Human melanocytic nevi are benign lesions harboring activated oncogenes, including BRAF. Nevi can infrequently progress to melanomas, but the underlying mechanism is unclear. In this study by Peeper and colleagues, the authors demonstrate that PTEN depletion abrogates BRAFV600E-induced senescence in human fibroblasts and melanocytes and that depletion of PTEN in murine BRAFV600E-driven nevi prompted tumor progression. In addition, genetic analysis showed that the PI3K pathway was often activated in melanomas relative to their adjacent nevi. PI3K inhibition in melanoma cells suppressed proliferation and induced a senescence-associated tumor suppressor. Thus, this works provides novel insights into the molecular mechanisms contributing to melanomagenesis.
Keywords: senescence, nevus, melanoma, BRAFV600E, PTEN, AKT, PI3K pathway
Abstract
Human melanocytic nevi (moles) are benign lesions harboring activated oncogenes, including BRAF. Although this oncogene initially acts mitogenically, eventually, oncogene-induced senescence (OIS) ensues. Nevi can infrequently progress to melanomas, but the mechanistic relationship with OIS is unclear. We show here that PTEN depletion abrogates BRAFV600E-induced senescence in human fibroblasts and melanocytes. Correspondingly, in established murine BRAFV600E-driven nevi, acute shRNA-mediated depletion of PTEN prompted tumor progression. Furthermore, genetic analysis of laser-guided microdissected human contiguous nevus–melanoma specimens recurrently revealed identical mutations in BRAF or NRAS in adjacent benign and malignant melanocytes. The PI3K pathway was often activated through either decreased PTEN or increased AKT3 expression in melanomas relative to their adjacent nevi. Pharmacologic PI3K inhibition in melanoma cells suppressed proliferation and induced the senescence-associated tumor suppressor p15INK4B. This treatment also eliminated subpopulations resistant to targeted BRAFV600E inhibition. Our findings suggest that a significant proportion of melanomas arise from nevi. Furthermore, these results demonstrate that PI3K pathway activation serves as a rate-limiting event in this setting, acting at least in part by abrogating OIS. The reactivation of senescence features and elimination of cells refractory to BRAFV600E inhibition by PI3K inhibition warrants further investigation into the therapeutic potential of simultaneously targeting these pathways in melanoma.
Nevi (moles) are benign melanocytic lesions that usually remain permanently growth-arrested for several decades, in spite of the fact that they commonly harbor a mutant allele of BRAF (Pollock et al. 2003) or NRAS (Bauer et al. 2007). The most frequent BRAF mutation observed results in a valine-to-glutamic acid change at position 600 (BRAFV600E), which renders this protein kinase constitutively active (Davies et al. 2002). We showed previously that expression of BRAFV600E induces premature senescence in human melanocytes and human diploid fibroblasts (HDF) (Michaloglou et al. 2005). Correspondingly, nevi display several hallmarks of senescence: stable proliferation arrest, increased expression of the CDK4/6 inhibitor p16INK4A, and induction of senescence-associated β-galactosidase (SA-β-Gal) activity (Michaloglou et al. 2005). As this occurs in the absence of gross telomere shortening, we and others have proposed that oncogene-induced senescence (OIS) is an in vivo tumor-suppressive mechanism that prevents melanomagenesis (Bennett 2003; Mooi and Peeper 2006).
The stable proliferative arrest seen in nevi is of high biological and clinical relevance, as it represents a key difference from melanoma, which expands excessively (Mooi and Krausz 2007). It is widely believed that a substantial percentage of melanomas arise from melanocytic nevi (Mooi and Krausz 2007). Indeed, several groups have provided genetic evidence that supports a progression model (Demunter et al. 2001; Bogdan et al. 2003; Yazdi et al. 2003; Dadzie et al. 2009). From the model outlined above, we and others deduced previously that abrogation of OIS of nevus cells may act as a rate-limiting event for melanomagenesis (Bennett 2003; Mooi and Peeper 2006). Favoring this hypothesis, nevi and melanomas are commonly and significantly histologically associated (Stolz et al. 1989; Smolle et al. 1999; Bevona et al. 2003 and references therein). Furthermore, melanomas and nevi are found nonrandomly in close proximity (Smolle et al. 1999).
However, the molecular mechanism underlying malignant transformation from nevus to melanoma is not yet resolved. The primary molecular engine driving melanomagenesis is the activation of the ERK pathway, mainly due to oncogenic mutation of BRAF (Davies et al. 2002; Pollock et al. 2003) or NRAS (Raybaud et al. 1988; van't Veer et al. 1989). Other frequent genetic events include the loss of CDKN2A and ARF, amplification of CCND1 or CDK4 (Kamb et al. 1994; Nobori et al. 1994; Freeman et al. 2003; Curtin et al. 2005), and, as identified more recently, alterations in MMP8, GRM3, ERBB4, GRIN2A (Palavalli et al. 2009; Prickett et al. 2009, 2011; Wei et al. 2011), and MITF (Garraway et al. 2005; Yokoyama et al. 2011). Loss of p16INK4A has long been suspected to play a critical role in the abrogation of OIS. However, although its involvement in melanomagenesis is undisputed (Curtin et al. 2005; COSMIC database, http://www.sanger.ac.uk/genetics/CGP/cosmic), and a role in replicative senescence has been reported (Gray-Schopfer et al. 2006), the available evidence supports a redundant role for p16INK4A in senescence induced by mutant BRAF or NRAS in vitro (Michaloglou et al. 2005; Denoyelle et al. 2006; Haferkamp et al. 2009) and in vivo (Dhomen et al. 2009).
Another common genetic event in melanoma is the activation of the PI3K pathway (Dhawan et al. 2002; Stahl et al. 2004). Elevated AKT activity is observed in 17% of benign nevi, 43% of dysplastic nevi, 49% of primary melanomas, and 77% of metastatic melanomas, correlating increased PI3K pathway with melanoma progression (Dai et al. 2005). Mutations in PIK3CA are rare in melanoma (Omholt et al. 2006), however, which suggests that other alterations contribute to activation of the pathway. PTEN is functionally lost in the majority of melanomas by either mutation, loss of heterozygosity (LOH) and chromosomal loss, methylation-induced transcriptional silencing, or microRNA-dependent mechanisms (Guldberg et al. 1997; Birck et al. 2000; Zhou et al. 2000; Tsao et al. 2003; Mirmohammadsadegh et al. 2006). Interestingly, concurrent mutation in BRAF and diminished expression of PTEN are common in melanomas (Tsao et al. 2000, 2004; Daniotti et al. 2004; Lin et al. 2008). The PI3K pathway can be activated in melanoma also by increased AKT3 activity owing to overexpression or mutation (Stahl et al. 2004; Davies et al. 2008). Furthermore, AKT3 was shown to cooperate with BRAFV600E in the transformation of murine melanocytes in vitro through mechanisms that involve phosphorylation of BRAFV600E by AKT3 (Cheung et al. 2008).
Here, we put to the test the hypothesis that abrogation of BRAFV600E-induced senescence contributes to melanomagenesis and set out to identify the key players that are mechanistically involved.
Results
PTEN depletion abrogates BRAFV600E-induced senescence in cultured human fibroblasts
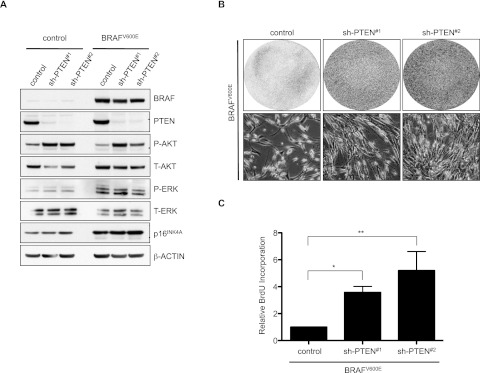
To investigate the possible role of PI3K pathway activation in evasion of BRAFV600E-induced senescence, we first focused on any effect of PTEN loss. As expected, PTEN depletion by two independent shRNAs from primary human fibroblasts resulted in activation of a critical downstream effector, AKT (as measured by P-AKT) (Fig. 1A). Consistent with our previous results (Michaloglou et al. 2005), ectopic expression of BRAFV600E in HDF induced a robust cell cycle arrest, which was accompanied by an increase in p16INK4A (Fig. 1A). Importantly, depletion of PTEN effectively abrogated BRAFV600E-induced senescence (Fig. 1B,C). We corroborated the participation of PI3K pathway activation in abrogation of BRAFV600E-induced senescence by ectopic expression of either wild-type or activated PIK3CA or activated AKT1 in independent HDF strains. This also effectively prevented BRAFV600E-induced senescence in fibroblasts (Supplemental Fig. 1A–F), indicating a causal role for this signaling route in the growth inhibitory action of BRAFV600E, consistent with previous results (Courtois-Cox et al. 2006). The increase in cell number seen in proliferation assays was not due to decreased cell death (which is not induced in HDF by BRAFV600E) (data not shown), but reflected decreased senescence and increased DNA replication as a function of PI3K activation, as evident from SA-β-Gal activity and bromodeoxyuridine (BrdU) incorporation assays (Fig. 1C; Supplemental Fig. 1B,C).
Figure 1.
PTEN depletion abrogates BRAFV600E-induced senescence in cultured human fibroblasts. TIG3 HDF stably expressing one of two nonoverlapping PTEN shRNAs (#1 or #2) as well as hTERT (to avoid confounding effects due to replicative senescence; hTERT does not interfere with BRAFV600E-induced senescence) were transduced with BRAFV600E-encoding retrovirus and selected for integration of the construct. Empty vectors were used as controls. (A) Samples were analyzed by Western blot for the indicated proteins 8 d post-infection. β-Actin served as a loading control. (B) Samples were seeded at equal densities and fixed and stained 8 d post-infection. (C) Samples were analyzed for BrdU incorporation 3 d post-infection. Data are represented as mean with standard deviation (SD) (paired ratio two-tailed t-test; [*] P = 0.0001; [**] P = 0.0006).
PTEN depletion abrogates BRAFV600E-induced senescence in cultured human melanocytes
Given the prominent role of BRAFV600E in nevi and melanomas and the frequent reduction of PTEN levels seen in melanomas (Tsao et al. 2000, 2004; Daniotti et al. 2004; Lin et al. 2008), we set out to determine the role of PTEN in the senescence program of primary human melanocytes in the context of oncogenic BRAF. These melanocytes were isolated from neonatal foreskins and characterized for melanocytic markers and the ability to undergo OIS (Michaloglou et al. 2005). Of note, similar to primary mammary epithelial cells (Brenner et al. 1998), primary melanocytes appear to be under selective pressure to lose p16INK4A during in vitro propagation (Bennett 2003). To avoid spontaneous mutations in p16INK4A or undefined genes, these experiments were performed with p16INK4A-depleted melanocytes (which still undergo BRAFV600E-induced senescence), but similar results were obtained with wild-type melanocytes (data not shown).
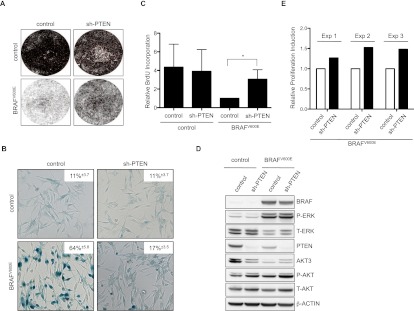
Consistent with previous results (Michaloglou et al. 2005; Denoyelle et al. 2006), BRAFV600E efficiently triggered senescence in melanocytes (Fig. 2A–C). shRNA-mediated PTEN depletion, however, prevented BRAFV600E cells from undergoing OIS. This was illustrated by increased cell proliferation, a significant rise in DNA replication, and decreased SA-β-Gal activity relative to BRAFV600E-expressing melanocytes (Fig. 2A–C).
Figure 2.
PTEN depletion abrogates BRAFV600E-induced senescence in cultured human melanocytes. (A) Primary human melanocytes stably expressing sh-p16INK4A and either control or sh-PTEN (#1) were transduced with control or BRAFV600E-encoding lentivirus and pharmacologically selected for successful integration of the construct. Empty vectors were used as controls. Samples were seeded at equal densities and fixed and stained 15 d post-infection. (B) Samples were fixed and analyzed for SA-β-Gal activity 15 d post-infection. Data are represented as percentage of cells ± SD (paired two-tailed t-test; P = 0.0001 for the bottom panel). (C) Samples were analyzed for BrdU incorporation 15 d post-infection. Data are represented as mean ± SD (paired ratio two-tailed t-test; [*] P = 0.0001). (D) Samples were analyzed by Western blot for protein expression as indicated. β-Actin served as a loading control. (E) Melanocytes stably expressing sh-p16INK4A were first transduced with BRAFV600E-encoding lentivirus, subsequently transduced with either control or sh-PTEN, and analyzed for BrdU incorporation 8 d post-infection. The relative ratios of three independent experiments are illustrated in the graph. Statistical analysis was performed by a paired ratio two-tailed t-test; P = 0.025.
As expected, BRAFV600E expression resulted in strong ERK activation, while PTEN depletion induced phosphorylation of AKT, which was further enhanced by BRAFV600E (Fig. 2D). Up-regulation of P-AKT was a common feature among BRAF mutant melanoma cell lines but did not always correlate to PTEN levels, consistent with the idea that PTEN-independent signaling also feeds into AKT (Supplemental Fig. 2A). Less expected was the observation that BRAFV600E strongly suppressed the accumulation of AKT3 in human melanocytes. This was at least partially restored in PTEN-depleted melanocytes evading OIS. Of note, we were unsuccessful in overexpressing PI3K or AKT family members (either wild-type or activated forms) in melanocytes, most likely because of toxicity. This is consistent with (but does not formally explain) the observation that mutational activation of PIK3CA is rare in melanoma but more frequent in several other human tumor types and suggests that reduction of PTEN dosage is not identical to an increase in PI3K signaling.
Although consistent with the hypothesis that abrogation of OIS contributes to melanomagenesis, the results above do not necessarily reflect the physiologic situation. Indeed, it is conceivable that PTEN deficiency in vivo occurs after the acquisition of the BRAFV600E mutation rather than prior to it. In an attempt to better mimic such a scenario, we first introduced BRAFV600E to trigger melanocyte senescence. Subsequently, melanocytes were transduced with lentivirus targeting PTEN. Upon its depletion, we again observed a consistent bypass of OIS (Fig. 2E; Supplemental Fig. 2B). Although this effect was moderate in vitro, such a senescence override is likely to be relevant in vivo. We conclude, therefore, that PTEN depletion leads to abrogation of BRAFV600E-induced senescence in human melanocytes.
Abrogation of BRAFV600E-induced senescence by PTEN depletion is dose-dependent
While some studies show that PTEN deficiency induces senescence in fibroblasts (Chen et al. 2005), it appears that its expression levels play an essential role: Slight differences in PTEN expression affect cell survival and proliferation (Alimonti et al. 2010). To address the importance of PTEN expression in melanocytes, we titrated lentiviruses expressing sh-PTEN in the presence or absence of BRAFV600E.
The bypass of BRAFV600E-induced senescence upon PTEN depletion correlated with PTEN expression levels, as judged by the ability to incorporate BrdU and SA-β-Gal activity (Fig. 3A–C). Importantly, determination of cell numbers revealed that there was an optimum: Too much PTEN depletion no longer resulted in a net increase in cell number, because of cytotoxic effects (Fig. 3D; data not shown). Too little PTEN depletion, on the other hand, failed to impair the senescence program. Thus, there was a window of PTEN expression that allowed BRAFV600E-expressing melanocytes to evade senescence. Furthermore, whereas BRAFV600E overexpression in melanocytes triggered up-regulation of the senescence-associated tumor suppressor p15INK4B, cells bypassing OIS down-regulated expression of p15INK4B. This correlated with both the dosage of PTEN and BrdU incorporation (Fig. 3A,B). We conclude, therefore, that there is an optimal window of PTEN expression allowing BRAFV600E-expressing melanocytes to abrogate OIS bypass: Too high levels do not allow for proliferation, whereas too low levels are cytotoxic.
Figure 3.
Abrogation of BRAFV600E-induced senescence by PTEN depletion is dose-dependent. Primary human melanocytes stably expressing sh-p16INK4A and either control or sh-PTEN (#1) were transduced with control or BRAFV600E-encoding lentivirus and selected for integration of the construct. Empty vectors were used as controls. (A) Samples were analyzed by Western blot for protein expression as indicated. β-Actin served as a loading control. (B) Samples were analyzed for BrdU incorporation 15 d post-infection. Data are represented as mean ± SD (one-way ANOVA/Bonferroni's multiple comparison test; P < 0.05). (C) Samples were fixed and analyzed for SA-β-Gal activity 15 d post-infection. Data are represented as percentage of cells ± SD (one-way ANOVA/Tukey's multiple comparison). For both B and C, BRAFV600E sh-PTEN1:10 and BRAFV600E sh-PTEN1:25 samples are significantly different from BRAFV600E controls (P < 0.05). (D) Samples were seeded at equal densities and fixed and stained 15 d post-infection. Crystal violet staining was extracted and quantified; similar results were obtained in duplicate experiments.
Acute in vivo PTEN depletion in BRAFV600E-expressing nevi drives tumor formation
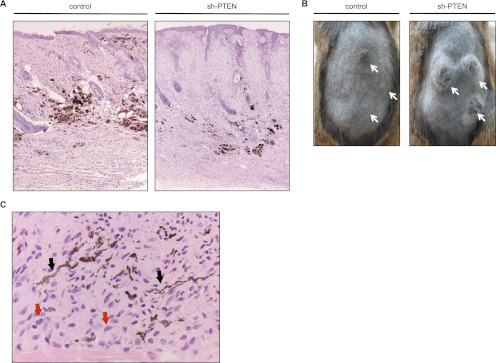
To validate the role of PTEN in the context of BRAFV600E in an in vivo setting, we made use of a knock-in BRAFV600E mouse model that allows for melanocyte-specific and inducible expression of BRAFV600E (Tyr::CreER; BRafCA) (Dankort et al. 2009). Upon 4-OHT (4-hydroxytamoxifen) treatment, these mice develop nevus-like lesions that are characterized by hyperplasia of melanocytes in the dermis and hair follicles with the presence of melanophages (Fig. 4A; Dankort et al. 2009). This represents a physiologically relevant setting to investigate whether, also in mice, PTEN depletion triggers proliferation of BRAFV600E-expressing melanocytes. To this end, we injected a lentivirus carrying sh-PTEN into these lesions. This commonly led to tumor formation at the sites of injection (Fig. 4B; Supplemental Fig. 3A). Focal pigmentation areas (dendritic melanocytes and melanophages) were observed and confirmed by H&E staining (Fig. 4A,C) and electron microscopy (data not shown). Histologically, these tumors consisted predominantly of oval to elongated cells with oval monochromatic nuclei and ill-defined pale cytoplasm. A minority of cells contained microgranular melanin pigment, which highlighted dendritic processes in some. Scattered groups of larger, polygonal, pigmented cells were seen as well. Necrosis was absent. The tumor cells infiltrated diffusely into the cutaneous and subcutaneous tissues, including striated muscle.
Figure 4.
Acute in vivo PTEN depletion in BRAFV600E-expressing nevi drives tumor formation. (A) H&E staining of mouse skin injected with lentivirus carrying either control or shRNA constructs. The sh-PTEN-injected lesion is more cellular and larger; both lesions show focal melanotic pigmentation indicative of the melanocytic origin. (B) Photographs of mice bearing tumors 18.5 wk after intradermal injection of sh-PTEN-carrying lentivirus. Adult mice received a sublethal total body irradiation (5 Gy) 3 d prior to lentivirus application to reduce an immune response eradicating virus-infected cells. shRNA targeting PTEN (sh-PTEN#3) or empty vector control were delivered by intradermal injection of lentivirus into the dorsal skin of 4-OHT-treated Tyr::CreER; BRafCA mice. Arrows indicate sites of injections. (C) High-power micrograph of melanocytic tumor of a sh-PTEN-injected mouse showing a population of plump to elongated cells (red arrows), some of which are laden with melanin pigment, highlighting the characteristic melanocytic dendrites (black arrows).
Of note, the lesions developed within the first month after injection of sh-PTEN virus, but subsequently slowed down without progressing further. Indeed, mitotic figures were noted at that stage only occasionally. Mice that had received control vector showed no lesions at all or very small lesions. Confirming BRAFV600E expression in these sh-PTEN-induced lesions, rearrangement of the BRafCA allele was detected by PCR (Supplemental Fig. 3B). Lesions of mice treated with sh-PTEN showed enrichment of the BRAFV600E-encoding allele compared with control-treated mice, suggesting that cells expressing BRAFV600E constitute the lesions. These results indicate that PTEN depletion can cause BRAFV600E-expressing nevus cells to resume proliferation, providing a time window for tumorigenic expansion in vivo.
Significant conservation of BRAF and NRAS mutations during progression from nevi to melanomas
We next wished to extend our findings obtained in vitro and in mice to humans. Specifically, we examined the possibility that also in human melanocytic lesions, reduction in PTEN levels, resulting in activation of the PI3K pathway, represents a rate-limiting step for the outgrowth of BRAFV600E-expressing melanocytes. Therefore, we investigated a series of 21 human tissue sections with nevi in direct contiguity with melanomas. Because a nevus-to-melanoma progression model predicts conservation of the driver mutation, we first examined whether the cells of the nevus and the contiguous melanoma in each specimen were clonally related. Genetic evidence has been presented previously in support of a progression model (NRAS, KRAS, and BRAF mutations [Demunter et al. 2001; Yazdi et al. 2003; Dadzie et al. 2009] and LOH of 9p21 markers [Bogdan et al. 2003]), but, importantly, no statistical analysis was used to rule out random co-occurrence of mutations within these lesions.
We first attempted to identify the “driver” mutations in the nevus and melanoma cell compartments. We focused on the likely suspects BRAF and NRAS, as they are most commonly activated in melanomas as well as nevi. Seventeen specimens were considered suitable for laser capture microdissection, after which the mutational status of BRAF exon 15 and NRAS exon 3 (in which most activating mutations reside) (Forbes et al. 2006; COSMIC database, http://www.sanger.ac.uk/genetics/CGP/cosmic) was analyzed by genomic DNA PCR amplification and sequencing. In addition, areas with normal tissue in these sections were microdissected in all cases and processed along with the nevi and melanomas as controls for positive identification of the wild-type NRAS and BRAF alleles and for excluding contamination problems or the occurrence of polymorphisms.
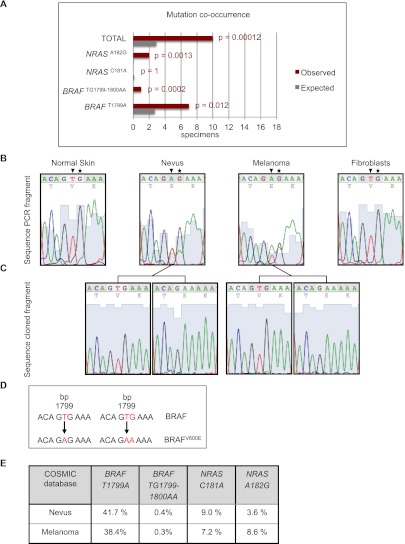
We found the common BRAFT1799A mutation (encoding BRAFV600E) in eight samples, seven of which showed co-occurrence of this mutation in both the nevi and their flanking melanomas (Fig. 5A, red bars; Supplemental Table 1). Interestingly, in one sample, we identified a rare BRAFTG1799–1800AA double mutation (also encoding BRAFV600E) in the melanoma as well as the associated nevus (Fig. 5B–D; Supplemental Fig. 4A–D for detailed information). In addition, activating NRAS mutations were detected in four samples, two of which showed co-occurrence in nevi and their contiguous melanomas. The presence of a BRAF or NRAS mutation in three melanomas but not in their contiguous nevi suggests that either the lesions within these specimens are clonally unrelated or the lesions harbor driver mutations other than those located in BRAF exon 15 and NRAS exon 3, with mutation in BRAF or NRAS occurring later, during melanoma progression. Finally, we identified four specimens with wild-type BRAF exon 15 and NRAS exon 3 sequences in all lesions. We cannot exclude, however, that some of these results are false negatives due to the very small lesion size in some cases.
Figure 5.
Significant conservation of BRAF and NRAS mutations during progression from nevi to melanomas. (A) Bar graph representing expected and observed frequencies of melanomas and their associated nevi harboring identical BRAF and NRAS mutations in nevi and contiguous melanomas. Seventeen specimens were suitable for laser capture microdissection, after which the mutational status of BRAF exon 15 and NRAS exon 3 (in which most activating mutations reside) was analyzed by genomic DNA PCR amplification and sequencing. Red bars (Observed) represent the mutational status observed for BRAF and NRAS in 17 nevi that are directly contiguous to melanomas. Gray bars (Expected) represent the calculated percentages of random (co-)occurrence. For statistical analysis, we carried out the “one-sample test” for proportions, with the null hypothesis being that the mutations occurred randomly. The “two-sided alternative test” was used to calculate P-values, which were <0.05 for the BRAFT1799A, BRAFTG1799–1800AA, and NRASA182G mutations, indicating that it is highly unlikely that the observed co-occurrence of these mutations (in red) has arisen due to chance. See Supplemental Table 1 for details. (B) A rare BRAF double mutation co-occurring in the nevus and the adjacent melanoma. Chromatograms of BRAF exon 15 PCR fragments amplified from genomic DNA of laser-microdissected normal skin, nevus, and melanoma and of a fibroblast cell line as a control. Arrowheads indicate nucleotide thymine 1799 in BRAF, which is often found mutated to an adenine, and asterisks indicate nucleotide guanine 1800, which is rarely found mutated to an adenine. (C) Chromatograms of cloned PCR fragments encompassing BRAF exon 15 show either the wild-type allele or the allele with the double mutation. (D) Schematic overview of the BRAFT1799A and BRAFTG1799–1800AA mutations, both encoding BRAFV600E. (E) To perform the statistical calculations, percentages of BRAFT1799A, BRAFTG1799–1800AA, NRASC181A, and NRASA182G mutations were extracted from the COSMIC database (http://www.sanger.ac.uk/genetics/CGP/cosmic) (Forbes et al. 2006). Melanomas and benign melanocytic nevi originating from skin were selected, excluding acral, mucosal, ocular, and genital origin; spitz; and blue nevi, as these types were not included in our specimen panel.
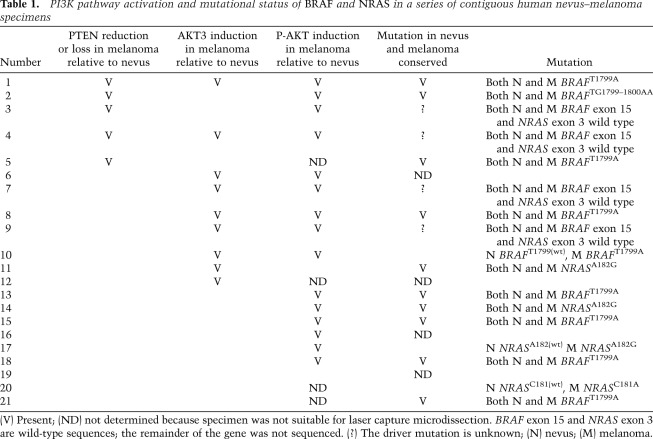
To evaluate the statistical significance of these findings, we collected from the COSMIC database (http://www.sanger.ac.uk/genetics/CGP/cosmic) the percentages of each mutation identified in the above-described specimens (Fig. 5E) and calculated for all cases the probability that identical mutations in nevi and melanomas could have occurred by chance (Supplemental Table 1). For each mutation identified in the 17 specimens, we calculated the expected frequencies of random co-occurrence in nevi and matched melanomas (i.e., Fig. 5A, gray bars labeled “Expected”) and compared these with the observed co-occurrence frequency obtained from actual mutational analysis (Fig. 5A, red bars labeled “Observed”). The predicted chance of random co-occurrence based on the percentages from the COSMIC database (http://www.sanger.ac.uk/genetics/CGP/cosmic) was then compared with the observed results of the mutational analysis. We found co-occurrence of BRAF and NRAS mutations in flanking nevi and melanomas that was statistically significant in 10 out of 17 specimens (P-value < 0.05), arguing that at least a considerable proportion of melanomas in this series are clonally related to their contiguous nevi.
Decrease in PTEN and increase in AKT3 and P-AKT expression in melanomas relative to their contiguous nevi
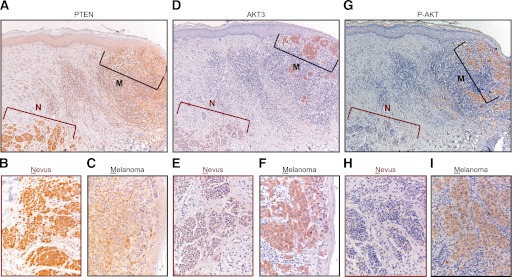
To investigate the role of PTEN reduction and PI3K pathway activation in human melanomagenesis in vivo, we analyzed the relative expression levels of PTEN by immunohistochemical staining of our series of contiguous nevus–melanoma specimens. As we had noted that BRAFV600E suppresses AKT3 accumulation in cultured melanocytes (Fig. 2D), we also included immunostaining for this factor as well as for P-AKT. Five out of 21 samples displayed reduced PTEN immunostaining in the melanoma compared with the associated nevus, including the sample with the rare BRAF double mutation (Fig. 6A–C; Table 1; Supplemental Fig. 5A–F). Conversely, an increase in AKT3 staining in the melanoma compared with the nevus was detected in nine specimens (Fig. 6D–F; Table 1; Supplemental Fig. 5G–I). In two specimens, both PTEN reduction and AKT3 increase were seen. Correspondingly, in the majority of the specimens analyzed, we detected induction of P-AKT in the melanoma relative to the nevus (Fig. 6G–I; Table 1). These analyses reveal recurrent activation of the PI3K pathway in melanomas relative to their contiguous nevi, with alterations in PTEN and AKT accounting for 12 out of 21 cases.
Figure 6.
Decrease in PTEN and increase in AKT3 and P-AKT expression in melanomas relative to their contiguous nevi. Representative immunohistochemical stainings of consecutive sections of a contiguous nevus–melanoma specimen with PTEN, AKT3, and P-AKT antibodies. Both nevus and melanoma compartments harbor the BRAFT1799A mutation, as determined by laser capture microdissection and sequence analysis. The melanoma compartment (M) exhibits clearly weaker PTEN (A–C) and stronger AKT3 (D–F) and P-AKT (G–I) immunostaining compared with the nevus compartment (N). B, E, and H show higher magnifications of the nevus, and C, F, and I, show higher magnifications of the melanoma. The cell-rich area underneath the melanoma compartment comprises mainly infiltrating lymphocytes.
Table 1.
PI3K pathway activation and mutational status of BRAF and NRAS in a series of contiguous human nevus–melanoma specimens
Pharmacologic PI3K inhibition induces cell cycle arrest in melanoma cells
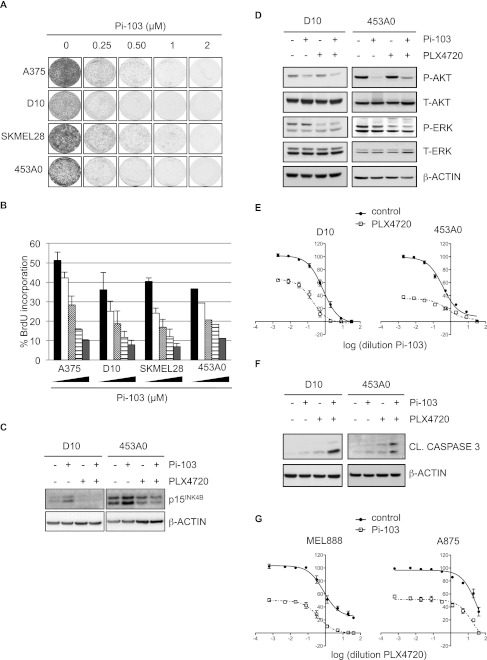
In view of the strong evidence linking PTEN reduction and PI3K pathway activation to melanoma, PI3K is receiving increasing interest as a possible therapeutic target. As our results indicate that activation of the PI3K pathway overrides BRAFV600E-induced senescence and represents a frequent event during melanomagenesis, we next investigated whether PI3K pathway inhibition could reactivate aspects of the senescence program in melanoma cells. We treated BRAFV600E melanoma cell lines with increasing concentrations of Pi-103, an inhibitor of PI3K and mTOR kinases. Cells treated with Pi-103 showed a profound decline in proliferative capacity, as measured by cell proliferation and BrdU incorporation assays (Fig. 7A,B), consistent with previous data (Marone et al. 2009). Correspondingly, the expression levels of p15INK4B increased upon Pi-103 treatment (Fig. 7C). Occasionally, but not consistently, we observed induction of SA-β-Gal activity (data not shown), suggesting that PI3K inhibition reactivates senescence incompletely, perhaps owing to the large number of genetic alterations typically seen in melanoma.
Figure 7.
PI3K inhibition induces cell cycle arrest in melanoma cells and enhances cytotoxicity of BRAFV600E inhibition. Melanoma cell lines were treated with Pi-103 and/or PLX4720. (A) Samples were kept in medium containing Pi-103 for 7 d and fixed and stained with crystal violet. (B) Samples were analyzed for BrdU incorporation 4 d after treatment with increasing concentrations of Pi-103 (0.25, 0.5, 1, and 2 μM). Error bars represent SEM (standard error of the mean) of three independent experiments. For 453A0 melanoma cells, a representative experiment is shown. (C) Samples were analyzed by Western blotting for p15INK4B 2 d after treatment with 0.25 μM Pi103 and/or 1 μM PLX4720. β-Actin served as a loading control. (D) Melanoma cell lines were treated with 0.5 μM Pi-103 and/or 2 μM PLX4720 for 4 h. Samples were analyzed by Western blotting for the indicated proteins. β-Actin served as a loading control. (E) Melanoma cell lines were treated with a dilution series of Pi-103 either alone or in combination with the BRAFV600E inhibitor PLX4720 at a concentration of 3 μM (D10) or 1 μM (453A0) for 3 d. Total cell numbers were determined with a cell titer blue assay. The Y-axis represents the percentage of living cells. (F) Cells were treated with 0.25 μM Pi-103 and/or 1 μM PLX4720 for 1 d (D10) or 3 d (453A0). Samples were analyzed by Western blotting for cleaved caspase 3. Apoptotic cells in the supernatant were included in the analysis. β-Actin served as a loading control. (G) Cells were treated with a dilution series of PLX4720 either alone or in combination with 0.5 μM Pi-103. Total cell numbers were determined with a cell titer blue assay. The Y-axis represents the percentage of living cells.
Pharmacologic PI3K inhibition enhances cytotoxicity of BRAFV600E inactivation
An exciting recent clinical development is the availability of mutant BRAF-specific inhibitors. Although, initially, dramatic responses are seen in melanoma patients, eventually, most of them show tumor progression. Intense investigations have revealed that tumor relapse occurs owing to a variety of resistance mechanisms, some of them implicating activation of the PI3K pathway as a key player in resistance to BRAF or MEK inhibition (Shao and Aplin 2010; Atefi et al. 2011; Paraiso et al. 2011). Therefore, there is a dire need to find combination therapy to delay or avoid resistance. In view of the results obtained for pharmacologic PI3K inhibition, we explored the possible therapeutic benefit of combining PI3K and BRAFV600E inhibition in melanoma cells.
As expected, treatment with Pi-103 and PLX4720 (a BRAFV600E inhibitor) inhibited the activity of AKT and ERK, respectively (Fig. 7D). More importantly, Pi-103 treatment profoundly cooperated with BRAFV600E inhibition in dose response curves (Fig. 7E; Supplemental Fig. 6A). Interestingly, whereas melanoma cells mainly arrested upon Pi-103 treatment, combination with PLX4720 induced cell death, as demonstrated by the cooperative induction of cleaved caspase 3 (Fig. 7F; Supplemental Fig. 6B). This was accompanied by the neutralization of p15INK4B induction by Pi-103, suggesting cross-talk between these two pathways (Fig. 7C).
We noted that the response to PLX4720 treatment varied among melanoma cells, and in some cases, a population of cells survived even high doses of this drug (Fig. 7G). We therefore treated also these resistant cell lines with increasing concentrations of PLX4720 and analyzed the possible additive effect of Pi-103. Interestingly, combined inhibition of the PI3K and BRAFV600E kinases efficiently eliminated the population of resistant melanoma cells (Fig. 7G; Supplemental Fig. 6C). We conclude from these results that while BRAF and PI3K pathway activation collaborate in melanomagenesis, pharmacologic PI3K inhibition reactivates senescence features and enhances the cytotoxicity of targeted mutant BRAF inhibition.
Discussion
To understand the molecular basis of melanomagenesis, it is imperative to identify the genetic changes that facilitate the different steps in progression from normal melanocytes to nevus and melanoma. A large proportion of melanomas harbor alterations in both the BRAF and PTEN genes, leading to their constitutive activation and inactivation, respectively. As we and others have previously postulated that abrogation of OIS in human nevi contributes to melanomagenesis, we here put to the test the hypothesis that reduction of PTEN plays a rate-limiting role in the progression from nevus to melanoma.
We employed four independent settings for this investigation: cultured human fibroblasts, cultured human melanocytes, a murine nevus model, and human contiguous nevus–melanoma specimens. First, we determined whether down-regulation of PTEN affects the senescence program that is elicited by BRAFV600E in vitro. In both primary human fibroblasts and melanocytes, PTEN silencing abolished BRAFV600E-induced senescence. These results are in agreement with and extend models previously proposed by us and others. For instance, senescent neurofibromas initiate a negative feedback loop in response to oncogenic stress, which inhibits AKT activation and results in the induction of senescence (Courtois-Cox et al. 2006). Furthermore, activation of the PI3K pathway by expression of myristoylated AKT1 attenuates RAS-induced senescence (Kennedy et al. 2011). These results suggest that this mechanism is conserved across cell types and shared among different OIS triggers. Hence, the abolishment of OIS by activation of the PI3K pathway may play a role also in tumor types other than melanoma.
In human tissue sections, it has been shown that normal melanocytes and most nevi exhibit PTEN expression, which is down-regulated in approximately a third of primary melanomas and becoming even more pronounced in melanoma metastases (Zhou et al. 2000; Whiteman et al. 2002; Tsao et al. 2003). This has led to the idea that PTEN reduction may be involved in melanoma progression rather than initiation (Whiteman et al. 2002). We analyzed PTEN expression in human contiguous nevus–melanoma specimens to investigate its role in melanoma initiation. By determining the mutational status of BRAF and NRAS in the benign and malignant melanocytic lesions within these specimens, we first investigated whether the nevi that are in direct contiguity with melanomas were clonally related. Indeed, we found co-occurrence of several different mutations in nevi and their associated melanomas. This includes the BRAFT1799A mutation that is commonly found in both nevi and melanomas but was found co-occurring in our data set in a significantly higher number of cases than expected by chance. Furthermore, we found rare mutations (NRASC181A and BRAFTG1799–1800AA) co-occurring in adjacent nevi and melanomas that are highly unlikely to have occurred independently. Although this is not formal proof, these results strongly suggest that at least a proportion of melanomas emerge from nevi.
More than half of the lesions we analyzed displayed activation of the PI3K pathway, as judged by loss of PTEN or gain of AKT3 immunopositivity in the melanoma compared with the nevus compartment. Of note, PTEN levels were reduced but appeared to be not completely shut down. This is consistent with our in vitro PTEN RNAi results and suggests a window for PTEN reduction that allows for melanoma development, in agreement with observations on PTEN dosage in cellular senescence and tumor development by others (Chen et al. 2005; Alimonti et al. 2010). As expected, in most of these cases, this was accompanied by increased P-AKT staining in the melanoma compartment. The observation that identical BRAF and NRAS mutations are present in both the nevi and flanking melanomas (at least in 10 out of 17 cases), with concomitant PTEN reduction or AKT activation in the melanoma, is compatible with a model in which BRAF/NRAS mutations precede the PTEN/AKT alterations. This is further supported by our in vitro observation that senescent human melanocytes expressing BRAFV600E are able to re-enter the cell cycle upon PTEN depletion. Together, our results suggest that not only in vitro, but also in lesions in vivo, activation of the PI3K pathway contributes to the abrogation of the OIS program of human melanocytes, thereby promoting the progression of nevi to melanomas. Clearly, although diminished PTEN expression or increased AKT3 production in melanomas was seen in roughly half of the cases, other pathways are likely to contribute to melanoma progression as well. We propose that alterations at several levels in the PI3K pathway represent a common feature of progression from nevus to melanoma.
We also observed that AKT3 expression levels were down-regulated upon BRAFV600E expression in human melanocytes. This is consistent with the previous suggestion that AKT3 is the critical AKT isoform in melanoma cell lines (Stahl et al. 2004) and that overexpression of activated AKT3 enhances anchorage-independent growth of melanoma cells (Cheung et al. 2008). Conversely, specific inhibition of AKT3 has been reported to induce apoptosis and inhibit the tumorigenic capacity of melanoma cells in a xenograft model (Stahl et al. 2004). Although they do not rule out contributions by the other AKT family members, our in vitro and in vivo data are consistent with these observations and support a prominent role of AKT3 in melanomagenesis.
Our finding that PTEN depletion and consequent activation of the PI3K pathway abrogates BRAFV600E-induced senescence is in agreement with and extends previous data on two BRAFV600E mouse models. Dhomen et al. (2009) showed that the induction of BRAFV600E in melanocytes results in senescent melanocytes displaying elevated SA-β-Gal activity, low Ki67 expression, and formation of (blue) nevi. After a year, half of these mice developed melanoma, presumably by acquiring additional (yet to be defined) mutations. When the BRAFV600E nevus-carrying mice were bred onto a p16INK4A-null background, they produced more tumors, which developed earlier, but nonetheless, these mice continued to develop nevi (Dhomen et al. 2009). This is consistent with the mosaic pattern of p16INK4A immunopositivity frequently seen in human nevi (Michaloglou et al. 2005) and the observation that p16INK4A-deficient individuals have nevi in addition to an increased propensity to develop melanoma (Pavel et al. 2003), arguing that also p16INK4A-independent mechanisms contribute to melanomagenesis. Goel et al. (2009) observed similar features in melanocytes residing in the skin of BRAFV600E transgenic mice, including increased levels of p16INK4A, p53, and PTEN and elevated SA-β-Gal activity. Consistent with our model, transgenic BRAFV600E mice that develop melanoma strongly up-regulate P-AKT levels (Goel et al. 2009).
Also, Dankort et al. (2009) found murine benign melanocytic hyperplasia driven by an endogenous BRAFV600E-encoding allele. These lesions were highly stable and failed to progress to melanoma for at least up to 20 mo. However, upon simultaneous activation of BRAFV600E and genetic inactivation of PTEN, mice quickly developed metastatic melanoma (Dankort et al. 2009). In our in vivo nevus injection model, we also observed cooperation between BRAFV600E expression and PTEN depletion in tumor formation, in accordance with Dankort et al. (2009). We used the same conditional BRAFV600E mouse model, but instead of concomitant induction of mutant BRAF expression and PTEN depletion, we first allowed the mouse to develop nevus-like lesions, which were later treated with sh-PTEN-carrying lentivirus. This strategy allowed us to analyze the order of events and demonstrate that tumors can emerge from BRAFV600E-arrested lesions in vivo in a PTEN-dependent manner. We cannot rule out that PTEN depletion in stromal cells contributed to this effect. In aggregate, these results support a model in which mutant BRAF and subsequent activation of the PI3K pathway cooperate in melanomagenesis in cultured melanocytes, in murine nevus cells, and in human lesions, with PTEN reduction and PI3K/AKT activation serving, at least in part, to abrogate OIS.
In recent years, several groups have detected senescence biomarkers in several preneoplastic lesions in mice and humans (Kuilman et al. 2010). Commonly, an initial “driver” mutation—either the activation of an oncogene or the loss of a tumor suppressor allele—triggers the establishment of a benign lesion. After this initial phase of cell proliferation, a senescence program is executed, causing the termination of tumor expansion. Only upon the emergence of additional tumorigenic alterations can malignant progression occur. OIS is now widely considered to represent a bona fide tumor suppressor mechanism, acting alongside cell death programs. We believe, therefore, that the field is now entering a second phase, in which we need to delineate the fundamental mechanistic aspects of OIS. As we show here, the nevus–melanoma model is ideally suited to unmask at least some of the key players involved. Similarly, BRAFV600E-driven nevus models in zebrafish and mice allow for the identification of melanoma progression genes (Ceol et al. 2011; Karreth et al. 2011). The factors and pathways identified in this way will likely be relevant for other tumor settings as well, as OIS is seen in many tissue types (Collado and Serrano 2010). One question that is particularly relevant in this context is the cell of origin within the nevus—or similar benign lesions, for that matter—that gives rise to the tumor. Is it a fully senescent cell that somehow acquired a secondary mutation, a cell that has stalled halfway through the senescence program, or perhaps a rare senescent (nevus) cell with special (stem cell) properties? This and related questions can be addressed in advanced animal models engineered for lineage tracing.
The critical role for the PI3K pathway in melanoma development may be explored for the development of more effective therapeutic approaches. Our results predict that inhibitors of the PI3K pathway may restrain the expansion of tumors with mutations in BRAF at least in part through restoration of OIS features, a concept that is receiving increasing interest (Nardella et al. 2011). Furthermore, in agreement with previous data (Shao and Aplin 2010; Atefi et al. 2011; Paraiso et al. 2011), we show that inhibition of PI3K can enhance the cytotoxicity of targeted BRAFV600E inhibition. In particular, the observation that a PI3K inhibitor can eliminate melanoma cells that resist killing by a BRAFV600E inhibitor is of obvious interest and merits further investigation.
Finally, the elucidation of the pathobiology of nevi will increase our understanding of the role of OIS in melanomagenesis and may identify biomarkers that allow a better distinction from melanoma in problem cases. There is a need to identify senescence biomarkers in benign lesions such as nevi; in particular, factors that do not merely accompany OIS, but contribute to the execution of the program. Most markers currently used, such as SA-β-Gal, are associated with the process but likely do not contribute to rate-limiting steps. Identification of new biomarkers is important not only to deepen our understanding of the role of OIS (abrogation) in tumorigenesis, but also for translation of fundamental findings to the clinic; for example, to create new diagnostic opportunities. Indeed, some nevi are difficult or impossible to distinguish with certainty from melanomas and vice versa. We show here that there is a common activation of the PI3K pathway in situ comparing nevi with their contiguous melanomas. These results merit further investigation into the possibility of using biomarkers like PTEN reduction and increases in AKT3 and P-AKT to allow a better distinction between nevi and melanomas in difficult cases.
Materials and methods
Cell culture and viral transduction
The HDF cell lines TIG3 and HCA2 (coexpressing the ecotropic receptor and hTERT) as well as Phoenix and HEK293T cells were maintained in DMEM (Gibco) supplemented with 9% fetal bovine serum (Greiner Bio-One), 2 mM glutamine, 100 U mL−1 penicillin, and 0.1 mg mL−1 streptomycin (all Gibco). The HDF cell line IMR90 (expressing the ecotropic receptor, hTERT, and shRNA for p16INK4A) was maintained in MEM + Earle's salts (Gibco) containing all supplements mentioned above and nonessential amino acids, 0.15% sodium bicarbonate, and 1 mM sodium pyruvate (all Gibco). Human melanocytes were isolated from the epidermis of neonatal foreskins (Michaloglou et al. 2005) and maintained in Medium 254 (Cascade Biologicals) supplemented with melanocyte growth supplement (HMGS, Cascade Biologicals), 100 U mL−1 penicillin, and 0.1 mg mL−1 streptomycin (Gibco). Melanoma cell lines (A375, D10, SKMEL28, 453A0, MEL888, and A875) were maintained in DMEM supplemented with 9% fetal bovine serum, 2 mM glutamine, 100 U mL−1 penicillin, and 0.1 mg mL−1 streptomycin.
Retroviral transductions were performed using Phoenix cells as producers. Retrovirus production employing Phoenix cells was performed as described (http://www.stanford.edu/group/nolan/retroviral_systems/phx.html). For production of lentivirus, HEK293T cells were refreshed with complete medium containing 25 mM chloroquine, transfected with 8 μg of lentiviral construct and 3 μg of each of the helper plasmids pMDLglpRRE, pHCMV-G, and pRSVrev and washed and refreshed in complete medium after 6 h. Lentivirus was frozen and diluted for transduction. For TIG3, HCA2, and IMR90 cell proliferation assays, cells were transduced with shRNA- or cDNA-encoding retrovirus and checked for successful proviral integration (by puromycin selection or GFP expression). Cells were plated at equal densities, subsequently transduced with BRAFV600E-encoding retrovirus, and selected (Blasticidin). DNA replication was measured by incubating cells for 3 h with BrdU 3 d post-infection. Cells were also seeded at equal numbers and fixed and stained with crystal violet, which was extracted by incubation with 10% acetic acid, and relative absorbance was measured with a TECAN infinite M200 scanner 8 d post-infection. Samples were harvested for Western blot analysis 8 d post-infection. Activity of SA-β-Gal was also measured 8 d post-infection (SA-β-Gal staining kit, Cell Signaling). Melanocytes were transduced with shRNA- or cDNA-encoding lentivirus, cultured for 1 wk with Medium 254 supplemented with HMGS-2 (−PMA, Cascade Biologicals), and subsequently transduced with BRAFV600E-encoding lentivirus and subjected to Blasticidin selection. Cells were seeded at equal numbers, analyzed for BrdU incorporation, fixed and stained with crystal violet, analyzed for SA-β-Gal activity, and harvested for Western blot analysis 15 d post-infection. Alternatively, cells were first transduced with BRAFV600E-encoding lentivirus, selected for 1 wk with Blasticidin, and subsequently transduced with sh-PTEN-encoding lentivirus. Cells were analyzed 8 d post-infection for BrdU incorporation and Western blot.
Plasmids
pRSPURO-sh-PTEN(#1 and #2), pMXGFP-PIK3CA(wt, K545, R1047, CAAX), and pMSCVBLAST-BRAFV600E were used for retroviral transduction. KH1GFP-sh-p16INK4A, KH1GFP-sh-PTEN(#1 and #3), and HIV-CSCGBLAST-BRAFV600E were used for lentiviral transductions. Corresponding empty vectors were used as controls. Sequences for sh-PTEN are described in the Supplemental Material.
Antibodies
Antibodies used for Western blot were against BRAF (sc-5284, Santa Cruz Biotechnology), PTEN (sc-7974, Santa Cruz Biotechnology), AKT3 (#4059, Cell Signaling), phospho-Ser 473-AKT (#9271, Cell Signaling), AKT1/2/3 (sc-8312, Santa Cruz Biotechnology), p16INK4A (JC8, Immunologic), p15INK4B (sc-612, Santa Cruz Biotechnology), p42/p44 (#9102, Cell Signaling), phospho-p42/p44 (#9106, Cell Signaling), cleaved caspase 3 (Asp175, Cell Signaling), and β-actin (AC-74 and A5316, Sigma).
In vivo administration of lentivirus
The dorsal skin of Tyr::CreER; BRafCA mice (Dankort et al. 2009) was treated with 4-OHT (20 mg mL−1 in EtOH) each second day during the first week after birth. Adult mice received a sublethal total body irradiation (5 Gy) 3 d prior to lentivirus application. shRNA targeting PTEN (sh-PTEN#1 and sh-PTEN#3) was delivered by intradermal injection of lentivirus into the dorsal skin of 4-OHT-treated Tyr::CreER; BRafCA mice. The animal experiments were performed following local and international regulations and ethical guidelines and have been authorized by the local experimental animal committee at The Netherlands Cancer Institute. PCR detection of BRAF allele rearrangements was performed as described (Dankort et al. 2007).
Immunohistochemistry
Twenty-one formalin-fixed paraffin-embedded human specimens harboring nevi in direct contiguity with melanomas were immunostained for PTEN (clone 6H2.1, DAKO), AKT3 (HPA026441, Sigma-Aldrich), and P-AKT (Ser 473) (736E11, Cell Signaling), as previously described (Michaloglou et al. 2005).
Laser capture microdissection and mutation analysis
Contiguous nevus–melanoma specimens were sectioned (8 μM) on P.A.L.M. pen membrane slides (Microlaser Technologies), which were pretreated according to the manufacturer's instructions with UV and poly-L-lysine, then deparaffinized and stained with hematoxyline. Laser capture microdissection was used to isolate normal tissue, nevus cell groups, and melanoma cell groups, which were identified by pathologist W.J. Mooi, based on consecutive H&E sections. Genomic DNA was isolated with QIAamp DNA microkit (Qiagen). PCR conditions (TaqPlus Precision PCR system, Stratagene) and primers for PCRs for BRAF exon 15 and NRAS exon 3 are described in the Supplemental Material. PCR products were sequenced using the BigDye Terminator cycle sequencing kit (Applied Biosystems) and an ABI 3730 automated capillary sequencer.
Drug treatments
Melanoma cell lines were treated with indicated concentrations of Pi-103 (Echelon Biosciences) and/or PLX4720 (Selleck). For dose response curves, samples were measured after 3 d of treatment with a cell titer blue assay (Promega), and fluorescence was measured with a TECAN infinite M200 scanner. For proliferation assays, cells were either analyzed for BrdU incorporation 4 d after treatment or seeded at equal densities and fixed and stained with crystal violet 7 d after treatment. Samples were harvested for Western blot analysis on the indicated days.
Acknowledgments
We thank N. Armstrong and M.L. Yurda for statistical analysis; Ji-Ying Song for scientific support; R. Kortlever, M. Voorhoeve, and K. Berns for kindly providing constructs; T. Kuilman for critical reading of the manuscript; and all members of the Peeper laboratory for their valuable input. This work was supported by a Melanoma Research Alliance grant to D.D. and M.M., grants from the Dutch Cancer Society (KWF Kankerbestrijding, including a Queen Wilhelmina Award program), and Vidi and Vici grants from the Netherlands Organization for Scientific Research (NWO) to L.C.W.V., P.A.P., C.M., M.A.S., and D.S.P.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.187252.112.
References
- Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, et al. 2010. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 120: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, Nazarian R, Chmielowski B, Glaspy JA, Comin-Anduix B, et al. 2011. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS ONE 6: e28973 doi: 10.1371/journal.pone.0028973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Curtin JA, Pinkel D, Bastian BC 2007. Congenital melanocytic nevi frequently harbor NRAS mutations but no BRAF mutations. J Invest Dermatol 127: 179–182 [DOI] [PubMed] [Google Scholar]
- Bennett DC 2003. Human melanocyte senescence and melanoma susceptibility genes. Oncogene 22: 3063–3069 [DOI] [PubMed] [Google Scholar]
- Bevona C, Goggins W, Quinn T, Fullerton J, Tsao H 2003. Cutaneous melanomas associated with nevi. Arch Dermatol 139: 1620–1624 [DOI] [PubMed] [Google Scholar]
- Birck A, Ahrenkiel V, Zeuthen J, Hou-Jensen K, Guldberg P 2000. Mutation and allelic loss of the PTEN/MMAC1 gene in primary and metastatic melanoma biopsies. J Invest Dermatol 114: 277–280 [DOI] [PubMed] [Google Scholar]
- Bogdan I, Smolle J, Kerl H, Burg G, Boni R 2003. Melanoma ex naevo: A study of the associated naevus. Melanoma Res 13: 213–217 [DOI] [PubMed] [Google Scholar]
- Brenner AJ, Stampfer MR, Aldaz CM 1998. Increased p16 expression with first senescence arrest in human mammary epithelial cells and extended growth capacity with p16 inactivation. Oncogene 17: 199–205 [DOI] [PubMed] [Google Scholar]
- Ceol CJ, Houvras Y, Jane-Valbuena J, Bilodeau S, Orlando DA, Battisti V, Fritsch L, Lin WM, Hollmann TJ, Ferre F, et al. 2011. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature 471: 513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. 2005. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M, Sharma A, Madhunapantula SV, Robertson GP 2008. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res 68: 3429–3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Serrano M 2010. Senescence in tumours: Evidence from mice and humans. Nat Rev Cancer 10: 51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Genther Williams SM, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K 2006. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell 10: 459–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, et al. 2005. Distinct sets of genetic alterations in melanoma. N Engl J Med 353: 2135–2147 [DOI] [PubMed] [Google Scholar]
- Dadzie OE, Yang S, Emley A, Keady M, Bhawan J, Mahalingam M 2009. RAS and RAF mutations in banal melanocytic aggregates contiguous with primary cutaneous melanoma: Clues to melanomagenesis. Br J Dermatol 160: 368–375 [DOI] [PubMed] [Google Scholar]
- Dai DL, Martinka M, Li G 2005. Prognostic significance of activated Akt expression in melanoma: A clinicopathologic study of 292 cases. J Clin Oncol 23: 1473–1482 [DOI] [PubMed] [Google Scholar]
- Daniotti M, Oggionni M, Ranzani T, Vallacchi V, Campi V, Di Stasi D, Torre GD, Perrone F, Luoni C, Suardi S, et al. 2004. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene 23: 5968–5977 [DOI] [PubMed] [Google Scholar]
- Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M 2007. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev 21: 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr, You MJ, Depinho RA, McMahon M, Bosenberg M 2009. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41: 544–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. 2002. Mutations of the BRAF gene in human cancer. Nature 417: 949–954 [DOI] [PubMed] [Google Scholar]
- Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, Lazar AJ, Gershenwald JE, Mills GB 2008. A novel AKT3 mutation in melanoma tumours and cell lines. Br J Cancer 99: 1265–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demunter A, Stas M, Degreef H, De Wolf-Peeters C, van den Oord JJ 2001. Analysis of N- and K-ras mutations in the distinctive tumor progression phases of melanoma. J Invest Dermatol 117: 1483–1489 [DOI] [PubMed] [Google Scholar]
- Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, Pointer JN, Gruber SB, Su LD, Nikiforov MA, et al. 2006. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol 8: 1053–1063 [DOI] [PubMed] [Google Scholar]
- Dhawan P, Singh AB, Ellis DL, Richmond A 2002. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-κB and tumor progression. Cancer Res 62: 7335–7342 [PubMed] [Google Scholar]
- Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V, Larue L, Pritchard C, Marais R 2009. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15: 294–303 [DOI] [PubMed] [Google Scholar]
- Forbes S, Clements J, Dawson E, Bamford S, Webb T, Dogan A, Flanagan A, Teague J, Wooster R, Futreal PA, et al. 2006. Cosmic 2005. Br J Cancer 94: 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, et al. 2003. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3: 117–130 [DOI] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA, Getz G, Berger AJ, Ramaswamy S, Beroukhim R, Milner DA, Granter SR, Du J, et al. 2005. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436: 117–122 [DOI] [PubMed] [Google Scholar]
- Goel VK, Ibrahim N, Jiang G, Singhal M, Fee S, Flotte T, Westmoreland S, Haluska FS, Hinds PW, Haluska FG 2009. Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene 28: 2289–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, Marais R, Wynford-Thomas D, Bennett DC 2006. Cellular senescence in naevi and immortalisation in melanoma: A role for p16? Br J Cancer 95: 496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J 1997. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res 57: 3660–3663 [PubMed] [Google Scholar]
- Haferkamp S, Scurr LL, Becker TM, Frausto M, Kefford RF, Rizos H 2009. Oncogene-induced senescence does not require the p16(INK4a) or p14ARF melanoma tumor suppressors. J Invest Dermatol 129: 1983–1991 [DOI] [PubMed] [Google Scholar]
- Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE, Skolnick MH 1994. A cell cycle regulator potentially involved in genesis of many tumor types. Science 264: 436–440 [DOI] [PubMed] [Google Scholar]
- Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al. 2011. In vivo identification of tumor-suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 147: 382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, McBryan T, Doyle B, McKay C, Oien KA, et al. 2011. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol Cell 42: 36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS 2010. The essence of senescence. Genes Dev 24: 2463–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WM, Baker AC, Beroukhim R, Winckler W, Feng W, Marmion JM, Laine E, Greulich H, Tseng H, Gates C, et al. 2008. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res 68: 664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone R, Erhart D, Mertz AC, Bohnacker T, Schnell C, Cmiljanovic V, Stauffer F, Garcia-Echeverria C, Giese B, Maira SM, et al. 2009. Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res 7: 601–613 [DOI] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS 2005. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436: 720–724 [DOI] [PubMed] [Google Scholar]
- Mirmohammadsadegh A, Marini A, Nambiar S, Hassan M, Tannapfel A, Ruzicka T, Hengge UR 2006. Epigenetic silencing of the PTEN gene in melanoma. Cancer Res 66: 6546–6552 [DOI] [PubMed] [Google Scholar]
- Mooi WJ, Krausz T 2007. Pathology of melanocytic disorders. Hodder Arnold, London [Google Scholar]
- Mooi WJ, Peeper DS 2006. Oncogene-induced cell senescence-halting on the road to cancer. N Engl J Med 355: 1037–1046 [DOI] [PubMed] [Google Scholar]
- Nardella C, Clohessy JG, Alimonti A, Pandolfi PP 2011. Pro-senescence therapy for cancer treatment. Nat Rev Cancer 11: 503–511 [DOI] [PubMed] [Google Scholar]
- Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA 1994. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 368: 753–756 [DOI] [PubMed] [Google Scholar]
- Omholt K, Krockel D, Ringborg U, Hansson J 2006. Mutations of PIK3CA are rare in cutaneous melanoma. Melanoma Res 16: 197–200 [DOI] [PubMed] [Google Scholar]
- Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, Davis S, Wang C, Cronin JC, Agrawal NS, et al. 2009. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet 41: 518–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, et al. 2011. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 71: 2750–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel S, Smit NP, van der Meulen H, Kolb RM, de Groot AJ, van der Velden PA, Gruis NA, Bergman W 2003. Homozygous germline mutation of CDKN2A/p16 and glucose-6-phosphate dehydrogenase deficiency in a multiple melanoma case. Melanoma Res 13: 171–178 [DOI] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, et al. 2003. High frequency of BRAF mutations in nevi. Nat Genet 33: 19–20 [DOI] [PubMed] [Google Scholar]
- Prickett TD, Agrawal NS, Wei X, Yates KE, Lin JC, Wunderlich JR, Cronin JC, Cruz P, Rosenberg SA, Samuels Y 2009. Analysis of the tyrosine kinome in melanoma reveals recurrent mutations in ERBB4. Nat Genet 41: 1127–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett TD, Wei X, Cardenas-Navia I, Teer JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A, et al. 2011. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet 43: 1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybaud F, Noguchi T, Marics I, Adelaide J, Planche J, Batoz M, Aubert C, de Lapeyriere O, Birnbaum D 1988. Detection of a low frequency of activated ras genes in human melanomas using a tumorigenicity assay. Cancer Res 48: 950–953 [PubMed] [Google Scholar]
- Shao Y, Aplin AE 2010. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res 70: 6670–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolle J, Kaddu S, Kerl H 1999. Non-random spatial association of melanoma and naevi—a morphometric analysis. Melanoma Res 9: 407–412 [DOI] [PubMed] [Google Scholar]
- Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, Kester M, Sandirasegarane L, Robertson GP 2004. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res 64: 7002–7010 [DOI] [PubMed] [Google Scholar]
- Stolz W, Schmoeckel C, Landthaler M, Braun-Falco O 1989. Association of early malignant melanoma with nevocytic nevi. Cancer 63: 550–555 [DOI] [PubMed] [Google Scholar]
- Tsao H, Zhang X, Fowlkes K, Haluska FG 2000. Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res 60: 1800–1804 [PubMed] [Google Scholar]
- Tsao H, Mihm MC Jr, Sheehan C 2003. PTEN expression in normal skin, acquired melanocytic nevi, and cutaneous melanoma. J Am Acad Dermatol 49: 865–872 [DOI] [PubMed] [Google Scholar]
- Tsao H, Goel V, Wu H, Yang G, Haluska FG 2004. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol 122: 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Veer LJ, Burgering BM, Versteeg R, Boot AJ, Ruiter DJ, Osanto S, Schrier PI, Bos JL 1989. N-ras mutations in human cutaneous melanoma from sun-exposed body sites. Mol Cell Biol 9: 3114–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Walia V, Lin JC, Teer JK, Prickett TD, Gartner J, Davis S, Stemke-Hale K, Davies MA, Gershenwald JE, et al. 2011. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat Genet 43: 442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman DC, Zhou XP, Cummings MC, Pavey S, Hayward NK, Eng C 2002. Nuclear PTEN expression and clinicopathologic features in a population-based series of primary cutaneous melanoma. Int J Cancer 99: 63–67 [DOI] [PubMed] [Google Scholar]
- Yazdi AS, Palmedo G, Flaig MJ, Puchta U, Reckwerth A, Rutten A, Mentzel T, Hugel H, Hantschke M, Schmid-Wendtner MH, et al. 2003. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol 121: 1160–1162 [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Woods SL, Boyle GM, Aoude LG, MacGregor S, Zismann V, Gartside M, Cust AE, Haq R, Harland M, et al. 2011. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature 480: 99–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XP, Gimm O, Hampel H, Niemann T, Walker MJ, Eng C 2000. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol 157: 1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]