro expansion step to augment low-level responses. The advent of the semi- or fully automated plate readers markedly increased the sensitivity and reproducibility of the IFN-γ ELISpot assay. Indeed, a lower limit detection threshold of less than 25 IFN-γ-producing T cells per million in peripheral blood mononuclear cells (PBMCs) has been reported [11].
The additional in vitro stimulation technique is specific to unmasking lower level responses as it has been shown to enhance the magnitude of IFN-γ ELISpot responses to cytomegalovirus peptides in serologically positive patients without generating false positive responses in serologically negative patients. The elicitation of these responses was 100% specific [12].
The IFN-γ ELISpot assay has been used extensively for the screening of immune responses in the development of vaccines for the prevention and treatment of many kinds of diseases including AIDS, malaria and TB [13,14]. While ELISpot responses to infectious disease antigens are easily detected, there has not been a strong correlation of IFN-γ-secreting cells and disease protection or pathogen-specific antibody responses [15,16]. It may be that the high baseline levels of immunity in patients with infectious disease preclude association of cellular immunity with disease progression or regression. Alternatively, clinically effective viral-specific immune responses may require the detection of more than a single type of cytokine-producing T cell. A modification of the IFN-γ ELISpot, which assesses the proliferative potential of IFN-γ-secreting cells by adding a culture step with peptide, has been shown to expand antigen-specific memory cells and may improve the correlative power of ELISpot in monitoring HIV treatment. Investigators demonstrated an inverse correlation of viremia with levels of HIV-specific memory T cells identified in this manner as well as a direct correlation of CD4+ counts with the ELISpot detected IFN-γ-secreting HIV-specific memory cells [17]. Of note, IFN-γ ELISpot responses were recently shown to correlate with resolution of vulvar intraepithelial neoplasia after the administration of a peptide-based human papillomavirus-specific vaccine (p = 0.02) [18].
Data have suggested that IFN-γ ELISpot responses induced by cancer vaccines may correlate with disease outcome. Clinical trials have shown a significant correlation of antigen-specific ELISpot responses with survival after a melanoma antigen-specific peptide-based vaccine in advanced-stage patients [19]. Moreover, the magnitude of antigen-specific IFN-γ-secreting cells, as measured by ELISpot, trended towards survival after the administration of a prostate-specific antigen vaccine in prostate cancer patients (p = 0.06) [20], as well as a human epidermal growth factor receptor 2 (HER2/neu) specific vaccine in breast cancer patients (p = 0.08) [21]. One of the major problems with attempting to define a correlation of clinical response with immune response as assessed by ELISpot is that, first, clinical responses generally occur in a minority of patients and, second, the clinical trials are grossly underpowered for a biomarker correlation to these responses. Unfortunately, larger randomized clinical trials, for the most part, do not include performance of ELISpot analysis. Larger studies with greater numbers of patients matched with appropriate controls will be needed to validate ELISpot or any other immunologic marker as a correlate to a clinical response.
Granzyme B ELISpot
One of the mechanisms of cell cytotoxicity involves the release of cytoplasmic granular proteins, including the pore-forming protein, perforin, and a family of serine proteases called granzymes, for example, granzyme B. Granzyme B is secreted mainly from CD8+ cytotoxic lymphocytes and natural killer cells and may induce the killing of virus-infected and tumor cells [22–25]. The detection of granzyme B-secreting cells via ELISpot is well established and previous studies have reported that granzyme B ELISpot has excellent correlation with the 51Cr-release assay for measuring cytotoxic activity of effector cells including cytotoxic lymphocytes and natural killer cells [26–28]. Compared to the IFN-γ ELISpot assay, the granzyme B ELISpot may be a more direct measure of cytotoxic cell activity because granzyme B is one of the primary molecules in effector cell-mediated killing.
TGF-β1 ELISpot
TGF-β plays an important role in tumor initiation and progression, functioning as both a suppressor and a promoter and also regulating infiltration of inflammatory/immune cells and cancer-associated fibroblasts in the tumor microenvironment. Development of an ELISpot assay for TGF-β1 was initially difficult as the majority of TGF-β1 was produced by mononuclear cells in the latent form. Recently, however, reagents have become available, such as TGF-β1 soluble receptor type II and antibodies to TGF-β1 that specifically recognize active TGF-β1 [29]. Investigators have reported that there was significant correlation between the frequency of TGF-β1-producing cells as measured by ELISpot and total secreted TGF-β1 as measured by ELISA in patients infected with Mycobacterium avium [30]. It has been suggested that TGF-β1 ELISpot assays are 100–200-fold more sensitive than ELISA in the detection of the cytokines from whole-cell secretions [31,32].
Fluorescent ELISpot
Fluorescent ELISpot assays using fluorophore-labeled detection antibodies have been shown to perform similarly to immuno-enzymatic ELISpots [33]. Moreover, using fluorescent-labeled capture antibodies allows detection of multiple cytokine production from the same cell. Careful optimization must be carried out to ensure that cross-reactivity between anticytokine antibodies is eliminated and that results from the multiple-cytokine assay are consistent with single-color assays done in the same experiment. A specialized plate reader is required for fluorospot assays [33].
Additional cytokines detectable using an ELISpot platform
Many other cytokines (IL-1β, -2, -4, -6, -8, -10, -12 and -17, and TNF-α) and chemokines have been evaluated by ELISpot assay in preclinical and clinical studies. ELISpot kits assessing these cytokines are now, for the most part, commercially available [34–38]. Recent approaches have combined the detection of two or more different cytokines simultaneously in a multicolor ELISpot with reproducible results leading to an increased range of detectable responses as well as the differentiation of the quality of the response. Some complicating factors in the use of the multicolor ELISpot are the necessity for greater amounts of cells per well, the increase in assay duration, and the potential decrease in sensitivity of the assay when multiple colors are used [38], although in some cases these have not been shown to limit the use of the assay. Other limiting factors may be the difficulty in discriminating dual-stained spots, the technical challenges involved in obtaining similarly defined spots for both cytokines and, in the case of the IL-2/IFN-γ dual-color assay, overcoming IL-2 dependence for IFN-γ secretion. Some of these limitations may be surmounted by choosing the fluorospot assay as the mode of analysis, but this assay is not without its own limitations as described previously.
Detecting polyfunctional T cells
Polyfunctional T cells may be important in mediating disease control [38]. In HIV trials in particular, single-color ELISpot assays were used along with intracellular cytokine staining to detect IL-2, IFN-γ and TNF-α, and when combined with limiting dilution assays to detect cytotoxic T-cell precursor frequency, polyfunctional T cells were shown to have increased following treatment. By using the generation of polyfunctional T cells as a marker for effective vaccination, investigators demonstrated a negative correlation between effective vaccination and HIV viral load, although as the patients in this study were also using antiretroviral therapy, the clinical effect of vaccination remains unclear [39]. The assessment of polyfunctional T cells is typically done using flow cytometry-based assays such as intracellular cytokine staining, and has been correlated with vaccination in different settings [40]. Assessing polyfunctional T cells by ELISpot to detect multiple cytokines may improve the correlation of immunological monitoring assays with clinical response and allow the evaluation of effective vaccination.
Translation of ELISpot from a research to a clinical tool
As stated earlier, ELISpot is a sensitive assay that can detect a low frequency of antigen-specific T cells; however, the multiple steps comprising the assay can lead to high variation and imprecision if the method is not validated. A variety of factors impact the performance characteristics of ELISpot; these include: cell recovery and viability, operator technique and proficiency, the different vendors and lots of reagents, experimental protocols used for assay execution, protein additives in media, manual or automated spot enumeration and data analysis.
One of the most critical steps in standardizing ELISpot is to ensure adequate cell recovery and viability after rescuing PBMCs from cryopreservation. This issue is particularly important depending on what cell populations are being used for the assay. If unfractionated PBMCs are used for ELISpot, the method of cryopreservation could impact the function of endogenous antigen-presenting cells thus decreasing T-cell activity. If T cells are to be purified prior to analysis, the method of cryopreservation could significantly impact the recovery of viable cells. As the purification process consumes many cells, fewer viable cells available for purification may mean that assays cannot be performed as designed. The adult AIDS Clinical Trials program demonstrated that retaining a cell viability of 70% or greater after thawing was associated with maintenance of functional responses [41]. Our group has reported that viability after thawing was significantly negatively impacted by the use of human AB serum in the cryopreservation media (p < 0.001) and the temperature of the cells after the thaw. Reconstituting the cells in media that was chilled to 4°C reduced viability by nearly 30% as compared with media warmed to 37°C [42]. ELISpot can be optimized for serum-free conditions as well [43]. A comparative study, performed at multiple different sites, using serum-free or serum-containing media, demonstrated no significant difference in the number of viable cells retrieved, the background of the assay or the ability to detect antigen-specific responses between the two types of media [43,44].
Once assay issues have been standardized, ELISpot has been shown to have a significant correlation with T-cell responses measured by another method, such as cytokine flow cytometry, in the same samples [45]. Comparative analyses have demonstrated that ELISpot has a higher coefficient of variation (CV) for intra-assay precision and inter-operator precision compared with flow cytometric-based assays such as MHC tetramer and cytokine flow cytometry [46]. Standardization of ELISpot around common validated protocols is necessary for the clinical application of the method to immunotherapy trials. In order to establish the feasibility of the assay to precisely monitor human vaccine clinical trials, the reproducibility of ELISpot in multiple laboratories has been studied in several proficiency panels. ELISpot assays can provide reproducible results among different laboratories when the assay procedure and data analysis are standardized [47,48]. With good laboratory practice and standard operating procedures (SOPs), an interlaboratory CV for the frequency of antigen-specific T cells was found to be less than 20% [49]. Other proficiency panel studies have shown that strict adherence to protocols that detail cell handling, ELISpot execution and plate reading can greatly improve the quality of the data generated by a poorly performing laboratory [50]. In particular, the issue of high assay background may be addressed through careful reagent choice [51], optimized sample processing and handling [45,52], a standard method of assay optimization using the same specimen set to ensure consistent results [51], and additional optimization strategies [53]. The choice of antigen-presenting cell used also affects background levels and ELISpot results [51].
There has been much discussion of how to define the ‘response to vaccination’ using the ELISpot assay. As mentioned previously, in clinical trials of both infectious disease and cancer vaccines, subjects frequently enter the study with significant detectable levels of antigen-specific T cells, as measured by ELISpot, prior to receiving the immune-based intervention. In many clinical trials, owing to the small number of patients enrolled and the limited number of samples, the data are not normally distributed and a Student’s T-test method is not suitable for defining positive response [54]. Different standards have been proposed to define positive responses induced by vaccines, such as fourfolds of negative control [55], and >50 spots per 106 PBMC [49]. In cancer vaccine trials, an additional criterion that is commonly used is the mean and two standard deviations (SDs) of pre-existing tumor antigen-specific T-cell responses; measuring a value greater than this criterion post-vaccination may be used to define whether a vaccine increased antigen-specific INF-γ-secreting cells [21], as two SDs is equivalent to a p-value of 0.05 [56]. A recent report has analyzed various statistical methods on a single sample set and has shown the strengths and weaknesses of different statistical approaches. The authors suggest the use of a nonparametric statistical test to define augmentation of an immune response as measured by IFN-γ ELISpot [54].
There are multiple published tools available to the user for the standardization and analysis of ELISpot assays. While reproducible data can be achieved with the adaptation of these tools, other issues need to be addressed in the development of a clinical laboratory that can maintain and track quality performance of the ELISpot over time.
Maintaining the quality performance of ELISpot
In addition to simply developing a SOP for the execution of ELISpot, other steps must be taken to ensure that not only does the assay perform as expected initially, but that it continues to perform to clinical standards over time. A documented training protocol, reagent validation, a readily available biorepository of normal and cancer donors, and regular quality assurance reporting and data analysis are all crucial parts of immune monitoring programs that maintain quality ELISpot performance. Many laboratories do not have the qualified personnel or the volume of assays necessary to make an investment in developing a quality program for ELISpot. However, there are many resources available, both at academic medical centers as well as in the private sector, for the contracting of clinical-grade ELISpot assays.
Laboratory training
A comprehensive training program covers both general institutional training as well as assay-specific training and includes performance standards. In the case of ELISpot, we find proficiency training requires a longer time to complete as compared with more automated assays (e.g., ELISA) as the ELISpot requires a higher level of technical proficiency. In general, for assay performance, the new or re-certifying employee observes the assay performed by an experienced technician with a validated technique. After observing the assay, the new employee will perform the assay under close supervision and step-by-step guidance from an experienced technician. The assay will be repeated under close supervision until the results and reproducibility of the assay fall within acceptable laboratory values of known standards. At that point, precision, accuracy and intra- and inter-assay variability should be assessed across a larger number of samples to ensure the new employee’s assay performance characteristics are in accordance with the rest of the laboratory.
In our Immunologic Monitoring Core Laboratory, samples that have been previously characterized for CMV responses and are derived from our repository are used for training. If the repeated results fall within the assay validation standards, the new employee is considered fully trained and allowed to perform clinical assays unsupervised. The plate controls (CMV-positive samples) for the new employee are monitored weekly during their probationary period for deviations. All plate controls on a study are trended monthly, and all employees must perform proficiency testing quarterly to maintain assay validation standards.
Training documentation
Laboratory training documentation is managed centrally to ensure compliance with federal, state and local health, safety and security regulations as well as job-specific requirements. An audit of individual employee training should be performed annually, and employees should be certified during the first 6 months of employment, re-certified at their 1-year employment anniversary, and reviewed at least annually thereafter. Each laboratory employee should complete SOP training when the SOPs are initially established as well as when they are modified. All original training data should be kept on file for review by laboratory supervisors and regulators. Ensuring that all assays performed in the laboratory are done by qualified technicians who can achieve comparable results is essential to a successful immune monitoring program.
Reagent validation
Prior to beginning a clinical trial, all reagents should be tested to ensure that assays performed using those reagents perform at historical standards. All efforts must be made to ensure that clinical trials can be completed with as few reagent lot changes as possible by ordering particular lot numbers in bulk. If new reagents are added during the conduct of a trial, a defined reference population must be used to compare the old, validated reagent with the new reagent to be introduced. Assay performance must conform to the standards set in order for the new reagent to be introduced into the study. Reagent validation reports should summarize the performance comparison between old and new reagents. Tagging data with reagent lot numbers in an experimental data management database simplifies this process.
Performance standards
All assays should include controls: typically, ELISpot assays include a ‘no antigen’ control in which the cells are plated in the media of choice, a positive control such as phytohemagglutinin (PHA) or phorbol myristate acetate (PMA), and a negative control, which can be an irrelevant antigen or a media-only well, preferably both if available cell number allows. A positive control sample should be run on each plate to track inter-assay variability as discussed previously. Because ELISpot is a sensitive assay with a relatively large detection range, it is also important to define different response levels. Variation of the assay often differs with different level of responses so assay validation must include assessments at the low, medium and high range of assay performance. Adherence to quality standards must be maintained across the full range of detectable responses.
Prior to assay use in a clinical trial, we refine the assays to the specific antigen system and establish performance characteristics. To define the normal range of response, we use PBMCs from 20 normal donors derived from our biorepository. This population is also used as our reference population for qualifying lots of reagents as discussed previously. In addition, these donors will be earmarked for plate controls and ongoing quality assurance throughout the course of the study. We evaluate these 20 donors for responses to our standard set of positive and negative controls as well as the antigens specific to the trial. No-antigen and media-only wells are used as background controls and CMV, Epstein–Barr virus and influenza virus (CEF) peptide mix, and PHA are positive controls. Assays are performed in six to eight well replicates. Once the assay is standardized, we also evaluate ten cancer patient samples to determine if there is a pre-existing response to the antigen. This data is helpful in providing methods to determine what is positive versus negative during the course of a trial. The definition of response in normal controls allows assessment of the sensitivity and specificity of the assay for the intervention. A leukapheresis program for collection and storage of control populations is critical for maintaining assay performance.
Continuing assay performance reporting
Frequent quality assurance monitoring such as monthly performance reports includes the standard positive and negative control results for every assay run that month. Average values and SDs are compared with historical results, and CV for all assays must remain within laboratory standard values. Deviation will trigger an investigation and further sample analysis will be halted until the problem is identified, documented and corrected. The control data are trended monthly to insure inter- and intra-assay performance. In addition, results of no-antigen wells along with mitogen controls should be trended to ensure each assay is performing within limits. Positive no-antigen wells and negative mitogen wells are commonly used as prospectively defined criteria for assay exclusion. If at any point the monthly or quarterly reports show assay performance that does not conform to the standards set, troubleshooting and assay testing is required to determine the source of the issue and to bring the assay back to standard.
These are just a few of the issues that should be considered when preparing to develop ELISpot as an immunologic monitoring tool for the assessment of human clinical trials of immune-based interventions. Further issues are addressed in recent publications assessing results across multiple institutions [57].
Expert commentary
The ELISpot assay is functional, highly quantitative, and has a large range of detection. This makes the assay suitable to a wide variety of clinical applications. ELISpot can assess cytokine release from antigen-specific cells as well as cytokine secretion from activated innate immune cells. The ability of the assay to be adapted to measure the response of multiple different cell types to a stimulus has made ELISpot an important tool in monitoring human immunity.
Although ELISpot is a cell-based assay, multiple studies have demonstrated that ELISpot can be perfected to meet rigorous performance standards. Multiple excellent SOPs are available for the conduct of ELISpot and reagents are available to expand the capability of the assays to assess different effector functions and even simultaneous secretion of a variety of cytokines from single cells.
Thus, ELISpot may not only provide a potency assay to assess the ability of a vaccine to induce an immune response, but also a biomarker of vaccine efficacy. Clinical trials should be designed to attempt to validate ELISpot as a biomarker of clinical response after immune intervention.
Five-year view
With the advent of several immune-based therapies appearing to have beneficial clinical effects, especially in cancer patients, the ability to monitor the immune response stimulated by those therapies will be critical. Assays will have to be simple, inexpensive and highly accurate to be able to be adapted to pivotal Phase III clinical trials. Evaluating the remarkable evolution of ELISpot from its origins into the immune monitoring tool that it has become today, there is no doubt there will be future modifications of the assay that further simplify and expand its capabilities. ELISpot remains a tool that can assess a large variety of immune effectors as well as the multiple functions of those effectors. Validation of ELISpot as a potential immunologic biomarker assay with prognostic significance would ensure ongoing adaptation of the assay as a primary mode for measuring human immunity in clinical trials.
Key issues.
Enzyme-linked immunosorbent spot (ELISpot) is a highly flexible assay and can be adapted to multiple readout formats.
ELISpot assays are quantitative and measure key cellular functions of immune system cells.
ELISpot has been used to assess both adaptive and innate immune responses.
The ELISpot assay can be standardized across multiple laboratories using common standard operating procedures, which have resulted in the translation of the assay from the laboratory to a clinical tool.
Preliminary data in cancer immunotherapy trials suggests that the magnitude of the ELISpot response may be associated with treatment-related clinical benefit.
Adaptation of ELISpot to clinical use requires development of a quality program as well as rigorous tracking of assay performance characteristics.
Acknowledgments
The authors thank Josh Aaseng for excellent assistance in manuscript preparation.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants R01CA136632 and P50CA083636, and also by the Ovarian Cancer Research Fund. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1••.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISpot) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65(1–2):109–121. doi: 10.1016/0022-1759(83)90308-3. Original paper describing enzyme-linked immunosorbent spot (ELISpot) [DOI] [PubMed] [Google Scholar]
- 2.Jerne NK, Nordin AA. Plaque formation in agar by single antibody-producing cells. Science. 1963;140(3565):405. [PubMed] [Google Scholar]
- 3.Gronowicz E, Coutinho A, Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- 4.Czerkinsky C, Prince SJ, Michalek SM, et al. Oral immunization with bacterial antigen induces IgA-secreting cells in peripheral blood in humans. Adv Exp Med Biol. 1987;216B:1709–1719. [PubMed] [Google Scholar]
- 5.Czerkinsky CC, Tarkowski A, Nilsson LA, et al. Reverse enzyme-linked immunospot assay (RELISpot) for the detection of cells secreting immunoreactive substances. J Immunol Methods. 1984;72(2):489–496. doi: 10.1016/0022-1759(84)90017-6. [DOI] [PubMed] [Google Scholar]
- 6.Taguchi T, McGhee JR, Coffman RL, et al. Detection of individual mouse splenic T cells producing IFN-γ and IL-5 using the enzyme-linked immunospot (ELISpot) assay. J Immunol Methods. 1990;128(1):65–73. doi: 10.1016/0022-1759(90)90464-7. [DOI] [PubMed] [Google Scholar]
- 7.Miyahira Y, Murata K, Rodriguez D, et al. Quantification of antigen specific CD8+ T cells using an ELISpot assay. J Immunol Methods. 1995;181(1):45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 8.Lalvani A, Brookes R, Hambleton S, et al. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186(6):859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herr W, Protzer U, Lohse AW, et al. Quantification of CD8+ T lymphocytes responsive to human immunodeficiency virus (HIV) peptide antigens in HIV-infected patients and seronegative persons at high risk for recent HIV exposure. J Infect Dis. 1998;178(1):260–265. doi: 10.1086/517449. [DOI] [PubMed] [Google Scholar]
- 10.Letsch A, Scheibenbogen C. Quantification and characterization of specific T-cells by antigen-specific cytokine production using ELISpot assay or intracellular cytokine staining. Methods. 2003;31(2):143–149. doi: 10.1016/s1046-2023(03)00124-5. [DOI] [PubMed] [Google Scholar]
- 11.Schmittel A, Keilholz U, Scheibenbogen C. Evaluation of the interferon-γ ELISpot-assay for quantification of peptide specific T lymphocytes from peripheral blood. J Immunol Methods. 1997;210(2):167–174. doi: 10.1016/s0022-1759(97)00184-1. [DOI] [PubMed] [Google Scholar]
- 12.Godard B, Gazagne A, Gey A, et al. Optimization of an elispot assay to detect cytomegalovirus-specific CD8+ T lymphocytes. Hum Immunol. 2004;65(11):1307–1318. doi: 10.1016/j.humimm.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Hill PC, Brookes RH, Fox A, et al. Longitudinal assessment of an ELISpot test for Mycobacterium tuberculosis infection. PLoS Med. 2007;4(6):e192. doi: 10.1371/journal.pmed.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kester KE, Cummings JF, Ockenhouse CF, et al. Phase 2a trial of 0, 1, and 3 month and 0, 7, and 28 day immunization schedules of malaria vaccine RTS,S/AS02 in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. 2008;26(18):2191–2202. doi: 10.1016/j.vaccine.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Lambotte O, Ferrari G, Moog C, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS. 2009;23(8):897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray CM, Mlotshwa M, Riou C, et al. Human immunodeficiency virus-specific γ interferon enzyme-linked immunospot assay responses targeting specific regions of the proteome during primary subtype C infection are poor predictors of the course of viremia and set point. J Virol. 2009;83(1):470–478. doi: 10.1128/JVI.01678-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calarota SA, Foli A, Maserati R, et al. HIV-1-specific T cell precursors with high proliferative capacity correlate with low viremia and high CD4 counts in untreated individuals. J Immunol. 2008;180(9):5907–5915. doi: 10.4049/jimmunol.180.9.5907. [DOI] [PubMed] [Google Scholar]
- 18.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood JM, Lee S, Moschos SJ, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/− granulocyte-monocyte colony-stimulating factor and/or IFN-α2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15(4):1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Disis ML, Wallace DR, Gooley TA, et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J Clin Oncol. 2009;27(28):4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafer-Weaver K, Sayers T, Strobl S, et al. The Granzyme B ELISpot assay: an alternative to the 51Cr-release assay for monitoring cell-mediated cytotoxicity. J Transl Med. 2003;1(1):14. doi: 10.1186/1479-5876-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinchieri G, Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984;50(5):489–513. [PubMed] [Google Scholar]
- 24.Smyth MJ, Trapani JA. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995;16(4):202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 25.Finn OJ, Lotze MT. A decade in the life of tumor immunology. Clin Cancer Res. 2001;7(Suppl 3):759S–760S. [PubMed] [Google Scholar]
- 26.Bleackley RC, Lobe CG, Duggan B, et al. The isolation and characterization of a family of serine protease genes expressed in activated cytotoxic T lymphocytes. Immunol Rev. 1988;103:5–19. doi: 10.1111/j.1600-065x.1988.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 27.Doherty PC, Christensen JP. Accessing complexity: the dynamics of virus-specific T cell responses. Annu Rev Immunol. 2000;18:561–592. doi: 10.1146/annurev.immunol.18.1.561. [DOI] [PubMed] [Google Scholar]
- 28.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 29.Assoian RK, Fleurdelys BE, Stevenson HC, et al. Expression and secretion of type β transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aung H, Sherman J, Tary-Lehman M, Toossi Z. Analysis of transforming growth factor-beta 1 (TGF-β1) expression in human monocytes infected with Mycobacterium avium at a single cell level by ELISpot assay. J Immunol Methods. 2002;259(1–2):25–32. doi: 10.1016/s0022-1759(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 31.Kabilan L, Andersson G, Lolli F, et al. Detection of intracellular expression and secretion of interferon-γ at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur J Immunol. 1990;20(5):1085–1089. doi: 10.1002/eji.1830200521. [DOI] [PubMed] [Google Scholar]
- 32.Hagiwara E, Abbasi F, Mor G, Ishigatsubo Y, Klinman DM. Phenotype and frequency of cells secreting IL-2, IL-4, IL-6, IL-10, IFN and TNF-α in human peripheral blood. Cytokine. 1995;7(8):815–822. doi: 10.1006/cyto.1995.0098. [DOI] [PubMed] [Google Scholar]
- 33.Gazagne A, Claret E, Wijdenes J, et al. A fluorospot assay to detect single T lymphocytes simultaneously producing multiple cytokines. J Immunol Methods. 2003;283(1–2):91–98. doi: 10.1016/j.jim.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Kalogerakou F, Albanidou-Farmaki E, Markopoulos AK, Antoniades DZ. Detection of T cells secreting type 1 and type 2 cytokines in the peripheral blood of patients with oral lichen planus. Hippokratia. 2008;12(4):230–235. [PMC free article] [PubMed] [Google Scholar]
- 35.Smedman C, Gardlund B, Nihlmark K, et al. ELISpot analysis of LPS-stimulated leukocytes: human granulocytes selectively secrete IL-8, MIP-1β and TNF-α. J Immunol Methods. 2009;346(1–2):1–8. doi: 10.1016/j.jim.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci. 2010;51(6):3083–3091. doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samaras V, Piperi C, Levidou G, et al. Analysis of interleukin (IL)-8 expression in human astrocytomas: associations with IL-6, cyclooxygenase-2, vascular endothelial growth factor, and microvessel morphometry. Hum Immunol. 2009;70(6):391–397. doi: 10.1016/j.humimm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 38••.Boulet S, Ndongala ML, Peretz Y, et al. A dual color ELISpot method for the simultaneous detection of IL-2 and IFN-γ HIV-specific immune responses. J Immunol Methods. 2007;320(1–2):18–29. doi: 10.1016/j.jim.2006.11.010. Excellent methods covering the development of dual-color ELISpot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valor L, Navarro J, Carbone J, et al. Immunization with an HIV-1 immunogen induces CD4+ and CD8+ HIV-1-specific polyfunctional responses in patients with chronic HIV-1 infection receiving antiretroviral therapy. Vaccine. 2008;26(22):2738–2745. doi: 10.1016/j.vaccine.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 41.Weinberg A, Zhang L, Brown D, et al. Viability and functional activity of cryopreserved mononuclear cells. Clin Diagn Lab Immunol. 2000;7(4):714–716. doi: 10.1128/cdli.7.4.714-716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Disis ML, dela Rosa C, Goodell V, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308(1–2):13–18. doi: 10.1016/j.jim.2005.09.011. Validated cryopreservation protocol for lymphocytes. [DOI] [PubMed] [Google Scholar]
- 43••.Mander A, Gouttefangeas C, Ottensmeier C, et al. Serum is not required for ex vivo IFN-γ ELISpot: a collaborative study of different protocols from the European CIMT Immunoguiding Program. Cancer Immunol Immunother. 2010;59(4):619–627. doi: 10.1007/s00262-009-0814-4. Protocol for the use of nonserum-containing media for ELISpot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janetzki S, Price L, Britten CM, et al. Performance of serum-supplemented and serum-free media in IFNγ ELISpot assays for human T cells. Cancer Immunol Immunother. 2010;59(4):609–618. doi: 10.1007/s00262-009-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maecker HT, Moon J, Bhatia S, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISpot. BMC Immunol. 2005;6:17. doi: 10.1186/1471-2172-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Maecker HT, Hassler J, Payne JK, et al. Precision and linearity targets for validation of an IFNγ ELISpot, cytokine flow cytometry, and tetramer assay using CMV peptides. BMC Immunol. 2008;9:9. doi: 10.1186/1471-2172-9-9. Comparison of the perfomance of ELISpot, tetramer and flow cytometry for detecting antigen-specific responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Caspell R, Karulin AY, et al. ELISpot assays provide reproducible results among different laboratories for T-cell immune monitoring – even in hands of ELISpot-inexperienced investigators. J Immunotoxicol. 2009;6(4):227–234. doi: 10.3109/15476910903317546. [DOI] [PubMed] [Google Scholar]
- 48.Boaz MJ, Hayes P, Tarragona T, et al. Concordant proficiency in measurement of T-cell immunity in human immunodeficiency virus vaccine clinical trials by peripheral blood mononuclear cell and enzyme-linked immunospot assays in laboratories from three continents. Clin Vaccine Immunol. 2009;16(2):147–155. doi: 10.1128/CVI.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samri A, Durier C, Urrutia A, et al. Evaluation of the interlaboratory concordance in quantification of human immunodeficiency virus-specific T cells with a γ interferon enzyme-linked immunospot assay. Clin Vaccine Immunol. 2006;13(6):684–697. doi: 10.1128/CDLI.00387-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Janetzki S, Panageas KS, Ben-Porat L, et al. Results and harmonization guidelines from two large-scale international ELISpot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol Immunother. 2008;57(3):303–315. doi: 10.1007/s00262-007-0380-6. Assessment of the performance of ELISpot across multiple institutions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janetzki S, Cox JH, Oden N, Ferrari G. Standardization and validation issues of the ELISpot assay. Methods Mol Biol. 2005;302:51–86. doi: 10.1385/1-59259-903-6:051. [DOI] [PubMed] [Google Scholar]
- 52.Weinberg A, Song LY, Wilkening CL, et al. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISpot assays. J Immunol Methods. 2010;363(1):42–50. doi: 10.1016/j.jim.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalyuzhny A, Stark S. A simple method to reduce the background and improve well-to-well reproducibility of staining in ELISpot assays. J Immunol Methods. 2001;257(1–2):93–97. doi: 10.1016/s0022-1759(01)00451-3. [DOI] [PubMed] [Google Scholar]
- 54••.Moodie Z, Price L, Gouttefangeas C, et al. Response definition criteria for ELISpot assays revisited. Cancer Immunol Immunother. 2010;59(10):1489–1501. doi: 10.1007/s00262-010-0875-4. Thoughtful analysis on defining positive responses by ELISpot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubey S, Clair J, Fu TM, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr. 2007;45(1):20–27. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 56.Pagano M, Gauvreau K. Principles of Biostatistics. 2. Duxbury Press; Pacific Grove, CA, USA: 2000. [Google Scholar]
- 57.Hanekom WA, Dockrell HM, Ottenhoff TH, et al. Immunological outcomes of new tuberculosis vaccine trials: WHO panel recommendations. PLoS Med. 2008;5(7):e145. doi: 10.1371/journal.pmed.0050145. [DOI] [PMC free article] [PubMed] [Google Scholar]