Abstract
This article reviews progress made in understanding the causes of stress urinary incontinence. Over the last century, several hypotheses have been proposed to explain stress urinary incontinence. These theories are based on clinical observations and focus primarily on the causative role of urethral support loss and an open vesical neck. Recently these hypotheses have been tested by comparing measurements of urethral support and function in women with primary stress urinary incontinence to asymptomatic volunteers who were recruited to be similar in age, race and parity. Maximal urethral closure pressure is the parameter that differs the most between groups being 43% lower in women with stress incontinence than similar asymptomatic women having as effect size of 1.6. Measures of urethral support effect sizes range from 0.5 to 0.6. Because any one objective measure of support may not capture the full picture of urethrovesical mobility, review of blinded ultrasounds of movements during cough were reviewed by an expert panel. The panel was able to identify women with stress incontinence correctly 57% of the time; just 7% above the 50% that would be expected by chance alone, confirming that urethrovesical mobility is not strongly associated with stress incontinence. Although operations that provide differential support to the urethra are effective, urethral support is not the predominant cause of stress incontinence. Improving our understanding of factors affecting urethral closure may lead to novel treatments targeting the urethra and improved understanding of the small but persistent failure rate of current surgery.
Keywords: stress urinary incontinence, urethra, urethral closure pressure, urethral support, pelvic floor disorders, female
Introduction
Since the first clinical description of what we now call stress urinary incontinence in 1912(1), no single individual has contributed more to our understanding and management of this common condition than Ed McGuire, MD. His observation that not all cases of stress incontinence could be attributed to problems with urethral support (Type III incontinence), development of the remarkably effective and simple pubovaginal sling operation, and description of the Valsalva leak point pressure which allows the competence of the sphincteric mechanism to be quantified, are important advances to our understanding of this disease. It is a great privilege to participate in this Festschrift in his Honor.
This article will provide a progress report on some of the research being conducted in the Pelvic Floor Research Group at the University of Michigan into the etiology of stress urinary incontinence. The history of attempts to answer the question: “Why do women have stress incontinence?” has spanned the last century and taken many turns. It is a story that is punctuated by astute observation, successful operations, and changes in theory that have occurred about once a generation. In 1912, Kelly described the open vesical neck seen with his urethrascope. He reported successful results of an operation to plicate the vesical neck. Not long after, Bonney observed abnormal displacement of the anterior vaginal wall in incontinent women in 1922 and proposed loss of urethral support as the cause of stress incontinence (2). He suggested that Kelly’s operation succeeded not because it narrowed the vesical neck, but because it improved urethral support and described an eponymous test to demonstrate the effect of improved support on stopping incontinence. Jeffcoate and Roberts in 1949 expanded Bonney’s ideas about vesical neck support loss by observing that many stress incontinent women had a loss of the urethrovesical angle (3). This technology gave rise to further examination and quantification of the urethrovesicopubic relationships (4,5). At the same time, the success of the Marshall, Marchetti, Krantz operation was noted (6). These authors were careful to point out that their operation was empirical (having been developed in men with post surgical stress incontinence) and did not come from any specific causal observation. Clinicians were quick, however, to say that the MMK worked because it restored the posterior urethrovesical angle (PUVA).
In 1960, Enhorning made meticulous and detailed measurements of intravesical and intraurethral pressure during a cough(7). He noted that intraurethral pressure mirrored abdominal pressure and described this phenomenon as “transmission” of abdominal pressure to the urethra. This transmission was reduced in women with stress incontinence. The demonstration of these pressure relationships and how they varied between individuals and along the urethra did not, however, identify the mechanism whereby pressure transmission occurred. Enhorning hypothesized loss of pressure transmission occurred because the urethra fell “below” the influence of abdominal pressure, preserving the idea that urethral support was the dominant factor in causing stress incontinence. However, the anatomical and structural factors responsible for these observations remained somewhat unknown. In 1976 Richardson described the paravaginal defect as the structural lesion that led to hypermobility (8) and reported surgical success in 1981(9). Petros and Ulmsten(10) and this author(11) proposed hypotheses of varying complexity concerning why urethral support would translate to improved closure and stress continence. McGuire made the important observation that not all incontinence had the same anatomical cause when he described Type III incontinence.
While astute and meaningful, this series of observations and hypotheses lack what any 9th grade science teacher would deem imperative in answering a question; an experimentum crusis, or a properly controlled experiment that tested the hypothesis in question. Hypothesis is a critically important aspect of science, but it is meaningless until experiment accepts or rejects the hypothesis. To answer the question “Why do women have stress urinary incontinence?” it would be necessary to compare anatomic and functional findings in stress incontinent women with those of properly matched asymptomatic continent controls. This type of study was not possible for early investigators because the technology to quantify different aspects of urethrovesical function were lacking. By the 1990’s however, sufficient progress had been made with imaging and pressure measurement. In addition, research support from the NIH (NIDDK, NICHD, and ORWH) for investigating incontinence and pelvic floor disorders became available and made such a study possible. Without this type of funding, it is unlikely that it would be possible to carry out the advanced imaging and urodynamics necessary in properly selected asymptomatic volunteers; studies would be dependent on a flawed experimental design; women (with other lower urinary tract symptoms) undergoing urodynamics for evaluation of incontinence but who did not have stress incontinence should not be considered asymptomatic controls.
Anatomy overall continence mechanism
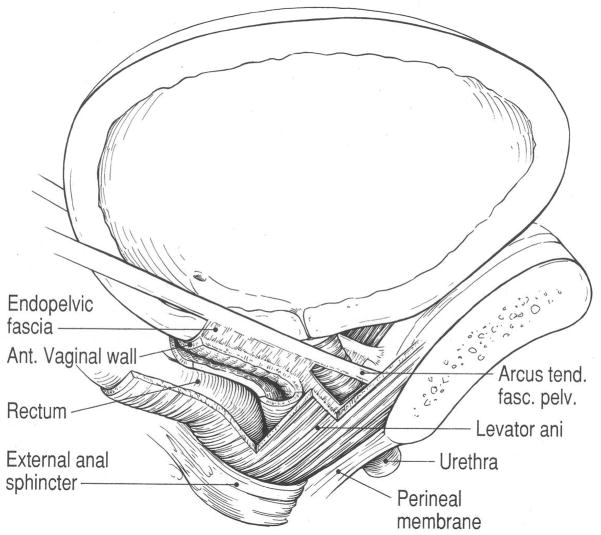
Any understanding of stress incontinence must begin with an accurate appreciation of the detailed anatomy of the continence mechanism and pelvic floor (Figure 0). The complete description of this detailed anatomy is beyond the scope of this article, but has recently been summarized(12). The stress continence control system consists of a sphincteric unit (including a multilayered urethra and alpha adrenergically innervated vesical neck) and a support system consisting of connective tissues interspersed with smooth muscle and the striated muscle of the levator ani. The urethral lumen is surrounded by several layers of muscle. In the region where it traverses the bladder wall, the smooth muscle of the trigonal ring surrounds the lumen. Below that level there is an outer striated urogenital sphincter muscle (rhabdosphincter), a middle thin circular smooth muscle, and an inner and surprisingly well developed longitudinal layer. The submucosa contains a remarkably prominent vasculature.
The supportive apparatus consists of the anterior vaginal wall and surrounding muscles and fascial tissues. The vaginal wall is connected laterally to the medial surface of the levator ani muscles (“pubovaginalis”); because of this connection, the contraction of this muscle affects urethral position. There is also a “paravaginal connection” of the vaginal wall to the tendineus arch of the pelvic fascia. These elements are arranged in a unique and complex 3-dimensional apparatus that is controlled by poorly understood neural mechanisms and subjected to remarkable forces. One can only watch and wonder what happens to prevent urinary incontinence as a gymnast lands a high bar dismount.
Causal factors associated with stress urinary incontinence
Knowing the anatomical components of the continence mechanism begets a list of structures whose function should be evaluated and compared. We have recently concluded the Research On Stress Incontinence Etiology (ROSE) study(13). This case-control study compared 103 women with daily and demonstrable stress incontinence to 108 asymptomatic controls proven to be stress continent; groups were matched for age, race, parity and hysterectomy status. Urethral closure pressure, urethral and pelvic organ support, levator ani muscle function, and intravesical pressure were measured and analyzed using logistic regression and multivariable modeling.
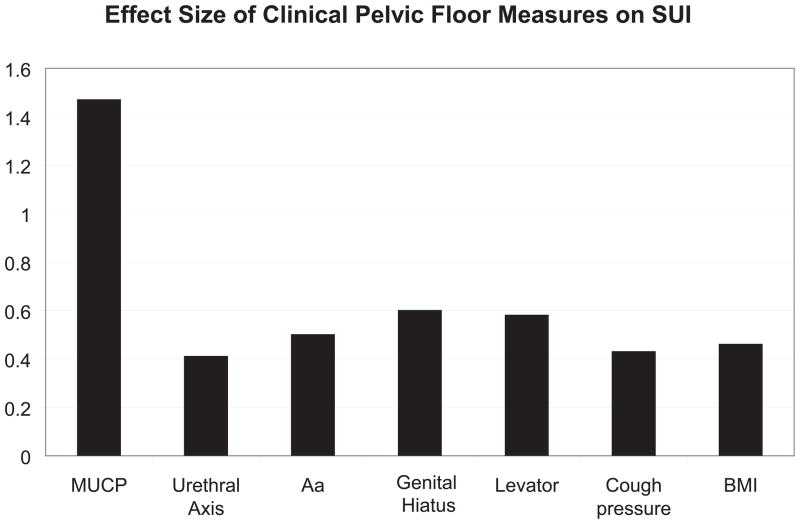
The degree to which different continence mechanism parameters differ between women with and without stress incontinence is shown in Table 1. Figure 1 shows the effect sizes (d) for differences in each measurement. Effect size permits differences between cases and controls to be compared in measurements taken in different units (e.g. cm H2O, degrees, cm, Newtons). It expresses the difference in the mean values between the two groups in standard deviation units. An effect size of 1, for example, means that the difference between the cases and controls was the same as the average standard deviation for the two groups.
Table I.
Comparison of Clinical Pelvic Floor Measures Between Stress Urinary Incontinent Women and Continent Volunteers. Data are reported as mean ± standard deviation or % (N).
| Stress Incontinent (N=103) | Continent (N=108) | P | Effect Size (d) | |
|---|---|---|---|---|
| MEASURE OF URETHRAL FUNCTION | ||||
| Maximum Urethral Closure Pressure (cm H2O) | 40.8 ± 17.1 | 70.2 ± 22.4 | <.0001 | 1.47 |
| MEASURES OF URETHROVAGINAL SUPPORT & PELVIC FLOOR STATUS | ||||
| Urethral Axis (degrees from horizontal) | ||||
| Cotton-tipped swab-rest | −0.8 ± 11.8 | −6.3 ± 15.1 | .004 | .41 |
| Cotton-tipped swab-strain | 29.5 ± 20.3 | 25.0 ± 19.2 | .10 | - |
| Pelvic Organ Prolapse (cm relative to the hymen with + denoting locations below) | ||||
| Point Aa* | −0.6 ± 0.8 | −1.0 ± 0.8 | <.0001 | .50 |
| Point C | −6.3 ± 1.7 | −6.3 ± 1.4 | .74 | - |
| Point Ap* | −1.4 ± 1.0 | −1. 4 ± 1.0 | .95 | - |
| Genital hiatus | 4.0 ± 1.0 | 3.4 ± 1.0 | <.0001 | .60 |
| Perineal body | 4.0 ± 1.4 | 3.8 ± 1.3 | .147 | - |
| Levator Function | ||||
| Vaginal Closure Force, Rest (Newtons) | 4.5± 2.7 | 4.5 ± 1.2 | .89 | - |
| Vaginal Closure Force, Augmented (Newtons) | 2.3 ± 1.7 | 2.8 ± 1.9 | .10 | - |
| Cotton-tipped swab axis-Muscle Contraction (degrees) | −11.6 ± 14.9 | −21.0 ± 17.4 | <.0001 | .58 |
| Levator Muscle Defects | (n=99) | (n=102) | .31 | |
| No defects % (N) | 60.8 (62) | 63.0 (68) | - | |
| Minor % (N) | 26.5 (27) | 19.4 (21) | - | |
| Major % (N) | 12.7 (13) | 17.6 (19) | - | |
| MEASURES RELATING TO INCREASED DEMANDS ON THE CONTINENCE SYSTEM | ||||
| Intravesical Pressure (cmH2O) | ||||
| At rest | 21.2 ± 5.9 | 19.6 ± 9.3 | .15 | - |
| With maximum cough | 143.2 ± 43.4 | 126.4 ± 34.3 | .002 | .43 |
| Body mass index (kg/m2)** | 30.4 ± 6.6 | 27.6 ± 5.6 | .001 | .46 |
Because Aa and Ba and Ap and Bp were similar in these populations, only the values for Aa and Ap are reported.
Repeated from Table 1 for reference in this table.
Figure 1.
The Continence Mechanism
Maximal urethral closure pressure was the parameter that had the greatest difference between the groups. It was 42% lower in cases (40.8± 17.1 cm H2O vs. 70.2± 22.4 cm H2O, d =1.47). This was the most prominent effect size (i.e. d=1.47) seen in the study. Lesser effect sizes were seen for parameters related to urethral support, including resting urethral axis and urethrovaginal support (Point Aa on POP-Q) (d= 0.41 and 0.50, respectively). Other pelvic floor parameters, including genital hiatus size and urethral axis during muscle contraction (d=0.60 and 0.58, respectively), differed and levator defect status did not. Maximum cough pressure, which is an assessment of forces placed on the continence mechanism, was also different among cases and controls (d=0.43). After adjusting for body mass index, the maximal urethral closure pressure alone correctly classified 50% of cases. Adding the best predictors for urethrovaginal support and cough strength to the model added 11% of predictive ability. This means that we had 61% of the answer to why women have stress incontinence.
We were surprised to find that urethral closure pressure is, by far, the parameter most characteristic of stress incontinence. We had measured urethral support in several ways; as axial mobility, support of the vagina adjacent to the urethra (POP-Q point Aa) and factors presumed to affect urethral support such as the strength of the levator ani muscle and genital hiatus size. Each of these factors was different between stress incontinent and continent controls, but not nearly as different as MUCP. Of course, there is always the concern that we had not assessed the correct parameter concerning urethral mobility. We therefore assembled an international expert panel to review ultrasound videos of subjects’ urethral mobility during coughing to identify which patterns they felt were most likely to be associated with stress incontinence.(14) To our surprise, none of the examiners were consistently able discern which women were stress incontinent and which were continent. The evaluators’ mean accuracy was 57%, only 7% better than that of random chance. This finding supports the concept that urethral support is not as important as previously thought.
Birth and Stress Urinary Incontinence
Women who have had two vaginal deliveries have an adjusted odds ratio of 2.4 for experiencing stress incontinence compared to women delivering by cesarean section (15). However, how birth alters the continence structures leading to this increased risk is still somewhat unclear. To evaluate the relative contributions of urethral mobility and urethral function to stress incontinence we conducted a case-control study with group matching including 80 primiparous women with self-reported new stress incontinence 9–12 months postpartum and 80 primiparous continent controls. Eighty nulliparous continent controls were evaluated as a comparison group to allow us to determine birth-related changes not associated with stress incontinence. Urethral function was measured with urethral profilometry, and vesical neck mobility was assessed with ultrasound and cotton swab test.
Urethral closure pressure in primiparous stress incontinent women (62.9±25.2 cm H20) was lower than in primiparous continent women (83.9±21.0, P<.001; d=0.91); primiparous continent women were similar to nulliparous women (90.3±25.0, P=.091). Vesical neck movement, measured during cough with ultrasonography, was the mobility measure most associated with stress incontinence; 15.6±6.2 mm in incontinent women compared with 10.9±6.2 in primiparous continent women (P<.001, d=0.76) and nulliparas (9.9±5.0, P=.322). Logistic regression disclosed the two-variable model (i.e. urethral closure pressure and vesical neck mobility) was more strongly associated with stress incontinence (max-rescaled R2=0.37, P<.001) than either single-variable model, (urethral closure pressure R2=0.25, P<.001; vesical neck movement R2=0.16 P<.001). In this cohort, visible damage to the levator ani muscle was twice as likely to be found in the stress incontinent women as it was in the continent women. This is in contrast to the findings of our ROSE study in older women (described above) in whom no difference in visible levator damage was seen.
Why is it that soon after vaginal delivery, urethral support and sphincter function seem to contribute more equally to the cause of stress incontinence while later in life declining sphincter function predominates? We believe this arises from two factors: 1) the fact that levator ani damage occurs only during vaginal birth and does not increase with age after that point and 2) the natural decline in maximal urethral closure pressure that occurs with age. Given the importance that urethral closure pressure plays in causing stress incontinence, it’s worth looking at some aspects of this topic.
The urethra
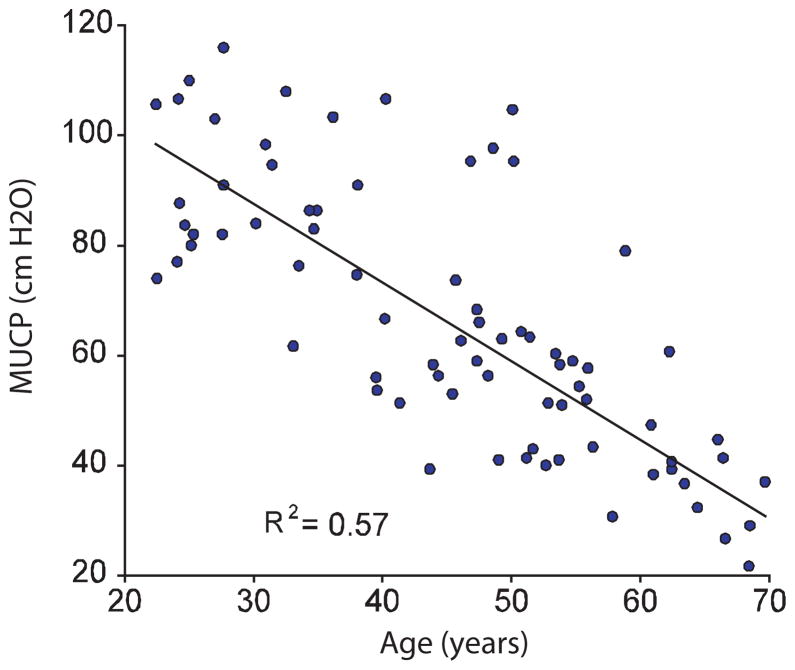
The urethra, as previously mentioned is a multilayered structure that consists of striated muscle, smooth muscle, connective tissue, a rich submucosal vascular plexus, and a lining epithelium. The combined actions of these tissues serve to create wall tension that compresses the lumen closed. Rud sought to estimate the contributions of these different layers(16). In a study of 5 continent women undergoing radical hysterectomy with a mean age 46 years, he made measurements of maximal urethral closure pressure at baseline, with striated muscle blockade, and after clamping the internal iliac vessels. This revealed that 33% of pressure was attributable to striated muscle activity, 28% to vascular factors and 39% to the remaining contributions of smooth muscle and connective tissues. In a subsequent study, he also revealed that after the age of 20 to 25, that urethral function decreased with increasing age(17). This decline included both the attributable effect of age as well as that of potential changes due to vaginal birth. Of course, examining urethral closure pressure among a full age spectrum of nulliparous women eliminates the potentially confounding effect vaginal birth (Figure 2). In this kind of a cohort, two factors become evident. First, the gradual decline maximum urethral closure pressure of approximately 15% per decade can be seen, but, just as importantly, the wide variation in MUCP among individuals of a similar age is evident. For example among women of 50 years, pressures as high as 110 and as low as 40 cm. H2O are seen in the absence of effects of vaginal birth. This remarkable variation between individuals is poorly understood.
Figure 2.
Effect Size of Clinical Pelvic Floor Measures on Stress Urinary Incontinence
The decline in striated muscle cells per year in the urethra may lead us to understand this phenomenon. In looking at the number of striated muscle cells visible in the ventral wall of the urethra (the portion adjacent to the pubic bone), we found a decline in the number of striated muscle cells that roughly parallels the decline in MUCP (Figure 3). This loss is most prominent in the proximal area of the urethra just below the vesical neck.
Figure 3.
Maximum Urethral Closure Pressure in Nulliparas
Circular smooth muscle (CSM) in the urethra also declines with age(18). We compared smooth muscle (stained for alpha actin) in mid-urethral hemiaxial sections from female cadavers aged 20–39 years (n = 12) with those from cadavers aged 70–89 years (n = 16). The circular smooth muscle was studied at 0 degrees (pubic bone side) and 180 degrees (vaginal side) and in between at 45, 90, and 135 degrees. Density of urethral CSM was 25%–50% higher in specimens aged 20–39 years, compared with those aged 70–89 years at 0, 135, and 180 degrees. In the younger group, higher fiber counts were observed at 135 and 180 degrees, and the CSM layer was thinner but not significantly so. These differences were not as dramatic as the striated muscle findings, as the circular smooth muscle layer is relatively thin, but these reductions in smooth muscle could be expected to contribute to the decline in urethral closure seen with advancing age.
How are we coming with our understanding of SUI cause?
Despite the progress made to date, our understanding is far from complete. The urethra is a dynamic structure and variations in closure are seen from second to second, minute to minute, day to day and year to year. The role of the submucosal vascular plexus is still poorly understood. The plexus occupies a sizeable space within the muscular tube of the urethra and without the vascular cushion, incontinence would surely be present. While nerve blockage greatly reduces urethral closure(19), the complex neural control mechanisms that allow for temporary and total relaxation during voiding, that somehow know when to re-establish normal muscle function are still not entirely understood. In addition, although it is clear that urethral support is not as important as previously thought, it is one of the major contributing factors for stress incontinence, especially among younger women who have functionally intact urethras. Understanding what it is about urethral support that lessens continence is vital. Racial disparities exist in incontinence, and the biological basis for these differences should help our understanding. Also, coming to a more complete understanding of how such factors as obesity relate to stress incontinence are needed.
Fortunately, we now have, or could develop, the investigative tools to answer these interesting questions.
Figure 4.
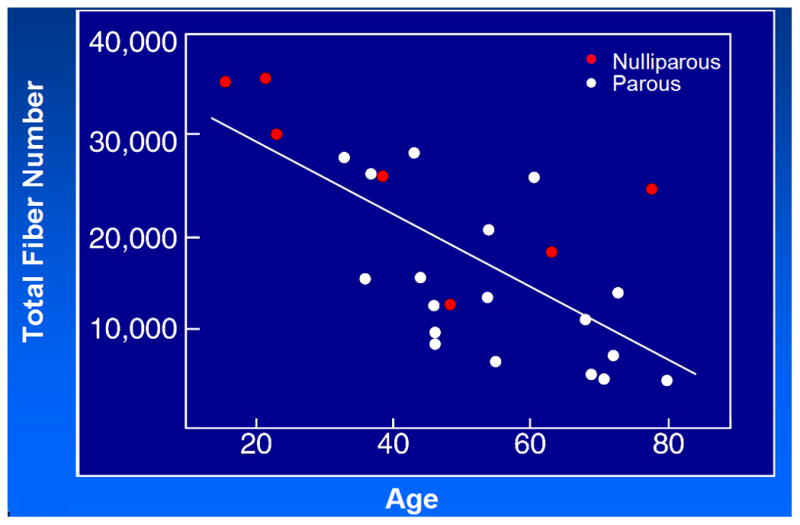
Total Fiber Number and Age – 1% of Fibers Lost Per Year
Acknowledgments
Financial Support from Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s P50 HD044406
Christopher Chapple led the review process.
References
- 1.Kelly HA, Dumm WM. Urinary incontinence in women, without manifest injury to the bladder: A report of cases. Surg Gynecol Obstet. 1914;18:444–50. [Google Scholar]
- 2.Bonney V. On diurnal incontinence of urine in women. J Obstet Gynecol Br Emp. 1923;30:358–65. [Google Scholar]
- 3.Jeffcoate TNA, Roberts H. Observations on stress incontinence of urine. Am J Obstet Gynecol. 1952;64:721–38. doi: 10.1016/s0002-9378(16)38792-0. [DOI] [PubMed] [Google Scholar]
- 4.Hodgkinson CP. Relationships of the female urethra in urinary incontinence. Am J Obstet Gynecol. 1953;65:560–573. doi: 10.1016/0002-9378(83)90612-9. [DOI] [PubMed] [Google Scholar]
- 5.Green TH. Development of a plan for the diagnosis and treatment of urinary stress incontinence. Am J Obstet Gynecol. 1962;83:632–48. doi: 10.1016/s0002-9378(16)35894-x. [DOI] [PubMed] [Google Scholar]
- 6.Marshall VF, Marchetti AA, Krantz KE. The correction of stress incontinence by simple vesicourethral suspension. Surg Gynecol Obstet. 1949;88:509–18. [PubMed] [Google Scholar]
- 7.Enhorning G. Simultaneous recording of the intravesical and intraurethral pressure. Acta Obstet Gynecol Scand (suppl) 1961;276:1–69. [PubMed] [Google Scholar]
- 8.Richardson AC, Lyons JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–73. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 9.Richardson AC, Edmonds PB, Williams NL. Treatment of stress urinary incontinence due to paravaginal fascial defect. Obstet Gynecol. 1981;57:357–62. [PubMed] [Google Scholar]
- 10.Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7–31. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 11.DeLancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–20. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 12.Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci. 2007 Apr;1101:266–96. doi: 10.1196/annals.1389.034. [DOI] [PubMed] [Google Scholar]
- 13.DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, Weadock WJ, Ashton-Miller JA. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008 Jun;179(6):2286–90. doi: 10.1016/j.juro.2008.01.098. discussion 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewicky-Gaupp C, Blaivas J, Clark A, McGuire EJ, Schaer G, Tumbarello J, Tunn R, DeLancey JO. "The cough game": are there characteristic urethrovesical movement patterns associated with stress incontinence? Int Urogynecol J Pelvic Floor Dysfunct. 2008 Oct 11; doi: 10.1007/s00192-008-0738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rortveit G, Daltveit AK, Hannestad YS, et al. Norwegian EPINCONT Study.Urinary incontinence after vaginal delivery or cesarean section. New England Journal of Medicine. 2003;348:900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 16.Rud T, et al. Factors maintaining the intraurethral pressure in women. Investigative Urology. 1980;17(4) [PubMed] [Google Scholar]
- 17.Rud T. Urethral pressure profile in continent women from childhood to old age. Acta Obstet Gynecol Scand. 1980;59(4):331–5. doi: 10.3109/00016348009154090. [DOI] [PubMed] [Google Scholar]
- 18.Clobes A, DeLancey JO, Morgan DM. Urethral circular smooth muscle in young and old women. Am J Obstet Gynecol. 2008 May;198(5):587.e1–5. doi: 10.1016/j.ajog.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thind P, Lose G. The effect of bilateral pudendal blockade on the static urethral closure function in healthy females. Obstet Gynecol. 1992 Dec;80(6):906–11. [PubMed] [Google Scholar]