Abstract
Background:
Neoadjuvant chemotherapy improves outcome in osteosarcoma. Determination of optimum regimens for survival, toxicity and prognostic factors requires randomised controlled trials to be conducted.
Patients and methods:
Between 1983 and 2002, the European Osteosarcoma Intergroup recruited 1067 patients with localised extremity osteosarcoma to three randomised controlled trials. Standard treatment in each was doxorubicin 75 mg/m2 and cisplatin 100 mg/m2. Comparators were addition of methotrexate (BO02/80831), a multidrug regimen (BO03/80861) and a dose-intense schedule (BO06/80931). Standard survival analysis methods were used to identify prognostic factors, temporal and other influences on outcome.
Results:
Five- and 10-year survival were 56% (95% confidence interval 53% to 59%) and 52%, respectively (49% to 55%), with no difference between trials or treatment arms. Median follow-up was 9.4 years. Age range was 3–40 years (median 15). Limb salvage was achieved in 69%. Five hundred and thirty-three patients received the standard arm, 79% completing treatment. Good histological response to preoperative chemotherapy, distal tumour location (all sites other than proximal humerus/femur) and female gender were associated with improved survival.
Conclusions:
Localised osteosarcoma will be cured in 50% of patients with cisplatin and doxorubicin. Large randomised trials can be conducted in this rare cancer. Failure to improve survival over 20 years argues for concerted collaborative international efforts to identify and rapidly test new treatments.
Keywords: adolescents, chemotherapy, osteosarcoma, randomised controlled trial
introduction
High-grade conventional osteosarcoma is a rare tumour with an age-standardised incidence of approximately 5 per million per year [1]. Half of all cases occur in 10- to 24-year-olds and it is the commonest primary bone tumour affecting young people. It has a high propensity to metastasise to the lungs and the prognosis for localised extremity osteosarcoma treated with surgery alone is poor (<20% 2-year survival) [2]. With the introduction of perioperative multi-agent chemotherapy in the 1980s, survival increased rapidly to 55% to 80% at 5 years [3–7]. However, further significant improvements in survival have not been reported.
A variety of combination chemotherapy regimens have been tested and it appears that only a limited number of cytotoxic agents are active against osteosarcoma. The most active are doxorubicin, cisplatin, methotrexate and ifosfamide, and regimens currently in use include at least two of these drugs. However, there is no consensus on the optimum combination [8]. This is at least partly due to a lack of data from randomised trials; historically, osteosarcoma clinical research has been conducted by single centres or small co-operative groups with a reliance on comparison with historical controls.
The European Osteosarcoma Intergroup (EOI) was formed in 1982 with the aim of conducting randomised controlled trials (RCTs). Founding members included the UK Medical Research Council (MRC), UK Children’s Cancer Study Group, Societé Internationale d’Oncologie Paediatrique (SIOP) and the European Organisation for Research and Treatment of Cancer (EORTC). To date, the EOI has completed three RCTs: MRC BO02/EORTC 80831, BO03/80861 and BO06/80931 [6, 9, 10]. In each, the control arm was a two-drug regimen of cisplatin plus doxorubicin and the treatment strategies investigated were the addition of methotrexate, use of a multidrug regimen and dose-intense chemotherapy.
Over 1000 patients participated in these trials and they present an important opportunity to record mature outcomes in a large osteosarcoma cohort. In order to identify time-dependent changes in outcome and key prognostic factors, we undertook a retrospective analysis examining patient factors, disease factors, treatment and survival.
patients and methods
patient selection
Between 1982 and 2002, 1067 patients aged ≤40 years with histologically proven high-grade localised extremity osteosarcoma were randomised in three consecutive international RCTs. Trial procedures including full eligibility criteria are as previously reported [6, 9, 10]. Ethics approval was granted at all institutions, and written consent was obtained from the patient or parent, in accordance with local regulatory guidelines.
We carried out a pooled analysis of all eligible patients (combined Consolidated Standards of Reporting Trials diagram, supplemental Figure S1, available at Annals of Oncology online). BO02 enrolled 307 patients between July 1983 and December 1986; of these, 198 had confirmed localised extremity osteosarcoma. We included 179 patients, excluding 19 patients electively treated with postoperative chemotherapy alone, in line with previous reports from this series [9, 11, 12]. BO03 registered 407 patients between September 1986 and February 1993, of whom 391 were eligible for randomisation; and in BO06, 504 patients were registered between May 1993 and September 2002, 497 of whom were eligible.
treatment details
The treatment regimens used in each trial are summarised in Table 1. In each, one arm of the randomisation was ‘standard’ treatment: six 3-weekly cycles of doxorubicin 75 mg/m2 plus cisplatin 100 mg/m2 (AP). In BO02, the research arm was four 4½-weekly cycles of cisplatin/doxorubicin plus high-dose methotrexate, 8 g/m2 per cycle; BO03 used a 44-week modified T10-like multidrug regimen [13]; in BO06, six 2-weekly cycles of granulocyte colony-stimulating factor-supported dose-intensified cisplatin/doxorubicin were given.
Table 1.
Treatment protocols by trial
| Trial | Chemotherapy | Timing of surgery | Duration of treatment |
| BO02/80831 | 6 × 3-weekly cycles | Week 9 | 18 weeks |
| doxorubicin 25 mg/m2/day × 3 and cisplatin 100 mg/m2 | |||
| versus | |||
| 4 × 4½-weekly cycles | Week 9 | 18 weeks | |
| doxorubicin 25 mg/m2/day × 3, cisplatin 100 mg/m2 and methotrexate 8 g/m2 plus leucovorin rescue | |||
| BO03/80861 | 6 × 3-weekly cycles | Week 9 | 18 weeks |
| doxorubicin 25 mg/m2/day × 3 and cisplatin 100 mg/m2 | |||
| versus | |||
| Multi-drug regimen | Week 7 | 44 weeks | |
| Weeks 1–2, 5–6, 12–13, 17–18 | |||
| vincristine 1.5 mg/m2 and methotrexate 8 g/m2 plus leucovorin rescue | |||
| Weeks 3, 14 | |||
| doxorubicin 25 mg/m2/day × 3 | |||
| Weeks 9, 26, 35, 44: | |||
| bleomycin 15 mg/m2/day × 2, cyclophosphamide 600 mg/m2/day × 2 and dactinomycin 0.6 mg/m2/day × 2 | |||
| Weeks 20, 23, 29, 32, 38 and 41 | |||
| doxorubicin 30 mg/m2/day × 2 and cisplatin 120 mg/m2 | |||
| BO06/80931 | 6 × 3-weekly cycles | Week 6 | 18 weeks |
| doxorubicin 25 mg/m2/day × 3 and cisplatin 100 mg/m2 | |||
| versus | |||
| 6 × 2-weekly cycles | Week 6 | 12 weeks | |
| doxorubicin 25 mg/m2/day × 3, cisplatin 100 mg/m2 and G-CSF |
G-CSF, granulocyte-colony stimulating factor.
data
Data were extracted from the primary trial datasets. Within the source trials, all data were collected prospectively using standardised case report forms. Baseline characteristics examined in this analysis included the following: year of randomisation, collaborative group, geographical location, age, gender, primary site (bone affected), proximal versus distal tumour (proximal defined as a proximal tumour of the humerus or femur; distal, all other sites) and histological subtype. Treatment-related factors included type and timeliness of surgery, treatment completion and histological response to preoperative chemotherapy. Good histological response was defined as ≥90% necrosis in the resected tumour specimen. The EOI pathology subcommittee reviewed diagnostic pathology and response assessment centrally. Treatment was considered completed if the full protocol-specified number of chemotherapy cycles had been given; reasons for stopping early were defined as progression, toxicity, patient choice and ‘other’.
statistical methods
This was a retrospective analysis, carried out on an intention-to-treat basis using standard time-to-event methodology (survival analysis) [14], examining prognostic factors for overall survival and progression-free survival (PFS). A two-sided significance level of 5% was adopted. Median follow-up was calculated by reverse censoring on overall survival. Overall survival was timed until death (from any cause) or patients were censored at date of last follow-up if death had not occurred. PFS was timed until the date of first event (local or metastatic disease progression or death but excluding apparent progression of local disease before primary surgery), or censoring occurred at date of last follow-up. Twenty-four patients had apparent progression of local disease before primary surgery but these events were not included in these analyses in recognition that early clinical distinction between response and progression is unreliable in osteosarcoma. The relative risks of each factor are summarised using hazard ratios (HR) from univariate and multivariate Cox regression models. HRs are expressed relative to patients in the baseline category of the factor of interest, so an HR <1.0 indicates a lower risk of the event for patients in that category compared with the baseline category. Variables were considered to be nominal. For the univariate models, survival was measured from date of randomisation or from date of surgery for factors measured at surgery (histological response, type and timeliness of surgery). In the multivariate analyses, survival was measured from date of surgery. Patients with missing data for the variable of interest were excluded from that particular univariate analysis. Data are assumed missing completely at random. Only patients with data available for all factors were incorporated in the multivariate analyses. All models were stratified by trial. Analyses were carried out using Stata 9 (StataCorp, College Station, TX).
results
Between 1982 and 2002, 1067 eligible patients were randomised in the three RCTs. Median follow-up is 9.4 years [interquartile range (IQR) 5.0–14.5] (Table 2) and 467 deaths and 584 PFS events have been reported.
Table 2.
Patient characteristics by trial
| BO02 (N = 179) |
BO03 (N = 391) |
BO06 (N = 497) |
Total (N
= 1067) |
|||||
| N | % | N | % | N | % | N | % | |
| Treatment arm | ||||||||
| Cisplatin + doxorubicin | 89 | 50 | 199 | 51 | 245 | 49 | 533 | 50 |
| Cisplatin + doxorubicin + HDMTX | 90 | 50 | 0 | 0 | 0 | 0 | 90 | 8 |
| Multi-drug regimen | 0 | 0 | 192 | 49 | 0 | 0 | 192 | 18 |
| Cisplatin + doxorubicin + G-CSF | 0 | 0 | 0 | 0 | 252 | 51 | 252 | 24 |
| Collaborative group | ||||||||
| UK/MRC | 112 | 63 | 267 | 68 | 228 | 46 | 607 | 57 |
| EORTC/SIOP | 67 | 37 | 64 | 16 | 151 | 30 | 282 | 26 |
| Other | 0 | 0 | 60 | 15 | 118 | 24 | 178 | 17 |
| Geographical location | ||||||||
| UK/Ireland | 112 | 63 | 263 | 67 | 228 | 46 | 603 | 57 |
| Mainland Europe | 64 | 36 | 68 | 17 | 151 | 30 | 283 | 27 |
| Other | 3 | 2 | 60 | 15 | 118 | 24 | 181 | 17 |
| Age at randomisation | ||||||||
| ≤10 years | 20 | 11 | 47 | 12 | 95 | 19 | 162 | 15 |
| 11–15 years | 68 | 38 | 134 | 34 | 195 | 39 | 397 | 37 |
| 16–20 years | 67 | 37 | 134 | 34 | 141 | 28 | 342 | 32 |
| 21–25 years | 16 | 9 | 45 | 12 | 32 | 6 | 93 | 9 |
| ≥26 years | 8 | 5 | 31 | 8 | 7 | 7 | 73 | 7 |
| Median (IQR) | 16 (13–18) | 16 (13–19) | 15 (12–18) | 15 (12–18) | ||||
| Minimum–maximum | 3–40 | 3–38 | 3–40 | 3–40 | ||||
| Sex | ||||||||
| Male | 102 | 57 | 261 | 67 | 293 | 59 | 656 | 62 |
| Female | 77 | 43 | 130 | 33 | 201 | 41 | 408 | 38 |
| Site of tumour | ||||||||
| Femur | 100 | 56 | 215 | 55 | 296 | 60 | 611 | 58 |
| Tibia | 47 | 26 | 101 | 26 | 116 | 24 | 264 | 25 |
| Fibula | 9 | 5 | 20 | 5 | 25 | 5 | 54 | 5 |
| Humerus | 23 | 13 | 49 | 13 | 48 | 10 | 120 | 11 |
| Radius | 0 | 0 | 3 | 1 | 5 | 1 | 8 | 1 |
| Ulna | 0 | 0 | 3 | 1 | 1 | 0 | 4 | 0 |
| Missing | 0 | n/a | 0 | n/a | 6 | n/a | 6 | n/a |
| Location of tumoura | ||||||||
| Proximal | 29 | 16 | 60 | 15 | 65 | 13 | 154 | 15 |
| Distal | 148 | 84 | 329 | 85 | 425 | 87 | 902 | 85 |
| Missing | 2 | n/a | 2 | n/a | 7 | n/a | 11 | n/a |
| Type of osteosarcoma | ||||||||
| Common type | 144 | 81 | 260 | 66 | 271 | 63 | 675 | 68 |
| Chondroblastic | 9 | 5 | 45 | 12 | 51 | 12 | 105 | 11 |
| Fibroblastic | 10 | 6 | 43 | 11 | 15 | 3 | 68 | 7 |
| Osteoclast rich | 3 | 2 | 8 | 2 | 9 | 2 | 20 | 2 |
| Anaplastic | 8 | 5 | 16 | 4 | 21 | 5 | 45 | 5 |
| Small cell | 1 | 1 | 2 | 1 | 5 | 1 | 8 | 1 |
| Telangiectatic | 0 | 0 | 10 | 3 | 29 | 7 | 39 | 4 |
| Other | 2 | 1 | 7 | 2 | 29 | 7 | 38 | 4 |
| Missing | 2 | n/a | 0 | n/a | 67 | n/a | 69 | n/a |
| Length of follow-up (years) | ||||||||
| Median (IQR) | 17.7 (16.5–19.0) | 12.9 (11.1–14.7) | 5.0 (3.1–7.2) | 9.4 (5.0–14.5) | ||||
Proximal, proximal tumour of the humerus or femur; distal = all other locations.
HDMTx, high-dose methotrexate; G-CSF, granulocyte colony stimulating factor; SIOP, Societé Internationale d’Oncologie Paediatrique; MRC, UK Medical Research Council; IQR, interquartile range.
patient characteristics
Patient characteristics are shown in Table 2. Median age was 15 years (IQR 12–18); 78% (832/1067) were aged 11–25 years and 62% were male. Primary tumours arising in the long bones of the lower limb accounted for 88% (929/1061) of cases. Tumour site was evenly distributed by age, except tibial tumours which were less common in >25 years [17% (12/72) versus 25% (252/989) in ≤25-year-olds].
Some differences were observed between the trials. There was a higher proportion of males in BO03, while more young patients (<15 years) were randomised into BO06. The most frequent histology was common type but more patients in the later two trials were classified into other subtypes. In particular, more chondroblastic tumours were seen in the later trials, the proportion of fibroblastic tumours was greater in BO03 and a higher (although still small) percentage of patients had telangiectatic and ‘other’ tumours in BO06.
treatment
Treatment received, by trial and treatment arm, is shown in supplemental Table S1 (available at Annals of Oncology online). Fifty per cent (533/1067) received standard AP chemotherapy, 79% (423/533) of whom completed treatment. Patients randomised to the multidrug regimen were much less likely to complete planned treatment, 40% (75/188) versus 81% (699/866) for all other treatment arms combined. They were more likely to progress on treatment [17% (32/188) versus 5% (43/866)] and to stop early due to toxicity or patient refusal [34% (65/188) versus 10% (83/866)]. Treatment completion rates in all arms declined with age being 85% for <11 years, 73% for 11- to 20-year-olds, 67% for 21- to 25-year-olds and 59% for >25-year-olds. This was principally due to toxicity and patient refusal rather than progression (data not shown).
The proportion of limb salvage operations increased significantly after BO02, in which 56% (100/178) of patients had limb salvage surgery compared with 72% (273/381) and 74% (334/453) in the subsequent trials. This was true for all primary sites (data not shown) and age groups (supplemental Table S2, available at Annals of Oncology online).
histological response
Data on histological response were available for 66% (699/1067) of patients overall but only for 35 from BO02 (Table S1). The overall proportion with a good histological response was 39% (272/699). Patients randomised to dose-intensified cisplatin/doxorubicin were most likely to report a good histological response, 50% (101/200).
recurrence
Isolated local recurrence occurred in 6% (67/1067) of patients and 47% (497/1067) developed metastases. This was consistent across all three trials (Table 3a). Local recurrence occurred more frequently in patients who had limb salvage surgery, 8% (54/707) versus 2% (5/305) in amputees; conversely, patients undergoing an amputation were more likely to develop metastatic disease, 56% (171/305) versus 43% (302/707) (Table 3b).
Table 3.
Pattern of recurrence
| BO02 (N = 179) |
BO03 (N = 391) |
BO06 (N = 497) |
Total (N = 1067) |
|||||
| N | % | N | % | N | % | N | % | |
| (A) By trial | ||||||||
| Local recurrence | 12 | 7 | 18 | 5 | 37 | 7 | 67 | 6 |
| Metastases | 79 | 44 | 190 | 49 | 228 | 46 | 497 | 47 |
| No recurrence | 88 | 49 | 183 | 47 | 232 | 47 | 503 | 47 |
| Amputation (N = 305) |
Limb salvage (N = 707) |
Missing (N = 55) |
Total (N = 1067) |
|||||
| N | % | N | % | N | % | N | % | |
| (B) By type of surgery | ||||||||
| Local recurrence | 5 | 2 | 54 | 8 | 8 | 15 | 67 | 6 |
| Metastases | 171 | 56 | 302 | 43 | 24 | 44 | 497 | 47 |
| No recurrence | 129 | 42 | 351 | 50 | 23 | 42 | 503 | 47 |
survival
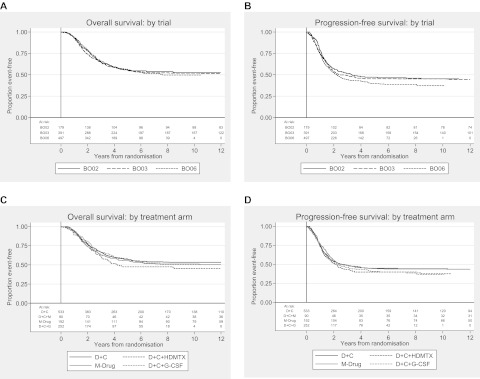
Five- and 10-year overall survival was 56% [95% confidence interval (CI) 53% to 59%] and 52%, respectively (49% to 55%), for the whole cohort and 5- and 10-year PFS was 43% (40% to 46%) and 42%, respectively (39% to 46%). There was no survival difference between trials or treatment arms (Figure 1).
Figure 1.
Kaplan–Meier plots for (A) overall survival and (B) progression-free survival (PFS) by trial and (C) overall survival and (D) PFS by treatment arm. D, doxorubicin; C, cisplatin; HDMTx, high-dose methotrexate; G-CSF, granulocyte colony-stimulating factor.
Univariate and multivariate analyses for overall survival are shown in Table 4. Female sex, distal tumour location and good histological response to preoperative chemotherapy were all prognostic for survival. Females had a 5-year overall survival of 61% (95% CI 56% to 66%) versus 53% (49% to 57%) for males. Five-year survival of patients with distal tumours was 58% (55% to 61%) versus 46% (38% to 54%) for proximal. Good histological response was a highly statistically significant prognostic factor with 5-year survival being 73% (67% to 78%) in those with a good histological response and only 47% (42% to 52%) for poor response. Age ≥26 was an adverse prognostic factor in the multivariate analysis compared with the younger age groups considered. There were trends towards improved survival for patients with chondroblastic, fibroblastic and anaplastic histology and tumour sites other than the femur/humerus.
Table 4.
Univariate and multivariate Cox models for overall survival
| Overall survival |
N | Univariate models |
Multivariate modela (N = 682) |
||||||||
| 5 years | 10 years | ||||||||||
| % | 95% CI | % | 95% CI | HR | 95% CI | P | HR | 95% CI | P | ||
| Year of randomisationb | 1067 | ||||||||||
| Each additional year (from 1983) | n/a | n/a | 1.00 | 0.98–1.01 | 0.678 | ||||||
| Collaborative group | 1067 | ||||||||||
| UK/MRC | 58 | 55–62 | 54 | 50–58 | 1.00 | 1.00 | |||||
| EORTC/SIOP | 51 | 45–56 | 48 | 42–53 | 1.20 | 1.00–1.45 | 0.053 | 1.16 | 0.59–2.29 | 0.660 | |
| Geographical location | 1067 | ||||||||||
| UK/Ireland | 59 | 55–63 | 54 | 50–58 | 1.00 | 1.00 | |||||
| Mainland Europe | 52 | 46–59 | 49 | 42–55 | 1.15 | 0.92–1.43 | 0.209 | 0.77 | 0.37–1.60 | 0.489 | |
| Other | 50 | 42–58 | 48 | 39–57 | 1.31 | 1.01–1.70 | 0.042 | 0.96 | 0.52–1.77 | 0.890 | |
| Age group | 1067 | ||||||||||
| ≤10 years | 62 | 53–69 | 61 | 52–68 | 1.00 | 1.00 | |||||
| 11–15 years | 54 | 49–60 | 50 | 44–55 | 1.32 | 0.98–1.78 | 0.071 | 1.30 | 0.88–1.93 | 0.188 | |
| 16–20 years | 54 | 48–59 | 50 | 44–55 | 1.31 | 0.97–1.78 | 0.081 | 1.36 | 0.91–2.03 | 0.136 | |
| 21–25 years | 63 | 52–72 | 56 | 44–66 | 1.09 | 0.72–1.64 | 0.695 | 1.12 | 0.64–1.96 | 0.696 | |
| ≥26 years | 54 | 41–65 | 47 | 35–59 | 1.45 | 0.96–2.21 | 0.079 | 1.89 | 1.11–3.20 | 0.019 | |
| Gender | 1064 | ||||||||||
| Male | 53 | 49–57 | 49 | 45–53 | 1.00 | 1.00 | |||||
| Female | 61 | 56–66 | 57 | 51–62 | 0.82 | 0.67–0.99 | 0.038 | 0.76 | 0.59–0.97 | 0.029 | |
| Site of tumourb | 1061 | ||||||||||
| Femur | 55 | 51–59 | 51 | 47–55 | 1.00 | ||||||
| Tibia | 61 | 54–67 | 56 | 50–63 | 0.81 | 0.64–1.01 | 0.061 | ||||
| Fibula | 60 | 45–72 | 60 | 45–72 | 0.76 | 0.48–1.20 | 0.244 | ||||
| Humerus | 46 | 37–55 | 40 | 31–50 | 1.28 | 0.98–1.68 | 0.073 | ||||
| Other | 90 | 47–99 | 77 | 35–94 | 0.29 | 0.07–1.16 | 0.080 | ||||
| Location of tumourc | 1056 | ||||||||||
| Proximal | 46 | 38–54 | 40 | 31–48 | 1.00 | 1.00 | |||||
| Distal | 58 | 55–61 | 54 | 50–58 | 0.68 | 0.54–0.86 | 0.001 | 0.73 | 0.54–0.99 | 0.045 | |
| Histological subtype | 998 | ||||||||||
| Common type | 57 | 53–61 | 53 | 49–57 | 1.00 | 1.00 | |||||
| Chondroblastic | 61 | 50–70 | 55 | 44–65 | 0.97 | 0.70–1.33 | 0.834 | 0.76 | 0.51–1.14 | 0.187 | |
| Fibroblastic | 58 | 45–69 | 54 | 41–65 | 0.94 | 0.64–1.37 | 0.742 | 0.89 | 0.55–1.45 | 0.650 | |
| Anaplastic | 64 | 47–77 | 59 | 42–72 | 0.85 | 0.52–1.39 | 0.530 | 0.66 | 0.35–1.26 | 0.208 | |
| Telangiectatic | 51 | 32–67 | 46 | 27–63 | 1.26 | 0.77–2.06 | 0.365 | 1.13 | 0.61–2.09 | 0.700 | |
| Other | 45 | 32–57 | 42 | 29–55 | 1.42 | 0.99–2.03 | 0.057 | 1.44 | 0.95–2.18 | 0.085 | |
| Histological responsea | 697 | ||||||||||
| Poor | 48 | 42–52 | 44 | 39–49 | 1.00 | 1.00 | |||||
| Good | 73 | 67–78 | 69 | 62–74 | 0.43 | 0.33–0.56 | <0.001 | 0.47 | 0.35–0.61 | <0.001 | |
| Type of surgerya | 1010 | ||||||||||
| Amputationd | 47 | 41–52 | 43 | 37–49 | 1.00 | 1.00 | |||||
| Limb salvage | 61 | 57–64 | 57 | 52–60 | 0.63 | 0.52–0.77 | <0.001 | 0.75 | 0.56–1.00 | 0.052 | |
| Timeliness of surgerya,e | 1004 | ||||||||||
| On time | 56 | 51–61 | 52 | 47–57 | 1.00 | 1.00 | |||||
| Early | 36 | 25–48 | 36 | 25–47 | 1.83 | 1.31–2.55 | <0.001 | 1.61 | 1.00–2.61 | 0.051 | |
| Late | 60 | 55–64 | 56 | 51–60 | 0.91 | 0.75–1.11 | 0.369 | 1.04 | 0.81–1.34 | 0.772 | |
Models stratified by trial (except for year of randomisation in univariate model).
Timed from date of surgery (includes multivariate model).
Not included in multivariate model due to overlap with other variables.
Proximal, proximal humerus/femur; distal, all other sites.
Includes rotationplasty and disarticulation.
On time, surgery occurred between 3 days earlier and 10 days later than specified in protocol; early, surgery occurred >3 days earlier than specified; late, surgery occurred >10 days later than specified.
CI, confidence interval; SIOP, Societé Internationale d’Oncologie Paediatrique.
Patients who underwent limb salvage surgery had improved 5-year survival compared with those undergoing an amputation, 61% (57% to 64%) versus 47% (41% to 52%), but this was of borderline significance at the 5% level in multivariate analysis. This was also true for timeliness of surgery where patients having surgery earlier than planned appear to have a worse prognosis than patients who had their surgery on time or late (26/31 who had their surgery earlier than planned also had an amputation).
Female gender, distal tumour location [5-year PFS 45% (41% to 48%) versus 32% (25% to 40%) for proximal] and good histological response [61% (55% to 67%) versus 34% (29% to 38%)] were also independent positive prognostic indicators for PFS (Table 5). Requiring surgery earlier than planned was an adverse factor [28% (19% to 39%) versus 45% (40% to 50%) if on time] and there was a suggestion of improved PFS with limb salvage versus amputation.
Table 5.
Univariate and multivariate Cox models for progression-free survival
| Univariate models |
Multivariate modela (N = 682) |
||||||
| N | HR | 95% CI | P | HR | 95% CI | P | |
| Year of randomisationb | 1067 | ||||||
| Each additional year (from 1983) | 1.01 | 1.00–1.03 | 0.104 | ||||
| Collaborative group | 1067 | ||||||
| MRC | 1.00 | 1.00 | |||||
| EORTC | 1.06 | 0.89–1.25 | 0.533 | 0.90 | 0.52–1.56 | 0.717 | |
| Geographical location | 1067 | ||||||
| UK/Ireland | 1.00 | 1.00 | |||||
| Mainland Europe | 0.99 | 0.82–1.21 | 0.952 | 0.89 | 0.49–1.62 | 0.709 | |
| Other | 1.20 | 0.96–1.51 | 0.113 | 1.04 | 0.64–1.69 | 0.878 | |
| Age group | 1067 | ||||||
| ≤10 years | 1.00 | 1.00 | |||||
| 11–15 years | 1.47 | 1.13–1.91 | 0.004 | 1.38 | 0.98–1.94 | 0.062 | |
| 16–20 years | 1.33 | 1.01–1.75 | 0.039 | 1.32 | 0.93–1.87 | 0.120 | |
| 21–25 years | 1.04 | 0.71–1.51 | 0.845 | 1.15 | 0.71–1.86 | 0.566 | |
| ≥26 years | 1.30 | 0.89–1.91 | 0.179 | 1.36 | 0.83–2.24 | 0.218 | |
| Gender | 1064 | ||||||
| Male | 1.00 | 1.00 | |||||
| Female | 0.84 | 0.71–1.00 | 0.052 | 0.79 | 0.64–0.99 | 0.036 | |
| Site of tumourb | 1061 | ||||||
| Femur | 1.00 | ||||||
| Tibia | 0.81 | 0.66–0.99 | 0.039 | ||||
| Fibula | 0.76 | 0.51–1.14 | 0.185 | ||||
| Humerus | 1.40 | 1.10–1.78 | 0.006 | ||||
| Other | 0.61 | 0.25–1.48 | 0.274 | ||||
| Location of tumourc | 1056 | ||||||
| Proximal site | 1.00 | 1.00 | |||||
| Distal site | 0.66 | 0.53–0.82 | <0.001 | 0.66 | 0.51–0.87 | 0.003 | |
| Type of osteosarcoma | 998 | ||||||
| Common type | 1.00 | 1.00 | |||||
| Chondroblastic | 1.13 | 0.86–1.48 | 0.390 | 0.95 | 0.68–1.31 | 0.739 | |
| Fibroblastic | 0.97 | 0.69–1.38 | 0.879 | 0.82 | 0.52–1.29 | 0.381 | |
| Anaplastic | 0.84 | 0.54–1.31 | 0.446 | 0.69 | 0.40–1.19 | 0.184 | |
| Telangiectatic | 0.92 | 0.57–1.49 | 0.748 | 0.88 | 0.49–1.58 | 0.657 | |
| Other | 1.25 | 0.90–1.74 | 0.187 | 1.14 | 0.77–1.68 | 0.508 | |
| Histological responsea | 697 | ||||||
| Poor | 1.00 | 1.00 | |||||
| Good | 0.44 | 0.35–0.56 | <0.001 | 0.48 | 0.38–0.61 | <0.001 | |
| Surgery typea | 1010 | ||||||
| Amputationd | 1.00 | 1.00 | |||||
| Limb salvage | 0.70 | 0.59–0.84 | <0.001 | 0.89 | 0.68–1.15 | 0.363 | |
| Timeliness of surgerya,e | 1004 | ||||||
| On time | 1.00 | 1.00 | |||||
| Early | 1.87 | 1.38–2.53 | <0.001 | 1.80 | 1.17–2.76 | 0.007 | |
| Late | 0.98 | 0.82–1.17 | 0.792 | 1.00 | 0.80–1.25 | 0.996 | |
Models stratified by trial (except for year of randomisation in univariate model).
Timed from date of surgery (includes multivariate model).
Not included in multivariate model due to overlap with other variables.
Proximal, proximal humerus/femur; distal, all other sites.
Includes rotationplasty and disarticulation.
On time, surgery occurred between 3 days earlier and 10 days later than specified in protocol; early, surgery occurred >3 days earlier than specified; late, surgery occurred >10 days later than specified.
CI, confidence interval; HR, hazard ratios.
treatment completion
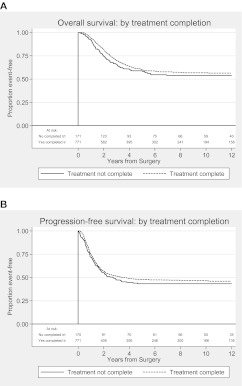
An exploratory univariate analysis of the effect of treatment completion on survival was also carried out. Patients who did not complete chemotherapy due to progressive disease were excluded. We found that the risk of death or progression was lower in patients who completed all protocol-specified cycles of chemotherapy compared with those who did not: HR for overall survival 0.64 (0.50–0.82, P < 0.001) and 0.65 (0.52–0.80, P < 0.001) for PFS (Figure 2).
Figure 2.
Kaplan–Meier plots for (A) overall survival and (B) progression-free survival by treatment completion. Treatment complete: all protocol-specified cycles of chemotherapy given and treatment not complete: any other number of cycles. Patients who stopped protocol treatment due to progression of disease were excluded from this analysis. Reasons for non-completion were toxicity, patient choice and ‘other’.
discussion
This cohort of >1000 patients with high-grade localised extremity osteosarcoma is the second largest yet reported [4] and has the second longest median follow-up [3]. It is the first mature cohort wholly comprised of patients treated within RCTs and represents a sustained commitment to the conduct of multicentre randomised trials in this rare cancer.
A potential criticism of a trial-based cohort is its generalisability and, in keeping with most osteosarcoma clinical trials, adults >40 years were not eligible. However, our cohort appears otherwise representative in terms of demographics and disease-related factors: the majority were aged 11–25 years and the ratio of males to females was 3:2; most tumours arose in the long bones of the leg and were of conventional histotype [15–17]. The increased frequency of histological sub-typing and assessment of histological response over time (which in contrast to most other studies were subject to central review) and the increased use of limb-sparing surgery are similar to those observed in contemporary cohorts [3, 4, 18]. Although the majority of recruitment was from Western Europe, patients were enrolled from 18 countries and a sizeable minority in the later trials (24% in BO06) were from other areas, including South Africa, Saudi Arabia and South America. This geographical range is a strength of our cohort compared with those from single institutions [3] or a smaller number of countries [4, 18].
A number of prognostic factors for localised extremity osteosarcoma have been proposed including: sex, age, histological subtype, tumour size and location, surgical resection margin, serum alkaline phosphatase and lactate dehydrogenase levels, expression of P-glycoprotein and Erb2 and histological response to preoperative chemotherapy [3, 4, 18–24]. However, published evidence is limited by methodological heterogeneity and contradictory results. Our results confirm the prognostic role of histological response, demonstrating a sustained absolute survival difference of 25% between good and poor responders but also show that 44% of patients with a poor histological response to these chemotherapy regimens may be cured. However, as previously observed in BO06, there is a caveat to this association: despite higher rates of good histological response being reported in patients receiving the dose-intense regimen, there was again no corresponding increase in survival [6]. Our results also add to evidence for female sex and distal tumour location being favourable prognostic indicators. The influence of age remains unclear, however. In this analysis, adults >25 years had the worst survival and children under 11 years the best but other studies have reported conflicting results [3, 20]. A previous combined analysis of BO02/BO03 suggested a trend to improved survival for chondroblastic histology [11]; this updated analysis was suggestive of a similar trend but again did not approach statistical significance. A number of other potential prognostic factors were not collected in the original trial datasets (tumour size, resection margin and alkaline phosphatase levels) and our models are limited by their exclusion.
We found that factors relating to surgery appear to influence prognosis. In this case, patients who required surgery earlier than planned and those having an amputation had worse overall and PFS, despite higher local recurrence rates in those who had limb salvage surgery. These are likely to be surrogate markers for chemotherapy-resistant disease, reflected by higher rates of subsequent metastatic disease in patients who underwent an amputation.
These long-term outcome data again failed to show a survival advantage for the research regimens over standard AP chemotherapy, in keeping with the primary trial results [6, 9, 10]. T10-like multidrug regimens and dose intensification are no longer employed in the treatment of osteosarcoma. However, the combination of high-dose methotrexate, cisplatin and doxorubicin (MAP) is used [5, 7, 18, 25, 26] and is the control arm of the current EURAMOS-1 trial (NCT00134030), a trans-Atlantic collaborative RCT in which the EOI is participating [27]. In hindsight, sub-optimal doses of cisplatin/doxorubicin and methotrexate were used in the research arm of BO02, which may have compromised its efficacy. AP is still used in routine clinical care and this combined analysis provides definitive evidence for its efficacy and tolerability: a patient with localised extremity osteosarcoma treated with AP has around a 40% chance of being progression free at 10 years and just over a 50% chance of being alive. In all three trials, >75% of patients randomised to AP completed chemotherapy.
However, our survival results are lower than those of contemporary series where 5-year overall survival rates of 65% to 74% have been reported [3, 4, 18]. The lowest figure here, 65% from the study by Bielack et al. [4], included patients with primary metastatic and axial disease. Their proportion of patients achieving a good histological response was also higher (55% to 66% versus 39%) and recurrence rates lower (<40% versus 54%). Although, as discussed earlier, there are differences between the setting of these studies and our RCTs, it is notable that these groups developed three- or four-drug combination chemotherapy regimens incorporating methotrexate and ifosfamide in addition to cisplatin–doxorubicin. In the analysis by Bacci et al. [3] to be treated with a two-drug regimen was an adverse prognostic factor. Recent trials using three- or four-drug regimens also consistently report response rates and survival times greater than those seen in our studies [7, 28]. Despite the lack of an RCT directly comparing AP with these regimens, it is reasonable to conclude that better results are achieved with at least three drugs, and AP should no longer be considered a standard chemotherapy for patients aged under 40 years with localised resectable osteosarcoma [29].
Disappointingly, but consistent with epidemiological studies [15–17], there was also no evidence of an improvement in survival over time. Over the 20-year period of these trials, there were major developments in oncological practice including the following: more accurate staging through the routine use of computed tomography and magnetic resonance imaging scanning; increasing surgical expertise in the management of metastatic disease, including repeat metastatectomy [30, 31] and improvements in supportive care during chemotherapy. Despite the introduction of more effective anti-emetics, nutritional support and growth factors, there was no increase in the proportion of patients receiving AP who completed chemotherapy. Although fewer patients stopped as a result of chemotherapy toxicity, a higher proportion stopped early through patient choice or for ‘other’ reasons. In a hypothesis-generating analysis, we have shown that failure to complete treatment was an adverse prognostic factor for survival. A similar relationship with local recurrence has been found in a recent retrospective analysis by the Cooperative Osteosarcoma Study group [32]. Poor treatment adherence has been proposed as one of the reasons that improvement in survival for adolescents and young adults (AYAs) with cancer has lagged behind that of children and older adults. The development of AYA-specific models of care and interventions may improve this but further research is needed [33].
conclusions
Our data, derived from 20 years of RCTs, show that >50% of patients diagnosed with localised extremity osteosarcoma can expect to achieve long-term survival. We have definitively shown that standard AP chemotherapy is tolerable and moderately effective. However, improvements in survival are needed and require globally collaborative clinical trials that can deliver results in a clinically relevant timeframe.
funding
This work was supported by the UK Medical Research Council via its core funding to the MRC Clinical Trials Unit and was in part carried out at University College Hospital NHS Foundation Trust, which receives funding through the National Institute of Health Research Comprehensive Biomedical Research Centre programme.
disclosure
MRS, RCJ, BU and JMH are employed by the UK Medical Research Council, which funded this study. JSW, AMcT, LT, VB, IJL, MAN, MvG, RJG, PCWH, AHMT and HG have no conflicts of interest to declare.
Supplementary Material
Acknowledgments
We are grateful to all staff and patients who supported European Osteosarcoma Intergroup randomised trials at over 100 institutions. We also thank the staff of the MRC Clinical Trials Unit and the EORTC Data Centre.
References
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Friedman MA, Carter SK. The therapy of osteogenic sarcoma: current status and thoughts for the future. J Surg Oncol. 1972;4:482–510. doi: 10.1002/jso.2930040512. [DOI] [PubMed] [Google Scholar]
- 3.Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 4.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 6.Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 7.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children's Oncology Group. J Clin Oncol. 2008;26:633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 8.Hogendoorn PC, Athanasou N, Bielack S, et al. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v204–v213. doi: 10.1093/annonc/mdq223. [DOI] [PubMed] [Google Scholar]
- 9.Bramwell VH, Burgers M, Sneath R, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: the first study of the European Osteosarcoma Intergroup. J Clin Oncol. 1992;10:1579–1591. doi: 10.1200/JCO.1992.10.10.1579. [DOI] [PubMed] [Google Scholar]
- 10.Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 11.Hauben EI, Weeden S, Pringle J, et al. Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer. 2002;38:1218–1225. doi: 10.1016/s0959-8049(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 12.Lewis IJ, Weeden S, Machin D, et al. Received dose and dose-intensity of chemotherapy and outcome in nonmetastatic extremity osteosarcoma. European Osteosarcoma Intergroup. J Clin Oncol. 2000;18:4028–4037. doi: 10.1200/JCO.2000.18.24.4028. [DOI] [PubMed] [Google Scholar]
- 13.Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Parmar MKB, Machin D. Survival Analysis: A Practical Approach. Chichester, New York: John Wiley; 1995. [Google Scholar]
- 15.Eyre R, Feltbower RG, James PW, et al. The epidemiology of bone cancer in 0–39 year olds in northern England, 1981–2002. BMC Cancer. 2010;10:357. doi: 10.1186/1471-2407-10-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gatta G, Capocaccia R, Stiller C, et al. Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol. 2005;23:3742–3751. doi: 10.1200/JCO.2005.00.554. [DOI] [PubMed] [Google Scholar]
- 17.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smeland S, Muller C, Alvegard TA, et al. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer. 2003;39:488–494. doi: 10.1016/s0959-8049(02)00747-5. [DOI] [PubMed] [Google Scholar]
- 19.Akatsuka T, Wada T, Kokai Y, et al. ErbB2 expression is correlated with increased survival of patients with osteosarcoma. Cancer. 2002;94:1397–1404. doi: 10.1002/cncr.10360. [DOI] [PubMed] [Google Scholar]
- 20.Bramer JA, van Linge JH, Grimer RJ, Scholten RJ. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol. 2009;35:1030–1036. doi: 10.1016/j.ejso.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Davis AM, Bell RS, Goodwin PJ. Prognostic factors in osteosarcoma: a critical review. J Clin Oncol. 1994;12:423–431. doi: 10.1200/JCO.1994.12.2.423. [DOI] [PubMed] [Google Scholar]
- 22.Glasser DB, Lane JM, Huvos AG, et al. Survival, prognosis, and therapeutic response in osteogenic sarcoma. The Memorial Hospital experience. Cancer. 1992;69:698–708. doi: 10.1002/1097-0142(19920201)69:3<698::aid-cncr2820690317>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Kawai A, Huvos AG, Meyers PA, Healey JH. Osteosarcoma of the pelvis. Oncologic results of 40 patients. Clin Orthop Relat Res. 1998;348:196–207. [PubMed] [Google Scholar]
- 24.Weeden S, Grimer RJ, Cannon SR, et al. The effect of local recurrence on survival in resected osteosarcoma. Eur J Cancer. 2001;37:39–46. doi: 10.1016/s0959-8049(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 25.Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the Istituto Ortopedico Rizzoli according to the Istituto Ortopedico Rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000;18:4016–4027. doi: 10.1200/JCO.2000.18.24.4016. [DOI] [PubMed] [Google Scholar]
- 26.Winkler K, Beron G, Delling G, et al. Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–337. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- 27.Marina N, Bielack S, Whelan J, et al. International collaboration is feasible in trials for rare conditions: the EURAMOS experience. Cancer Treat Res. 2009;152:339–353. doi: 10.1007/978-1-4419-0284-9_18. [DOI] [PubMed] [Google Scholar]
- 28.Smeland S, Bruland OS, Hjorth L, et al. Results of the Scandinavian Sarcoma Group XIV protocol for classical osteosarcoma: 63 patients with a minimum follow-up of 4 years. Acta Orthop. 2011;82:211–216. doi: 10.3109/17453674.2011.566141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anninga JK, Gelderblom H, Fiocco M, et al. Chemotherapeutic adjuvant treatment for osteosarcoma: where do we stand? Eur J Cancer. 2011 doi: 10.1016/j.ejca.2011.05.030. doi:10.1016/j.ejca.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 30.Buddingh EP, Anninga JK, Versteegh MI, et al. Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer. 2010;54:216–221. doi: 10.1002/pbc.22293. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari S, Briccoli A, Mercuri M, et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol. 2003;21:710–715. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 32.Andreou D, Bielack SS, Carrle D, et al. The influence of tumor- and treatment-related factors on the development of local recurrence in osteosarcoma after adequate surgery. An analysis of 1355 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. Ann Oncol. 2011;22:1228–1235. doi: 10.1093/annonc/mdq589. [DOI] [PubMed] [Google Scholar]
- 33.Butow P, Palmer S, Pai A, et al. Review of adherence-related issues in adolescents and young adults with cancer. J Clin Oncol. 2010;28:4800–4809. doi: 10.1200/JCO.2009.22.2802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.