Abstract
Objective
To determine whether high concentration oxygen increases the PaCO2 in the treatment of community-acquired pneumonia.
Design
Randomized controlled clinical trial in which patients received high concentration oxygen (8 L/min via medium concentration mask) or titrated oxygen (to achieve oxygen saturations between 93 and 95%) for 60 minutes. Transcutaneous CO2 (PtCO2) was measured at 0, 20, 40 and 60 minutes.
Setting
The Emergency Departments at Wellington, Hutt and Kenepuru Hospitals.
Participants
150 patients with suspected community-acquired pneumonia presenting to the Emergency Department. Patients with chronic obstructive pulmonary disease (COPD) or disorders associated with hypercapnic respiratory failure were excluded.
Main outcome variables
The primary outcome variable was the proportion of patients with a rise in PtCO2 ≥4 mmHg at 60 minutes. Secondary outcome variables included the proportion of patients with a rise in PtCO2 ≥8 mmHg at 60 minutes.
Results
The proportion of patients with a rise in PtCO2 ≥4 mmHg at 60 minutes was greater in the high concentration oxygen group, 36/72 (50.0%) vs 11/75 (14.7%), relative risk (RR) 3.4 (95% CI 1.9 to 6.2), P < 0.001. The high concentration group had a greater proportion of patients with a rise in PtCO2 ≥8 mmHg, 11/72 (15.3%) vs 2/75 (2.7%), RR 5.7 (95% CI 1.3 to 25.0), P = 0.007. Amongst the 74 patients with radiological confirmation of pneumonia, the high concentration group had a greater proportion with a rise in PtCO2 ≥4 mmHg, 20/35 (57.1%) vs 5/39 (12.8%), RR 4.5 (95% CI 1.9 to 10.6) P < 0.001.
Conclusions
We conclude that high concentration oxygen therapy increases the PtCO2 in patients presenting with suspected community-acquired pneumonia. This suggests that the potential increase in PaCO2 with high concentration oxygen therapy is not limited to COPD, but may also occur in other respiratory disorders with abnormal gas exchange.
Introduction
Community-acquired pneumonia is a common respiratory condition associated with significant morbidity and risk of mortality.1 Patients presenting with suspected community-acquired pneumonia routinely receive oxygen therapy, however there are no randomized controlled trials of the benefits or risks of this approach. Hence, it is not known whether high concentration oxygen therapy in this setting might increase arterial CO2 tension (PaCO2) similar to the physiological response to such therapy in chronic obstructive pulmonary disease (COPD) exacerbations.2,3 In support of this possibility, it has been demonstrated that in COPD the increase in PaCO2 with oxygen therapy may result not only from suppression of the hypoxic respiratory drive, but also from the release of hypoxic pulmonary vasoconstriction.4–7 This leads to worsening ventilation perfusion inequality, resulting in an increase in the physiological dead space and thus CO2 retention. Furthermore, there is evidence that oxygen therapy may also result in an increase in PaCO2 in severe asthma,8–10 suggesting that this physiological response may be present in other acute respiratory conditions in which ventilation/perfusion abnormalities are present.
In this randomized controlled trial, we sought to investigate the effects of high concentration oxygen therapy on PaCO2 in patients presenting to the Emergency Department (ED) with suspected community-acquired pneumonia. Comparison was made with oxygen therapy titrated as required to relieve hypoxaemia but avoid hyperoxaemia, with a target oxygen saturation of between 93 and 95%. The current study was designed to test the hypothesis that uncontrolled high concentration oxygen would result in an increase in the PaCO2 compared with the titrated oxygen regime.
Methods
Subjects
Patients aged 18 to 75 years presenting to the Wellington (tertiary referral public), Kenepuru and Hutt (secondary referral public) Hospital EDs with suspected community-acquired pneumonia were enrolled in the study. Inclusion criteria were the presence of cough, a respiratory rate >18 breaths per minute, and at least one systemic feature of sweating, rigors or fever >37.8°C, representing the criteria for the clinical diagnosis of pneumonia. Patients with a diagnosis of COPD, or disorders associated with hypercapnic respiratory failure such as neuromuscular disease, or obesity hypoventilation syndrome were excluded from the study, due to the potential for confounding. Patients presenting with respiratory failure requiring mechanical ventilation, acute ECG changes suggesting ischaemia or suspected neutropenic sepsis were also excluded. The study was approved by the Central Regional Ethics Committee, and informed consent was obtained from each patient.
Study Design
On presentation, if oxygen saturations were >92% whilst breathing room air, patients remained off oxygen for 10 minutes, while eligibility for inclusion was assessed and informed consent obtained. If the oxygen saturations were ≤92% on presentation, patients received titrated oxygen to achieve an oxygen saturation of 93 to 95% for this 10 minute period. Patients were then randomly assigned using a computer-generated method, to one of two oxygen regimes for one hour. All patients in the high concentration oxygen group received 8 L/min via a medium concentration mask (Hudson RCI, Durham NC, USA) which delivers a FiO2 of between 0.4 and 0.78.11,12 Patients in the titrated group received oxygen only if their saturation was ≤92% on room air, with oxygen titrated as required at 5 minute intervals, to achieve an oxygen saturation of 93 to 95% according to the protocol outlined in the online supplement. A computerized randomization sequence was generated by the biostatistician (MWe) and the patients were enrolled and assigned to their treatment group by the clinical research fellows (MWi, KP, BH, KW, SB and RBo). Allocation concealment was achieved by using a secure database which contained the randomization sequence. Allocation was revealed to the researchers only when the subjects name was entered. The clinical research fellows and patients could not be blinded to the treatment regimes, due to the requirement to titrate oxygen therapy in the control group. The administration of oxygen therapy prior to hospital attendance and randomization was not documented.
A full history was taken and each patient underwent a physical examination. Empirical antibiotics were administered in accordance with published guidelines,1 and other therapies such as analgesia and intravenous fluids were administered at the discretion of the attending investigator. All patients underwent a chest radiograph.
Measures
Transcutaneous partial pressure of carbon dioxide (PtCO2) was used to estimate arterial PaCO2 using a combined oxygen saturation/PtCO2 monitor (TOSCA, Radiometer, Basel, Switzerland). A sensor was attached via a low-pressure clip to the patient's earlobe and heated to 42°C to enhance blood flow and to ‘arterialize’ the underlying capillaries. Baseline measurements of PtCO2 and oxygen saturation were recorded. Patients remained in a semi-recumbent position during the study protocol.
Measurements of PtCO2, respiratory rate and heart rate were made at baseline (0 minutes) and at 20, 40 and 60 minutes. The oxygen saturation was measured continuously throughout the study period and recorded at 5 minute intervals.
Arterial blood gas (ABG) measurements were performed according to clinical need and were not part of the study protocol.
Statistical analysis
The pre-specified primary outcome variable was the proportion of patients with a PtCO2 >38 mmHg at 60 minutes. However, after initial recruitment and clinical monitoring of patients, it was apparent that this outcome was inappropriate to determine if a physiologically relevant increase in PtCO2 had occurred, as it was primarily determined by the presenting PtCO2. For this reason, the primary outcome was changed to the proportion of patients with a PtCO2 rise of ≥4 mmHg at 60 minutes and the proportion of patients with a PtCO2 rise of ≥4 mmHg and a PtCO2 ≥38 mmHg at 60 minutes was included as a secondary outcome variable. Other secondary outcome variables included the mean change from baseline PtCO2, the mean change in respiratory rate and heart rate, and the need for hospital admission. The proportion of patients with a PtCO2 rise of ≥8 mmHg was included as a post-hoc analysis. Whether the risk of a PtCO2 rise of ≥4 mmHg at 60 minutes was influenced by the presence or absence of a pulmonary infiltrate on the chest radiograph consistent with pneumonia, was tested by an interaction term in a logistic regression model.
The rate of change of PtCO2 was determined using a mixed linear model with random intercept and slope terms. Continuous outcome variables were analysed as change from baseline using independent sample t-tests or for achieved oxygen saturation, for which normality assumptions were not met, by a Mann-Whitney test. Analysis was by intention to treat. SAS version 9.1 and Minitab version 14 were used.
Based on previous research,10 we calculated that to detect a difference in the main outcome variable of 20% in the high concentration oxygen group and 5% in the titrated group, with power of 80% at a type 1 error rate of 5%, 75 patients were required in each group.
Results
Patient Characteristics
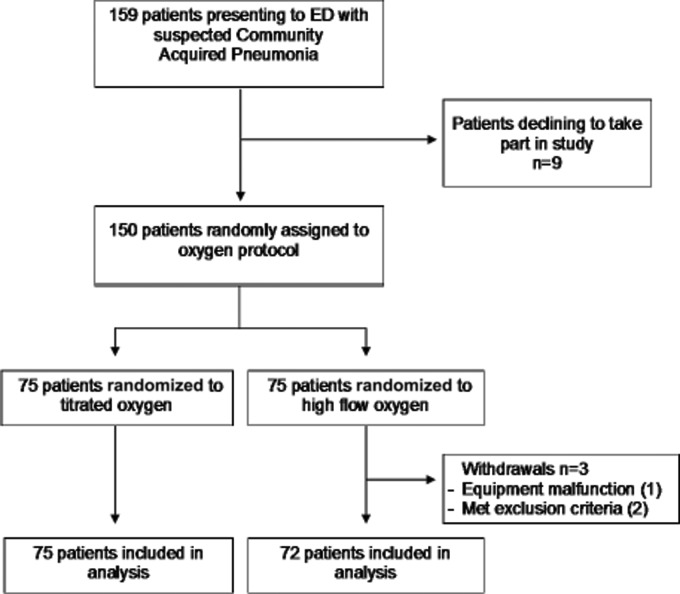
Eligible patients were recruited from July 2007 to April 2009. Figure 1 shows the flow of the 150 patients through the study, with 75 randomized to high concentration oxygen and 75 randomized to titrated oxygen. Three patients were withdrawn from the high concentration oxygen group prior to the administration of oxygen due to the inability to obtain PtCO2 recordings (n = 1) and two protocol violations which included the inadvertent enrolment of a patient with COPD and another with obesity hypoventilation syndrome. As a result, there were 72 and 75 patients included in the high concentration and titrated oxygen groups respectively. The two groups were similar in age, sex, respiratory rate, oxygen saturation, PtCO2, CRB-65 score and radiological confirmation of pneumonia at baseline, as outlined in Table 1. In 74/146 (50.7%) patients (one patient refused a chest radiograph), there was radiological confirmation of pneumonia, with the presence of a pulmonary infiltrate on the chest radiograph.
Figure 1.
Flow of subjects through the study
Table 1.
Baseline characteristics of patients presenting with suspected community-acquired pneumonia, according to randomized oxygen treatment group
| High concentration n = 72 | Titrated n = 75 | All n = 147 | |
|---|---|---|---|
| Sex, male | 32 | 28 | 60 |
| Age, yr | 45.2 (16.3) | 46.4 (16.3) | 45.8 (16.2) |
| Respiratory rate, breaths/min | 24.2 (6.0) | 24.6 (6.6) | 24.4 (6.3) |
| Heart Rate, beats/min | 90.5 (16.8) | 88.1 (18.2) | 89.3 (17.5) |
| SpO2, % | 96.3 (3.4) | 96.2 (3.2) | 96.2 (3.3) |
| PtCO2, mmHg | 32.7 (4.6) | 33.6 (5.9) | 33.1 (5.3) |
| PtCO2 ≥38 mmHg | 11/72 (15.3) | 18/75 (24.0) | 29/147 (19.7) |
| CRB-65 Score ≥2 | 7 (9.7) | 7 (9.3) | 14 (9.3) |
| Confirmed pneumonia | 35/72 (48.6) | 39/75 (52.0) | 74/147 (50.3) |
Values are mean (SD) for age, respiratory rate, heart rate, SpO2 and PtCO2, number of participants (percentage) for sex, confirmed pneumonia, PtCO2 ≥38 mmHg and CRB-65 score ≥2.
SpO2: Oxygen saturation; PtCO2: Transcutaneous carbon dioxide; PtCO2: ≥38 mmHg at baseline; CRB-65 Score: Pneumonia severity score (see text); Confirmed pneumonia: Presence of pulmonary infiltrate on chest radiograph.
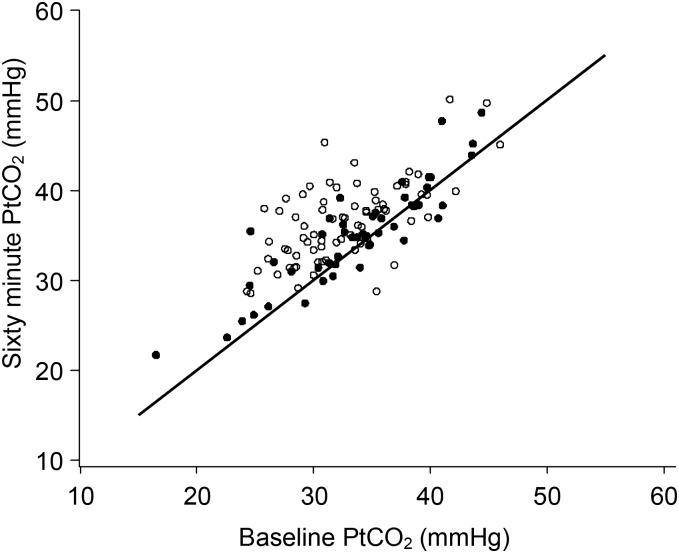
There was a wide range of PtCO2 levels at baseline ranging from 17 to 49 mmHg (Figure 2). Most (134/147) (91.2%) presented with an oxygen saturation >92% at baseline. In the titrated oxygen group, 68/75 (90.7%) patients did not require oxygen therapy throughout the 60 minute treatment period as their oxygen saturations remained >92%. In the titrated oxygen group, 6 of 75 (8%) patients required oxygen between 1 to 4 litres per minute via nasal prongs and 1 of 75 (1.4%) patient required >4 litres per minute via medium concentration mask to achieve oxygen saturations ≥93%. In the high concentration oxygen group, the oxygen saturation at 60 minutes was ≥99% in 65/72 (90.3%) of patients and was between 93 and 98% in the remaining 7/72 (9.7%) patients.
Figure 2.
The PtCO2 levels at baseline and after 60 minutes in the high concentration (o) and titrated (•) oxygen groups
Changes in PtCO2
The proportion of patients with an increase in PtCO2 of ≥4 mmHg at 60 minutes was significantly greater in the high concentration group, compared with the titrated oxygen group, 36/72 (50.0%) vs 11/75 (14.7%) with a relative risk of 3.4 (95% CI 1.9 to 6.2; P < 0.001) (Table 2). The proportion of patients with a rise in PtCO2 ≥4 mmHg was also greater in the high concentration group at the 20 and 40 minute time points (Table 3). In both groups the proportion of patients with a PtCO2 ≥4 mmHg progressively increased throughout the 60 minute time course.
Table 2.
The proportion of patients with a predetermined rise in PtCO2 from baseline at 60 minutes
| High concentration n (%) | Titrated n (%) | Relative risk (95% CI) | P value | |
|---|---|---|---|---|
| Change in PtCO2 ≥4 mmHg | 36 (50%) | 11 (14.7%) | 3.4 (1.9 to 6.2) | <0.001 |
| Change in PtCO2 ≥4 mmHg and PtCO2 ≥38 mmHg | 19 (26.4%) | 5 (6.7%) | 2.7 (1.2 to 6.0) | 0.01 |
| Change in PtCO2 ≥8 mmHg | 11 (15.3%) | 2 (2.7%) | 5.7 (1.3 to 25.0) | 0.007 |
Table 3.
The time course of the changes in PtCO2 in the treatment groups
| (i) The proportion of patients with a rise in PtCO2 ≥4 mmHg | ||||
| Time | High concentration n (%) | Titrated n (%) | Relative risk (95% CI) | P value |
| 20 minutes | 19 (26.4%) | 4 (5.3%) | 5.0 (1.8 to 13.8) | <0.001 |
| 40 minutes | 27 (37.5%) | 8 (10.7%) | 3.5 (1.7 to 7.2) | <0.001 |
| 60 minutes | 36 (50.0%) | 11 (14.7%) | 3.4 (1.9 to 6.2) | <0.001 |
| (ii) The mean change in PtCO2 (mmHg) | ||||
| Time | High concentration mean (SD) | Titrated mean (SD) | Difference (95% CI) | P value |
| 20 minutes | 1.9 (3.4) | −0.2 (2.7) | 2.1 (1.1 to 3.1) | <0.001 |
| 40 minutes | 2.9 (3.7) | 0.5 (3.6) | 2.4 (1.2 to 3.6) | <0.001 |
| 60 minutes | 3.6 (3.9) | 0.9 (3.7) | 2.7 (1.5 to 3.9) | <0.001 |
The change in PtCO2 from baseline was significantly greater in the high concentration oxygen compared with the titrated oxygen group, with a mean difference of 2.7 mmHg (95% CI 1.5 to 3.9; P < 0.001) at 60 minutes (Table 3). The rate of increase in PtCO2 in the high concentration group was 0.058 (95% CI 0.044 to 0.072) mmHg/min and in the titrated group was 0.017 (95% CI 0.0031 to 0.031) mmHg/min. The difference in the rate of change was 0.041 (95% CI 0.022 to 0.06, P < 0.001) mmHg/min.
The proportion of patients with a rise in PtCO2 ≥4 mmHg and a final PtCO2 ≥38 mmHg was significantly greater in the high concentration group with a relative risk of 2.7 (95% CI 1.2 to 6.0, P = 0.01). The proportion of patients with a rise in PtCO2 ≥8 mmHg was significantly greater in the high concentration group with a relative risk of 5.7 (95% CI 1.3 to 25.0, P = 0.007).
In patients with radiological confirmation of pneumonia, the high concentration oxygen group had a higher proportion with a rise in PtCO2 ≥4 mmHg (20/35 (57.1%) vs 5/39 (12.8%), relative risk 4.5 (95% CI 1.9 to 10.6), compared to those without consolidation 16/37 (43.2%) vs 6/36 (16.7%), relative risk 2.6 (95% CI 1.1 to 5.9), however, this interaction was not statistically significant, P = 0.28.
Arterial Blood Gases
ABGs were performed in 12 (8%) patients according to clinical need. The mean (SD) PaCO2 – PtCO2 difference was −0.9 (1.6) mmHg with limits of agreement of plus or minus 3.2 mmHg (−4.1 to +2.3).
Clinical variables
There was no significant difference in the change in respiratory rate (−2.9 vs −2.5 breaths per minute, high concentration vs titrated oxygen groups respectively, P = 0.63). The reduction in heart rate was greater in the high concentration compared to the titrated oxygen group (−6.8 vs −2.6 beats per minute, mean difference −4.2, 95%CI −7.3 to −1.2, P = 0.007). There were similar rates of hospital admission between the two treatment groups, 36/72 (50%) vs 37/75 (49.3%) for high concentration versus titrated oxygen respectively, relative risk 1.01 (95% CI 0.74 to 1.39, P = 0.94). One patient in the titrated group required admission to the intensive care unit.
Discussion
This randomized controlled trial has shown that high concentration oxygen therapy results in a significant increase in PtCO2 when administered to patients presenting to an emergency department with suspected community-acquired pneumonia. The three- and six-fold relative risks of an increase in PtCO2 of at least 4 mmHg and at least 8 mmHg respectively, suggests that this effect may potentially be of both physiological and clinical significance.
There are a number of methodological issues relevant to the interpretation of the study findings. The first is that we used a TOSCA transcutaneous CO2 monitor to measure PaCO2 rather than the ‘gold standard’ ABG test. This method was chosen as it allowed continuous PtCO2 monitoring without the discomfort of arterial blood gas sampling or the risk of hand ischemia associated with indwelling radial artery cannulae. The TOSCA has been shown to be an accurate means of estimating both PaCO2 and oxygen saturations from a single probe in healthy subjects,13,14 in COPD15 and in critically ill patients.16 This was confirmed in our subset of patients who had simultaneous ABG and PtCO2 recordings. The PtCO2 device accurately assessed PaCO2 without significant bias and with clinically acceptable limits of agreement when compared to the ABG measurement, thus validating our methodology.
By necessity the study was unblinded, as there was a clinical requirement for the investigator to have knowledge of the oxygen saturations in order to titrate the oxygen therapy in the ‘control’ treatment group, and an ethical requirement for the investigator to monitor the patient's progress with knowledge of the oxygen administered.
The decision to administer oxygen for 60 minutes was based on the evidence that CO2 retention in COPD4–7 and asthma8–10,17 may occur within this period. However, further increases in PaCO2 may occur beyond this time period and, as a result, the magnitude of the risk of an increased PtCO2 with high concentration oxygen therapy may have been underestimated in our study. This was suggested by our observation that the difference in PtCO2 between the high concentration and titrated oxygen groups progressively increased throughout the 60 minute period. This is clinically relevant as relief of dyspnoea in pneumonia can take many days and oxygen therapy may be continued until the dyspnoea resolves. Previous studies suggest that in some patients a progressive increase in PaCO2 occurs with more prolonged exposure to oxygen therapy.18,19
Our pre-specified analysis plan was to use the proportion of subjects with a PtCO2 >38 mmHg at 60 minutes as the primary outcome variable. However, in the early phase of recruitment it was apparent that the pre-specified primary outcome variable did not reflect a physiological increase in PtCO2, as it was primarily determined by the presenting PtCO2, and for this reason we registered a change in the primary outcome variable to the proportion of subjects with a PtCO2 rise of ≥4 mmHg. We acknowledge that changing the primary outcome variable after the start of the study raises the possibility of creating a biased assessment of the outcome of the trial. However, no formal interim statistical analysis, of either the pre-specified outcome variable or the new main outcome variable, was carried out prior to this decision and although the study itself was not masked as to treatment allocation, the decision was made without reference to the randomized allocation of the research participants.
Patients were excluded if they had a diagnosis of COPD due to the known effect of high concentration oxygen in exacerbations of this disorder.2 We are confident that patients with established COPD were not included in this study based on their history. We also had immediate access to electronic medical records which document any previous hospital admission, out-patients consultations and spirometry where available, allowing further investigation of a diagnosis of COPD. However, given that we did not perform spirometry on enrolment or prior to discharge, patients with a new diagnosis of airway obstruction may have been included in the study. In clinical practice, when patients present with symptoms to suggest pneumonia, spirometry is rarely available at the first point of oxygen therapy which is usually in the ambulance or ED, thus our study replicates what happens in clinical practice. Indeed, it is likely that greater increases in PtCO2 may occur in an unselected population of patients with community-acquired pneumonia, which is more likely to include those with concomitant COPD or other disorders associated with chronic respiratory failure.
Patients presenting with suspected (rather than confirmed) community-acquired pneumonia were enrolled in the study, as oxygen therapy is usually administered to breathless patients presenting with suspected pneumonia rather than after the diagnosis is confirmed by chest radiography. About half of the patients had pneumonia subsequently confirmed by the presence of consolidation on the chest radiograph. Most of the other patients were likely to have had lower respiratory tract infections, and radiological confirmation of pneumonia would have been possible in a proportion if high-resolution computed tomography had been undertaken.20,21 This may explain why the presence of radiologically confirmed pneumonia made no significant difference to the risk of a raised PtCO2 with high concentration oxygen therapy. When the analysis was restricted to subjects with radiologically confirmed pneumonia, there was a 4.5-fold increased risk of a rise in PtCO2 ≥4 mmHg with high concentration oxygen therapy.
In the absence of a clear view on what represents a ‘physiologically’ or ‘clinically’ significant increase in PtCO2 we chose specific levels of PtCO2 increases from baseline. The primary outcome variable was a rise of ≥4 mm Hg which was considered to represent an effect of physiological significance given that it is a higher rise that would be expected from the Haldane effect alone.22 In addition, a change in PtCO2 of ≥ 4mmHg is higher than the limits of agreement demonstrated in our published validation study of the PtCO2 device (−3.9 mmHg to +3.7 mmHg)23 meaning it is likely to represent a true increase. The secondary outcome variable of a rise in PtCO2 of ≥8 mmHg was considered to represent an effect of potential clinical significance. The study was not powered to investigate differences in clinical outcomes, in particular the adverse effects of hypercapnia or hyperoxaemia. This issue needs to be addressed in a large study of an unselected group presenting with severe community-acquired pneumonia.
Both treatment groups were hypocapnic on presentation indicating a degree of hyperventilation. Over the course of the 60 minutes, there was a modest reduction in respiratory rate of <3 breaths per minute, with no significant difference between the two groups. This suggests that the greater increase in PtCO2 in the high flow group could not be attributed to differential effects on minute ventilation. High flow oxygen resulted in a reduction in heart rate, which is a well recognised cardiovascular response to oxygen therapy.24 We consider that this effect is not a marker of clinical benefit, as it is associated with a reduction in cardiac output, increase in blood pressure, and reduction in coronary artery blood flow.24,25
In pneumonia, hypoxaemia is primarily due to intrapulmonary shunting and ventilation-perfusion inequalities.26–29 Treatment with high concentration oxygen in pneumonia has minimal effect on intrapulmonary shunting, however it has the potential to significantly worsen ventilation-perfusion mismatch through release of hypoxic pulmonary vasoconstriction. This could potentially lead to an increase in the physiological dead space and is likely to represent the predominant mechanism responsible for the increase in PtCO2 observed. Importantly, these data suggest that high concentration oxygen therapy may have the potential to cause an increase in PaCO2 across a wide range of respiratory conditions with abnormal gas exchange. Indeed, this physiological response to high concentration oxygen therapy has now been reported in stable COPD,5,7 exacerbations of COPD,2–4,6 asthma,8–10,17 obesity hypoventilation syndrome,30,31 and diffuse pulmonary fibrosis or infiltration.32
We conclude that high concentration oxygen increases the PtCO2 in patients with community acquired pneumonia. This suggests that the potential increase in PaCO2 with high concentration oxygen therapy is not limited to COPD, but may also occur in other respiratory disorders with abnormal gas exchange.
DECLARATIONS
Competing interests
None declared
Funding
Health Research Council of New Zealand, Wellington Hospitals & Health Foundation, Royal Australasian College of Physicians, Asthma & Respiratory Foundation of New Zealand
Ethics approval
Central Regional Ethics Committee, Wellington, New Zealand
Clinical trials registration
ACTRN12607000196448
Guarantor
MW
Contributorship
Meme Wijesinghe, Kyle Perrin, and Bridget Healy recruited patients; Mark Weatherall undertook statistical analyses; All authors contributed to the design of the study and writing of the manuscript
Acknowledgements
We gratefully acknowledge the contributions of Kirsten Wadsworth, Richard Bowditch and Susan Bibby in the recruitment of patients. Meme Wijesinghe, Bridget Healy and Kyle Perrin are Research Fellows funded by the Wellington Hospitals & Health Foundation and the Health Research Council of New Zealand. The TcCO2 devices were funded by the Royal Australasian College of Physicians, the Asthma & Respiratory Foundation of New Zealand, and the Health Research Council of New Zealand
Reviewer
Julie Morris
References
- 1.Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64:ii1–iii55 [DOI] [PubMed] [Google Scholar]
- 2.Murphy R, Driscoll P, O'Driscoll R Emergency oxygen therapy for the COPD patient. Emerg Med J 2001;18:333–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.New A Oxygen: kill or cure? Prehospital hyperoxia in the COPD patient. Emergency Medicine Journal 2006;23:144–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubier M, Murciano D, Milic-Emili J, et al. Effects of the administration of O2 on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. American Review of Respiratory Disease 1980;122:747–54 [DOI] [PubMed] [Google Scholar]
- 5.Dick C, Liu Z, Sassoon C, Berry R, Mahutte C O2-induced change in ventilation and ventilatory drive in COPD. Am J Respir Crit Care Med 1997;155:609–14 [DOI] [PubMed] [Google Scholar]
- 6.Robinson TD, Freiberg DB, Regnis JA, Young IH The Role of Hypoventilation and Ventilation-Perfusion Redistribution in Oxygen-induced Hypercapnia during Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2000;161:1524–9 [DOI] [PubMed] [Google Scholar]
- 7.Sassoon CS, Hassell KT, Mahutte CK Hyperoxic-induced hypercapnia in stable chronic obstructive pulmonary disease. American Review of Respiratory Disease 1987;135:907–11 [DOI] [PubMed] [Google Scholar]
- 8.Chien JW, Ciufo R, Novak R, et al. Uncontrolled Oxygen Administration and Respiratory Failure in Acute Asthma. Chest 2000;117:728–33 [DOI] [PubMed] [Google Scholar]
- 9.Perrin K, Wijesinghe M, Weatherall M, Beasley R High flow oxygen causes carbon dioxide retention in severe asthma: a randomized controlled trial. Respirology 2009;14:A42 [Google Scholar]
- 10.Rodrigo GJ, Rodriquez Verde M, Peregalli V, Rodrigo C Effects of short-term 28% and 100% oxygen on PaCO2 and peak expiratory flow rate in acute asthma: a randomized trial. Chest 2003;124:1312–7 [DOI] [PubMed] [Google Scholar]
- 11.Garcia JA, Gardner D, Vines D, Shelledy D, Wettstein R, Peters J The oxygen concentrations delivered by different oxygen therapy systems. Chest 2005;128:389S-b-90 [Google Scholar]
- 12.Milross J, Young IH, Donnelly P The oxygen delivery characteristics of the Hudson Oxy-one face mask. Anaesth Intensive Care 1989;17:180–4 [DOI] [PubMed] [Google Scholar]
- 13.Kocher S, Rohling R, Tschupp A Performance of a digital PCO2/SPO2 ear sensor. J Clin Monit Comput 2004;18:75–9 [DOI] [PubMed] [Google Scholar]
- 14.Parker SM, Gibson GJ Evaluation of a transcutaneous carbon dioxide monitor (“TOSCA”) in adult patients in routine respiratory practice. Respir Med 2007;101:261–4 [DOI] [PubMed] [Google Scholar]
- 15.Cox M, Kemp R, Anwar S, Athey V, Aung T, Moloney E Non-invasive monitoring of CO2 levels in patients using NIV for AECOPD. Thorax 2005;61:363–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatevossian RG, Wo CC, Velmahos GC, Demetriades D, Shoemaker WC Transcutaneous oxygen and CO2 as early warning of tissue hypoxia and hemodynamic shock in critically ill emergency patients. Crit Care Med 2000;28:2248–53 [DOI] [PubMed] [Google Scholar]
- 17.Field GB The effects of posture, oxygen, isoproterenol and atropine on ventilation-perfusion relationships in the lung in asthma. Clinical Science 1967;32:279–88 [PubMed] [Google Scholar]
- 18.Massaro DJ, Katz S, Luchsinger PC Effect of various modes of oxygen administration on the arterial gas values in patients with respiratory acidosis. Br Med J 1962;2:627–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warrell DA, Edwards RH, Godfrey S, Jones NL Effect of controlled oxygen therapy on arterial blood gases in acute respiratory failure. Br Med J 1970;1:452–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reittner P, Muller NL, Heyneman L, et al. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol 2000;174:37–41 [DOI] [PubMed] [Google Scholar]
- 21.Syrjala H, Broas M, Suramo I, Ojala A, Lahde S High-resolution computed tomography for the diagnosis of community-acquired pneumonia. Clin Infect Dis 1998;27:358–63 [DOI] [PubMed] [Google Scholar]
- 22.Lenfant C Arterial-alveolar difference in PCO2 during air and oxygen breathing. Journal of Applied Physiology 1966;21:1356–62 [DOI] [PubMed] [Google Scholar]
- 23.Perrin K, Wijesinghe M, Weatherall M, Beasley R Assessing PaCO2 in acute respiratory disease: accuracy of a transcutaneous carbon dioxide device. Intern Med J 2011; 41:630–3 [DOI] [PubMed] [Google Scholar]
- 24.Bodetoft S, Carlsson M, Arheden H, Ekelund U Effects of oxygen inhalation on cardiac output, coronary blood flow and oxygen delivery in healthy individuals, assessed with MRI. Eur J Emerg Med 2011;18:25–30 [DOI] [PubMed] [Google Scholar]
- 25.Farquhar H, Weatherall M, Wijesinghe M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J 2009;158:371–7 [DOI] [PubMed] [Google Scholar]
- 26.Gea J, Roca J, Torres A, Agusti AG, Wagner PD, Rodriguez-Roisin R Mechanisms of abnormal gas exchange in patients with pneumonia. Anesthesiology 1991; 75:782–9 [DOI] [PubMed] [Google Scholar]
- 27.Lampron N, Lemaire F, Teisseire B, et al. Mechanical ventilation with 100% oxygen does not increase intrapulmonary shunt in patients with severe bacterial pneumonia. Am Rev Respir Dis 1985;131:409–13 [DOI] [PubMed] [Google Scholar]
- 28.Light RB Pulmonary pathophysiology of pneumococcal pneumonia. Semin Respir Infect 1999;14:218–26 [PubMed] [Google Scholar]
- 29.Rodriguez-Roisin R, Roca J Update '96 on pulmonary gas exchange pathophysiology in pneumonia. Semin Respir Infect 1996;11:3–12 [PubMed] [Google Scholar]
- 30.Barrera F, Hillyer P, Ascanio G, Bechtel J The distribution of ventilation, diffusion, and blood flow in obese patients with normal and abnormal blood gases. Am Rev Respir Dis 1973;108:819–30 [DOI] [PubMed] [Google Scholar]
- 31.Wijesinghe M, Williams M, Perrin K, Weatherall M, Beasley R The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest 2010;139:1018–24 [DOI] [PubMed] [Google Scholar]
- 32.Said SI, Banerjee CM Venous admixture to the pulmonary circulation in human subjects breathing 100 per cent oxygen. J Clin Invest 1963;42:507–15 [DOI] [PMC free article] [PubMed] [Google Scholar]