Abstract
Rationale: Survivors of critical illness suffer significant limitations and disabilities.
Objectives: Ascertain whether severe sepsis is associated with increased risk of so-called geriatric conditions (injurious falls, low body mass index [BMI], incontinence, vision loss, hearing loss, and chronic pain) and whether this association is measured consistently across three different study designs.
Methods: Patients with severe sepsis were identified in the Health and Retirement Study, a nationally representative cohort interviewed every 2 years, 1998 to 2006, and in linked Medicare claims. Three comparators were used to assess an association of severe sepsis with geriatric conditions in survivors: the prevalence in the United States population aged 65 years and older, survivors’ own pre-sepsis levels assessed before hospitalization, or survivors’ own pre-sepsis trajectory.
Measurements and Main Results: Six hundred twenty-three severe sepsis hospitalizations were followed a median of 0.92 years. When compared with the 65 years and older population, surviving severe sepsis was associated with increased rates of low BMI, injurious falls, incontinence, and vision loss. Results were similar when comparing survivors to their own pre-sepsis levels. The association of low BMI and severe sepsis persisted when controlling for patients’ pre-sepsis trajectories, but there was no association of severe sepsis with injurious falls, incontinence, vision loss, hearing loss, and chronic pain after such controls.
Conclusions: Geriatric conditions are common after severe sepsis. However, severe sepsis is associated with increased rates of only a subset of geriatric conditions, not all. In studying outcomes after acute illness, failing to measure and control for both preillness levels and trajectories may result in erroneous conclusions.
Keywords: severe sepsis, long-term outcomes, geriatric conditions, cohort studies, trajectory bias
At a Glance Commentary
Scientific Knowledge on the Subject
Survivors of critical illness in general, and severe sepsis in particular, have a substantial burden of symptoms after their acute illness.
What This Study Adds to the Field
Many common problems of aging—the so-called geriatric conditions—have a very high prevalence among survivors of severe sepsis, but they are not caused by severe sepsis. However, common study designs that did not fully control for pre-sepsis levels and trajectories of illness might have reported a false-positive association.
A substantial body of research has demonstrated that survivors of critical illness face extensive physical, psychological, cognitive, and social deficits in the aftermath of their critical illness (1). For example, survivors of acute respiratory distress syndrome (ARDS) have prolonged 6-minute walk times, executive dysfunction, other neuropsychiatric deficits, and diminished ability to return to work (2–13). Numerous studies have found quality of life among survivors of critical illness (14, 15), severe sepsis (16, 17), and ARDS (4–8, 13, 18) to be abnormally low.
Patients surviving critical illness have limitations and disabilities somewhat characteristic of an older adult population. Rubenfeld recently postulated a “progeric hypothesis” that the sequelae of critical illness mirror accelerated aging (19). This hypothesis suggests that severe sepsis may be associated with the development of so-called geriatric conditions (15, 20, 21). These are conditions common among older adults, multifactorial in etiology, and associated with disability, often contributing to decreased quality of life (20–23). The following have been previously studied as geriatric conditions: dementia, falls, incontinence, cachexia, poor vision, poor hearing, and chronic pain. These result from a variety of different aging processes: brain dysfunction (dementia), sarcopenia (cachexia, mobility impairment) (24), and multidimensional problems stemming from multiple possible mechanisms (chronic pain) or the interaction of multiple problems (incontinence, vision, hearing). Supporting the progeric hypothesis, previous research found that survivors of severe sepsis had increased rates of cognitive dysfunction and limitations in activities of daily living (25).
Important source data on survivors of critical illness have come from research studies using inception cohorts. This common study design involves empanelling patients at time of diagnosis and following them forward to document their outcomes. Cohort empanelment at diagnosis precludes prospective collection of prediagnosis information. Retrospective collection of prediagnosis data can be difficult (26, 27). If outcomes are worse than “expected,” it is sometime concluded that the condition caused the poor outcomes, although those arguments may only be implied.
Our study has two objectives. The first is to test the hypothesis that severe sepsis leads to an increase in the prevalence of geriatric conditions among survivors. The second is to examine the concordance between different study designs evaluating the impact of critical illness on survivors. Using a unique, nationally representative cohort of older Americans who were interviewed repeatedly for many years before their development of severe sepsis, we compare three study designs. First, we replicate a typical inception cohort design, comparing the prevalence of geriatric conditions among survivors of severe sepsis to population norms. Second, we use an alternative inception cohort design, in which prospectively collected pre-sepsis assessment of survivors is compared with the first post-sepsis assessment. Third, we expand our use of pre-sepsis data, taking advantage of multiple years of prospective pre-sepsis assessment of geriatric conditions, to determine whether severe sepsis is associated with the development of new geriatric conditions, net of the baseline trajectory in the development of these conditions.
Methods
Data Source
The Health and Retirement Study (HRS) is an ongoing cohort nationally representative of Americans over the age of 50 years. Begun in 1992, more than 27,000 individuals have contributed 200,000 hours of data-collection interviews. The cohort is reinterviewed biennially, including detailed questions about their health. The HRS has achieved a very high follow-up rate, using proxies when respondents are unable to complete the survey unassisted. Reinterview rates routinely exceed 90 to 95% (28). Additionally, on reaching age 65 and Medicare eligibility, 16,772 HRS respondents have consented for their HRS data to be linked with their Medicare claims data.
We studied all respondents with at least one HRS interview during 1998 to 2004 and for whom there were subsequent claims-based data on a hospitalization for severe sepsis during 1998 to 2005. All patients were followed through death or the 2006 HRS survey. Our analyses focus on hospitalizations that respondents survived until at least one follow-up interview—the “survivors” cohort. Further characterization of this severe sepsis survivors cohort has been previously published (25).
Ascertainment of Severe Sepsis
We relied on a claims-based definition of severe sepsis, which has been widely used (25, 29–34) and clinically validated (29, 35). Consistent with an international consensus conference definition of severe sepsis, this claims-based definition requires evidence of both an infection and new-onset organ dysfunction during a single hospitalization (36). If a patient had more than one distinct septic hospitalization, each hospitalization was included.
Ascertainment of Outcomes: Geriatric Conditions
Geriatric conditions were ascertained based on interviews with respondents or their proxies as previously described (20, 22). We examined the active or severe forms:
Falls: any fall resulting in injury, or three or more falls in the previous 2 years.
Incontinence: incontinence requiring an undergarment, or incontinence 15 or more days each month.
Low body mass index (BMI): below 18.5 kg/m2 (based on self-report height and weight).
Poor vision: poor eyesight or blindness despite use of corrective lenses.
Poor hearing: poor hearing despite use of hearing aids.
Severe pain: “often” troubled with severe pain.
Analyses
Three analytic approaches were used. In the first, we replicated a cohort of patients, empaneled on the day of hospital admission for severe sepsis and followed forward in time to their first post-sepsis HRS interview. The prevalence of each geriatric condition in this survivors cohort was compared with the corresponding age-group–matched national population estimate of prevalence, using a chi-square test. These national prevalence estimates were assessed using the same survey instrument at a similar point in time (22).
In the second approach, a pre-/post- design in the same cohort was used. Respondents with severe sepsis were compared with their own pre-sepsis measurement of the outcome variables. These pre-sepsis assessments were collected prospectively, thus limiting the potential bias of retrospective assessment of the geriatric conditions before the sepsis episode (26, 27). We present both unadjusted and adjusted models. Only eventual survivors were included in pre-sepsis measurements, not all pre-sepsis patients. In the adjusted models, respondents were matched to their own pre-sepsis measurement, and only within-person variance was examined; that is, respondents served as their own controls.
In the third approach, in the same cohort, multiple years of pre-sepsis longitudinal data through the first post-sepsis assessment were analyzed. Here, a within-person fixed effect model was used (also called a latent growth curve model) (37, 38). (The term “fixed effect” is used here in its econometric/panel data sense, not the somewhat distinct sense in which the term is used in biostatistics [39].) Respondents again served as their own controls, and only within-person variance was assessed. Each respondent provided full control for all characteristics of that respondent that did not change over time. These models are estimated for a dichotomous outcome, using clogit in Stata v.12 (StataCorp LP; College Station, TX). The independent variable, time from the interview to severe sepsis, was measured as a continuous variable. The effect of severe sepsis was estimated as a dichotomous indicator variable, representing the extent to which the probability of the geriatric condition was greater than expected based on the pre-sepsis linear time trend. Similar models estimated the effects of severe sepsis on the total number of geriatric conditions, using xtpoisson, fe in Stata. Consistent with the Journal’s policy, please see a previously published online Appendix for additional details (25).
There are few missing data in the HRS for the outcome variables and no missing data, by construction, for the exposure variable. (Details are available from the authors on request.) Therefore, we conducted a complete case analysis (40).
We performed two-sided significance testing, with a P value less than 0.05 considered significant. In regression models, SEs are adjusted for the small number of patients with more than one hospitalization. The University of Michigan Institutional Review Board approved this work.
Results
The severe sepsis cohort was composed of 516 respondents who survived to a follow-up survey, with 623 severe sepsis hospitalizations among them (Table 1). The median age at hospitalization was 77 years (interquartile range [IQR], 70–83 yr). The mean length of hospital stay was 10.6 days; 43% spent time in a critical care unit. The mean organ dysfunction score was 1.1. The median time from hospital admission for severe sepsis to follow-up assessment for geriatric conditions was 0.9 years, (25th percentile, 0.4; 75th percentile, 1.4).
TABLE 1.
DESCRIPTIVE STATISTICS FOR THE COHORT
| Male, % | 45.1 |
| Age at sepsis, yr | 76.9 (SD, 8.8) |
| Black, % | 20.5 |
| Hispanic, % | 7.1 |
| Length of stay, d | 10.6 (SD, 10.0) |
| Mechanical ventilation, % | 19.7 |
| Dialyzed, % | 4.3 |
| Underwent major surgery, % | 30.4 |
| Used critical care, % | 43.2 |
| Organ dysfunction score | 1.1 |
N = 623 hospitalizations.
The prevalence of geriatric conditions among severe sepsis survivors was high, especially in comparison with the overall U.S. older adult population (Table 2). For example, 32% (95% CI, 28–36%) of severe sepsis survivors reported falling, in contrast to 9.6% of older Americans generally (P < 0.001). Similarly, incontinence, low BMI, and poor vision (P < 0.001) were significantly more common among severe sepsis survivors than older Americans. The prevalence of poor hearing was higher among the overall older adult population than among severe sepsis survivors (P < 0.001).
TABLE 2.
COMPARISON OF THE SURVIVORS COHORT TO U.S. POPULATION PREVALENCE OF GERIATRIC CONDITIONS AMONG THOSE AGED 65 YEARS AND OLDER
| General Population Aged 65+ yr |
Survivors of Severe Sepsis |
||
| Proportion (22) | Proportion (95% CI) | Difference vs. General Population | |
| Incontinence | 0.13 | 0.24 (0.21–0.27) | P < 0.001 |
| Low BMI | 0.03 | 0.07 (0.05–0.09) | P < 0.001 |
| Poor hearing | 0.26 | 0.15 (0.12–0.18) | P < 0.001 |
| Poor vision | 0.08 | 0.20 (0.17–0.23) | P < 0.001 |
| Severe pain | Not reported | 0.12 (0.10–0.15) | Not tested |
| Injurious fall | 0.10 | 0.32 (0.28–0.36) | P < 0.001 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval.
General population data were assessed using the same instrument, previously published (22). Severe chronic pain was not assessed in those data. P values are from a chi-square goodness of fit test.
A similar picture emerges when comparing the prevalence of geriatric conditions after severe sepsis to survivors’ own prospectively collected pre-sepsis reports. Four of six geriatrics conditions showed a significant increase in prevalence after hospitalization, compared with their prevalence before severe sepsis (Table 3, first three columns). The prevalence of incontinence, low BMI, poor vision, and poor hearing all increased significantly in unadjusted models, with substantial increases in both relative and absolute risk. Fully adjusted models, in which survivors served as their own controls, produced similar patterns (Table 3, last two columns). Clinically and statistically significant increases were seen in all of the geriatrics conditions, including a 63% increase in the odds of incontinence and nearly 90% increases in the odds of poor hearing and poor vision.
TABLE 3.
PROPORTION OF GERIATRIC CONDITIONS AFTER SEPSIS COMPARED TO SURVIVORS’ OWN PRE-SEPSIS LEVELS
| Before Severe Sepsis |
After Severe Sepsis | Unadjusted Chi-Square | Matched Analysis | Matched Analysis | |
| Pre-/Post- | Proportion (95% CI) | Proportion (95% CI) | P Value | OR (95% CI) | P Value |
| Incontinence | 0.19 (0.16–0.22) | 0.24 (0.21–0.27) | 0.029 | 1.63 (1.13–2.35) | 0.008 |
| Low BMI | 0.02 (0.02–0.04) | 0.07 (0.05–0.09) | <0.001 | 7.75 (2.61–23.0) | <0.001 |
| Poor hearing | 0.10 (0.08–0.13) | 0.15 (0.12–0.18) | 0.013 | 1.88 (1.15–3.08) | 0.012 |
| Poor vision | 0.15 (0.12–0.18) | 0.20 (0.17–0.23) | 0.019 | 1.86 (1.20–2.89) | 0.006 |
| Severe pain | 0.12 (0.10–0.15) | 0.12 (0.10–0.15) | 0.975 | 1.00 (0.66–1.53) | 1.000 |
| Injurious fall | 0.29 (0.25–0.33) | 0.32 (0.28–0.36) | 0.196 | 1.25 (0.91–1.70) | 0.170 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; OR = odds ratio.
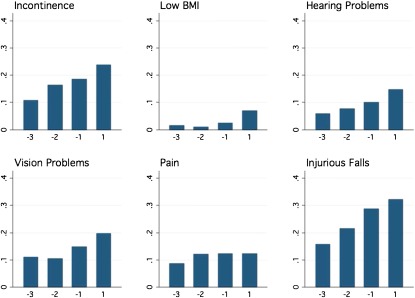
However, use of full longitudinal data yielded a different interpretation. Figure 1 depicts unadjusted population prevalences. Negative numbers on the horizontal axis represent biennial surveys before severe sepsis, with −1 being the last pre-sepsis survey, −2 being the second-to-last, and so on. Except for low BMI, a pre-sepsis increase in the prevalence of each geriatric condition is evident. No difference was evident between the interval within which the sepsis hospitalization occurred and the other intersurvey intervals. These findings were confirmed in the fully adjusted regression models (Table 4); for five of the six geriatric conditions, severe sepsis was not associated with a change in the pre-sepsis trajectory of development of the conditions. The point estimates for these effects are small, suggesting that this lack of association is not due to statistical imprecision. In contrast, the odds of low BMI are statistically significantly higher after severe sepsis than before. There was also no association between severe sepsis and the total number of geriatric conditions, after controlling for the baseline trajectory (P = 0.906).
Figure 1.
Prevalence of geriatric conditions among survivors of severe sepsis, unadjusted. The −1 survey is the last survey conducted before severe sepsis (median, 1.19 yr before sepsis; interquartile range [IQR]: 0.7–1.7 yr), the −2 is the second-to-last (median, 3.21 yr before sepsis; IQR, 2.7–3.7 yr), and the −3 survey was a median of 5.16 yr before sepsis (IQR, 4.7–5.6 yr). The post-sepsis survey was a median of 0.92 yr after severe sepsis (IQR, 0.4–1.4 yr). BMI = body mass index.
TABLE 4.
ASSOCIATION OF SEVERE SEPSIS WITH PREVALENCE OF GERIATRIC CONDITIONS WHEN FULL LONGITUDINAL DATA ARE CONSIDERED
| Pre-sepsis Trajectory |
Effect of Severe Sepsis |
|||
| OR per yr (95% CI) | P Value | OR vs. Pre-sepsis (95% CI) | P Value | |
| Incontinence | 1.21 (1.07–1.37) | 0.002 | 1.02 (0.61–1.71) | 0.939 |
| Low BMI | 1.09 (0.78–1.51) | 0.607 | 5.60 (1.86–16.9) | 0.002 |
| Poor hearing | 1.23 (1.07–1.41) | 0.004 | 1.32 (0.68–2.57) | 0.414 |
| Poor vision | 1.25 (1.11–1.40) | <0.001 | 1.16 (0.69–1.94) | 0.571 |
| Severe pain | 1.15 (1.01–1.30) | 0.030 | 0.70 (0.40–1.23) | 0.217 |
| Fall | 1.19 (1.06–1.33) | 0.002 | 0.89 (0.58–1.38) | 0.610 |
For definition of abbreviations, see Table 3.
Within-person fixed effect (also known as “latent growth curve”) regressions. Interpretive example: With each passing year, patients in this cohort had a 21% increase in their odds of developing incontinence. This increase was statistically significant at P = 0.002. However, there was no additional increase in their odds of developing incontinence if they had been hospitalized for severe sepsis (odds ratio, 1.02) relative to their likelihood of developing incontinence before severe sepsis after controlling for the pre-sepsis trend.
We performed extensive sensitivity testing. Previous research has suggested that critical illness may be associated with sarcopenia, whereas Table 4 showed increased rates of low BMI but no significant increase in falls. In post hoc review, it was noted that bed-bound patients lack the opportunity to fall. Thus, if severe sepsis were associated with a substantial increase in patients no longer ambulating, there would be decreased opportunity among survivors to fall. Parallel analyses to Table 4 showed that severe sepsis was associated with a significant increase in difficulty walking across a room (unadjusted: 39.5% before severe sepsis, 63.2% after [P < 0.001]; odds ratio [OR], 2.79 [95% CI, 1.56–5.00], P = 0.001 when controlling for pre-sepsis trajectory). Likewise, severe sepsis was associated with a significant increase in difficulty lifting 10 pounds (unadjusted: 54.8% before severe sepsis, 68.2% after [P < 0.001]; OR, 2.00 [95% CI, 1.31–3.05]; P = 0.001 when controlling for pre-sepsis trajectory). However, no association between severe sepsis and falling was found among patients reporting no difficulty walking across the room (OR, 0.55 [95% CI, 0.25–1.2]; P = 0.144 when controlling for pre-sepsis trajectory). Furthermore, restricting analyses to only the first severe sepsis hospitalization in the data for each patient produced substantively similar results (see Tables E1 and E2 in the online supplement).
Discussion
Our investigation of a nationwide, prospectively assessed cohort of older Americans found that many geriatric conditions were very common after hospitalization for severe sepsis. This reinforces previous findings that cognitive impairment and limitations in activities of daily living are more common after severe sepsis (25). These results demonstrate an increase in low BMI among survivors, and in post hoc analyses, decreases in the ability to walk across a room and lift 10 pounds. However, the prevalence of five other major geriatric conditions—injurious falls, incontinence, chronic pain, and vision and hearing impairment—were not increased after severe sepsis when patients’ pre-sepsis trajectories of illness were considered. Failure to measure and control for these pre-sepsis trajectories might lead to the spurious inference of a clinically and statistically significant association with severe sepsis. These findings have both clinical and methodological implications.
Clinical Implications
These findings enrich our growing understanding of survivorship after critical illness (1, 41, 42). First, they demonstrate the large burden of consequential conditions carried by survivors. Incontinence, pain, and sensory loss have an important impact on patients and their caregivers. Evidence-based interventions to provide amelioration exist (43–47), but physicians frequently fail to diagnose or manage geriatric conditions (21). Taken together, this suggests that interventions to improve quality of life for survivors might benefit from an increased geriatric focus.
The second clinical contribution of these findings is to suggest that the negative long-term effects of severe sepsis may be focused in sarcopenia and brain dysfunction, in contrast to other facets of aging. Previous work has demonstrated a marked increase in moderate to severe cognitive impairment after severe sepsis (24). The present results demonstrate significant increases in cachexia, weakness, and, as previously reported (25), limitations in walking after severe sepsis. This contrasts with a lack of association of severe sepsis with development of geriatric conditions not associated with sarcopenia or brain dysfunction. Previous findings in the same cohort had emphasized the overall high incidence of disability after severe sepsis (25). If aging were a unitary process, then the current findings would contradict those previous results. Instead, the current findings provide support for a “brains and muscles” focus—as opposed to other aging processes—potentially narrowing the range of likely biologic mechanisms for the prevalent problems of survivorship. Further research on the progeric hypothesis must, however, explain the unexpected null findings with regard to self-reported falls. It is not clear whether this null result is caused by limitations in self-report for this outcome, unmeasured behavioral adaptation to the limits of sarcopenia (48), or other mechanisms.
The findings of sarcopenia and cognitive impairment suggest that severe sepsis produces frailty in survivors. Testing this hypothesis requires a clear conceptualization of frailty, of which there are at least two distinct, well-established options: a “biologic syndrome” model developed by Fried and her collaborators (49–57) and a “burden” model proposed by Rockwood and his collaborators (58–60). The biological syndrome model conceptualizes frailty in terms of “decreased physical reserves and resistance to stressors, resulting from cumulative declines across multiple physiologic systems, and causing vulnerability to adverse outcomes” (61, 62). Sarcopenia plays a crucial role in the Fried and colleagues conceptualization, but it is not a sufficient condition (63). In contrast, the burden model views frailty as an accumulated burden of symptoms, diseases, conditions, disability, and the like (58–60). The two conceptualizations do not identify the same group of patients as frail (64), and it is not clear how well either conceptualization correlates with ICU physicians’ bedside assessment and informal usage of the term. Understanding the relationship between severe sepsis and frailty may offer a promising way to refine the progeric hypothesis by specifying which processes of aging are most relevant at the cellular, organismal, individual, and social levels.
The third clinical contribution of these findings is to emphasize that some of the burdens of survivorship may not be caused by severe sepsis or ICU care. From a prevention perspective, this suggests that some of the burdens of survivorship cannot be avoided by actions in the ICU, as they predate ICU admission. From a discharge and remediation perspective, the issue of causality is less important than the magnitude of the unmet need—for sepsis survivors, that magnitude seems substantial for geriatric conditions.
Methodological Implications
Methodologically, these results serve as a caution against the overinterpretation of studies based on inception cohorts that lack adequate measures of prediagnosis trajectory of outcomes of interest. Substantial anecdotal experience suggests that analyses of the type presented in Tables 2 and 3 may be interpreted causally, even as they are formally acknowledged to be “only associations.” The present results demonstrate that this leap to a causal interpretation can be hazardous.
There are at least four mechanisms that may generate this problem of spurious inference from the prospective cohort design. They are not mutually exclusive.
Nonconstant probability of developing the outcome: If the probability of developing the outcome is changing over time for any reason, this will confound any pre-/post- comparison for outcomes of disease.
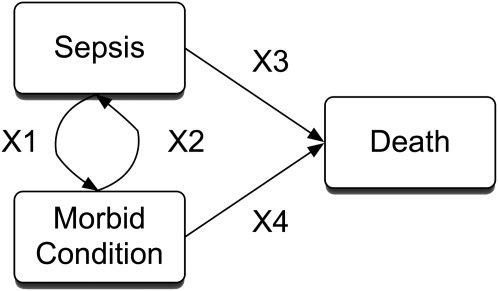
Reciprocal causation: Many morbid outcomes after acute illnesses are of interest precisely because they increase the subsequent risk of health problems (Figure 2). In our example, one can explain post hoc how severe sepsis may lead to incontinence (particularly via urogenital care during the hospitalization) but also how incontinence places a patient at increased risk for urinary tract infection and severe sepsis. This specific example of selection emphasizes the fact that neither severe sepsis nor critical illness are random events, nor do they occur in a strictly representative sample of the general population.
Outcome of interest does not fully manifest the risk status: To avoid these problems, some studies exclude any patient not yet manifesting the outcome of interest. Such an approach would not have solved the spurious inference problem in the present results, because the underlying propensity to develop the geriatric condition cannot be measured, only whether or not the outcome has already occurred.
Outcome of interest is an absorbing state: Some outcomes may be states from which recovery is not possible. Thus, the prevalence of this outcome in a cohort of survivors can only stay the same or increase. This may be because recovery is not possible (e.g., death), rare in practice (e.g., incontinence, hearing problems, vision problems), or is an artifact of the way the outcome is measured (e.g., “has a doctor ever told you that you had a stroke?”).
Figure 2.
Reciprocal causation. In long-term outcome studies, our primary interest is typically in estimating X1. Note that X2 is usually neglected, and X3 and X4 are of key interest when considering truncation by death.
In pediatrics, changes in trajectories—rather than absolute levels—are routinely taken to be the key outcome of interest, as in height and weight growth curves. For many outcomes of interest for adults, particularly older patients, similar trajectories may be common and must be controlled to generate appropriate inferences.
In principle, these challenges could be overcome in a cohort study by obtaining sufficient data on not only the level but also the trajectory of symptoms before the onset of acute illness. Such information would need to be reliable despite the challenges of retrospective collection. In practice it has proven challenging to obtain sufficiently reliable measurements of immediate prehospitalization function for critically ill patients (26, 27). Advances in survey techniques, including facilitated recall prompted by medical record review, may mitigate this potential source of bias in cohort studies unable to prospectively collect prehospitalization data. Our results highlight the potential value of innovative approaches in this area.
Randomized controlled trials continue to be the gold standard for demonstrating causal relationships, but the present results imply a caution in randomized controlled trial interpretation. Many survivor outcomes of interest may be systematically underdiagnosed in routine practice (47, 65, 66). As such, an intervention to improve such an outcome after critical illness might yield a positive trial, even if the critical illness does not cause the outcome—but instead because critical illness serves as a marker for a large burden of an underdiagnosed problem or propensity to develop that problem. For example, antidepressants might improve quality of life after critical illness even if critical illness does not increase rates of depression but rather because depression was undertreated among those who became critically ill.
Limitations
This work has important limitations to consider. First, the HRS is a biennial survey. As such, it is useful for studying medium- to long-term adaptation after critical illness, not the acute phase of recovery (15). Second, we used a claims-based definition of severe sepsis, rather than detailed clinical case ascertainment. Third, our negative conclusions about the associations of severe sepsis and most geriatric conditions may have been influenced by truncation by death (67), in which the extreme phenotype of loss of function cannot be observed because it is lethal. This potential bias can also be considered as informative censoring. We note, however, that such truncation by death did not preclude our ability to detect associations between severe sepsis and disability, cognitive decline, and low BMI, suggesting it should be less of a problem for incontinence or hearing and vision loss. Finally, we have demonstrated that potential spurious inferences can be a problem for a range of outcomes of interest after acute illness, but we cannot determine which such inferences are actually false without more data.
Conclusions
In sum, survivors of severe sepsis have a substantial burden of geriatric conditions in addition to their other medical problems. Some of these problems may be caused or exacerbated by severe sepsis, such as low BMI, disability, and cognitive impairment. However, severe sepsis is not associated with increased rates of five other geriatric conditions. This finding seems to rule out a direct causal link from severe sepsis to these geriatric conditions, while also serving as evidence of the complexity and heterogeneity of these multifactorial conditions over time. This lack of association might have been incorrectly assessed in other cohort designs, in which rates among survivors are compared with a general population or even to the cohort's own baseline measurements. A risk for spurious inference about long-term outcome from acute illness may be present in the medical literature when baseline trajectories are not adequately controlled. As we strive to meet our patients’ many needs, we must carefully assess the causal evidence for the etiology of those needs, while also looking skeptically at when such causal evidence is truly necessary to motivate changes in clinical practice.
Supplementary Material
Acknowledgments
The authors thank Laetitia Shapiro, A.M., at the University of Michigan, for her expert programming; she was financially compensated for her work. They also thank Rodney Hayward, M.D., of the University of Michigan and seminar participants at the University of Washington/Harborview Division of Pulmonary and Critical Care Medicine for insightful critiques; they received no compensation for their work.
Footnotes
Supported by National Institutes of Health grants K08 HL091249 (T.J.I.), K08 AG031837 (C.C.), K12 RR023250 (G.N.), and R01 AG030155, as well as the Society of Critical Care Medicine's 2010 Vision Grant, Claude D. Pepper Older Americans Independence Center at the University of Maryland (P30-AG028747), the John A. Hartford Foundation Center of Excellence in Geriatrics at the University of Michigan, the Ann Arbor VA Geriatric Research, Education and Clinical Center (GRECC), and by pilot support from the Michigan Institute for Clinical and Health Research (MICHR), UL1RR024986. The National Institute on Aging provides funding for the Health and Retirement Study (U01 AG09740), which is performed at the Institute for Social Research, University of Michigan. Consultative support was provided by the Measurement Core of the Michigan Diabetes Center (NIDDK P60 DK-20572). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government. The funders played no role in the design, interpretation, or decision to publish the analysis presented here.
Author Contributions: Drafting and critical revision: T.J.I., G.N., K.M.L., and C.C. Approval of final manuscript: T.J.I., G.N., K.M.L., and C.C. Conception/design: T.J.I., G.N., K.M.L., and C.C. Acquisition of data: T.J.I., K.M.L., and C.C. Analysis/interpretation: T.J.I., G.N., K.M.L., and C.C. Statistical analysis: T.J.I. Obtaining funding: T.J.I., G.N., K.M.L., and C.C.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201109-1660OC on February 9, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med 2011;39:371–379 [DOI] [PubMed] [Google Scholar]
- 2.Adhikari NK, McAndrews MP, Tansey CM, Matte A, Pinto R, Cheung AM, Diaz-Granados N, Barr A, Herridge MS. Self-reported symptoms of depression and memory dysfunction in survivors of ARDS. Chest 2009;135:678–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung AM, Tansey CM, Tomlinson G, Diaz-Granados N, Matte A, Barr A, Mehta S, Mazer CD, Guest CB, Stewart TE, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:538–544 [DOI] [PubMed] [Google Scholar]
- 4.Dowdy DW, Eid MP, Dennison CR, Mendez-Tellez PA, Herridge MS, Guallar E, Pronovost PJ, Needham DM. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med 2006;32:1115–1124 [DOI] [PubMed] [Google Scholar]
- 5.Granja C, Morujao E, Costa-Pereira A. Quality of life in acute respiratory distress syndrome survivors may be no worst than in other ICU survivors. Intensive Care Med 2003;29:1744–1750 [DOI] [PubMed] [Google Scholar]
- 6.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 2003;348:683–693 [DOI] [PubMed] [Google Scholar]
- 7.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011;364:1293–1304 [DOI] [PubMed] [Google Scholar]
- 8.Hopkins RO, Herridge MS. Quality of life, emotional abnormalities, and cognitive dysfunction in survivors of acute lung injury/acute respiratory distress syndrome. Clin Chest Med 2006;27:679–689 [DOI] [PubMed] [Google Scholar]
- 9.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JJF. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 2005;171:340–347 [DOI] [PubMed] [Google Scholar]
- 10.Hopkins RO, Weaver LK, Pope D, Orme JJF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 1999;160:50–56 [DOI] [PubMed] [Google Scholar]
- 11.Lee CM, Herridge MS, Gabor JY, Tansey CM, Matte A, Hanly PJ. Chronic sleep disorders in survivors of the acute respiratory distress syndrome. Intensive Care Med 2009;35:314–320 [DOI] [PubMed] [Google Scholar]
- 12.Mikkelsen ME, Shull WH, Biester RC, Taichman DB, Lynch S, Demissie E, Hansen-Flaschen J, Christie JD. Cognitive, mood and quality of life impairments in a select population of ARDS survivors. Respirology 2009;14:76–82 [DOI] [PubMed] [Google Scholar]
- 13.Orme JJF, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, Crapo RO, Weaver LK. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med 2003;167:690–694 [DOI] [PubMed] [Google Scholar]
- 14.Cuthbertson BH, Roughton S, Jenkinson D, Maclennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Crit Care 2010;14:R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sacanella E, Perez-Castejon JM, Nicolas JM, Masanes F, Navarro M, Castro P, Lopez-Soto A. Functional status and quality of life 12 months after discharge from a medical ICU in healthy elderly patients: a prospective observational study. Crit Care 2011;15:R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettila V, for the Finnsepsis Study Group Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med 2009;37:1268–1274 [DOI] [PubMed] [Google Scholar]
- 17.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 2010;38:1276–1283 [DOI] [PubMed] [Google Scholar]
- 18.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA 1999;281:354–360 [DOI] [PubMed] [Google Scholar]
- 19.Rubenfeld GD. What should be the research agenda for the next decade? Session D04: Long term outcomes after the intensive care unit. Presented at the American Thoracic Society International Conference; May 13–18, 2011, Denver, CO [Google Scholar]
- 20.Cigolle CT, Lee PG, Langa KM, Lee YY, Tian Z, Blaum CS. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med 2011;26:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007;55:780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med 2007;147:156–164 [DOI] [PubMed] [Google Scholar]
- 23.Halter J, Ouslander J, Tinetti M, Studenski S, High K, Asthana S. Hazzard's geriatric medicine and gerontology. New York: McGraw-Hill Medical; 2009.
- 24.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010;304:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scales DC, Tansey CM, Matte A, Herridge MS. Difference in reported pre-morbid health-related quality of life between ARDS survivors and their substitute decision makers. Intensive Care Med 2006;32:1826–1831 [DOI] [PubMed] [Google Scholar]
- 27.Gifford JM, Husain N, Dinglas VD, Colantuoni E, Needham DM. Baseline quality of life before intensive care: a comparison of patient versus proxy responses. Crit Care Med 2010;38:855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Health and Retirement Study. Sample sizes and response rates. 2008 [accessed 2009 October 27]. Available from: http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf.
- 29.Angus D, Linde-Zwirble W, Lidicker J, Clermont G, Carcillo J, Pinsky M. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 30.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med 2001;29:S109–S116 [DOI] [PubMed] [Google Scholar]
- 31.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis. Am J Respir Crit Care Med 2007;177:279–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Doig CJ, Ghali WA, Donaldson C, Johnson D, Manns B. Detailed cost analysis of care for survivors of severe sepsis. Crit Care Med 2004;32:981–985 [DOI] [PubMed] [Google Scholar]
- 33.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Palmer OM, Barnato AE, Weissfeld LA, Angus DC. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA 2010;303:2495–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weycker D, Akhras KS, Edelsberg J, Angus DC, Oster G. Long-term mortality and medical care charges in patients with severe sepsis. Crit Care Med 2003;31:2316–2323 [DOI] [PubMed] [Google Scholar]
- 35.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA 2010;304:1833–1834 [DOI] [PubMed] [Google Scholar]
- 36.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 37.Allison PD. Fixed effects regression methods for longitudinal data using SAS. Cary, NC: SAS Publishing; 2005.
- 38.Allison PD. Fixed effects regression models. Thousand Oaks, CA: Sage; 2009.
- 39.Farewell VT. Fixed effects. In: Armitage P, ed. Encyclopedia of Biostatistics. New York: John Wiley; 1998: p. 1533.
- 40.Allison PD. Missing data. Thousand Oaks, CA: Sage; 2002.
- 41.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med 2010;153:204–205 [DOI] [PubMed] [Google Scholar]
- 42.Needham DM, Feldman DR, Kho ME. The functional costs of ICU survivorship: collaborating to improve post-ICU disability. Am J Respir Crit Care Med 2011;183:962–964 [DOI] [PubMed] [Google Scholar]
- 43.Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc 2001;49:664–672 [PubMed] [Google Scholar]
- 44.American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009;57:1331–1346 [DOI] [PubMed] [Google Scholar]
- 45.Michael YL, Whitlock EP, Lin JS, Fu R, O'Connor EA, Gold R. Primary care-relevant interventions to prevent falling in older adults: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med 2010;153:815–825 [DOI] [PubMed] [Google Scholar]
- 46.Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med 2008;148:459–473 [DOI] [PubMed] [Google Scholar]
- 47.Yueh B, Shapiro N, MacLean CH, Shekelle PG. Screening and management of adult hearing loss in primary care: scientific review. JAMA 2003;289:1976–1985 [DOI] [PubMed] [Google Scholar]
- 48.Brown CJ, Roth DL, Allman RM, Sawyer P, Ritchie CS, Roseman JM. Trajectories of life-space mobility after hospitalization. Ann Intern Med 2009;150:372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res 2009;12:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boyd CM, Ricks M, Fried LP, Guralnik JM, Xue Q-L, Xia J, Bandeen-Roche K. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women's Health and Aging Study I. J Am Geriatr Soc 2009;57:1757–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci 2009;64:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc 2009;57:1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leng SX, Hung W, Cappola AR, Yu Q, Xue QL, Fried LP. White blood cell counts, insulin-like growth factor-1 levels, and frailty in community-dwelling older women. J Gerontol A Biol Sci Med Sci 2009;64:499–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 2009;64:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilhelm-Leen ER, Hall YN, Tamur MK, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med 2009;122:664–71e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci 2010;65:407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiner AP, Aragaki AK, Gray SL, Wactawski-Wende J, Cauley JA, Cochrane BB, Kooperberg CL, Woods NF, LaCroix AZ. Inflammation and thrombosis biomarkers and incident frailty in postmenopausal women. Am J Med 2009;122:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 2007;62:738–743 [DOI] [PubMed] [Google Scholar]
- 59.Canadian Study of Health and Aging Working Group Canadian study of health and aging: study methods and prevalence of dementia. CMAJ 1994;150:899–913 [PMC free article] [PubMed] [Google Scholar]
- 60.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:M255–M263 [DOI] [PubMed] [Google Scholar]
- 62.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156 [DOI] [PubMed] [Google Scholar]
- 63.McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care 2011;15:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc 2009;57:830–839 [DOI] [PubMed] [Google Scholar]
- 65.Calkins DR, Rubenstein LV, Cleary PD, Davies AR, Jette AM, Fink A, Kosecoff J, Young RT, Brook RH, Delbanco TL. Failure of physicians to recognize functional disability in ambulatory patients. Ann Intern Med 1991;114:451–454 [DOI] [PubMed] [Google Scholar]
- 66.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord 2009;23:306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McConnell S, Stuart EA, Devaney B. The truncation-by-death problem: what to do in an experimental evaluation when the outcome is not always defined. Eval Rev 2008;32:157–186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.