Abstract
Since the 1989 discovery that mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene cause cystic fibrosis (CF), there has been substantial progress toward understanding the molecular basis for CF lung disease, leading to the discovery and development of new therapeutic approaches. However, the earliest impact of the loss of CFTR function on airway physiology and structure and its relationship to initial infection and inflammation are poorly understood. Universal newborn screening for CF in the United States represents an unprecedented opportunity for investigating CF clinical manifestations very early in life. Recently developed animal models with pulmonary phenotypic manifestations also provide a window into the early consequences of this genetic disorder. For these reasons, the National Heart, Lung, and Blood Institute (NHLBI) convened a working group of extramural experts, entitled “Future Research Directions in Early CF Lung Disease” on September 21–22, 2010, to identify future research directions of great promise in CF. The priority areas identified included (1) exploring pathogenic mechanisms of early CF lung disease; (2) leveraging newborn screening to elucidate the natural history of early lung disease; (3) developing a spectrum of biomarkers of early lung disease that reflects CF pathophysiology, clinical outcome, and response to treatment; (4) exploring the role of genetics/genomics (e.g., modifier genes, gene–environmental interactions, and epigenetics) in early CF pathogenesis; (5) defining early microbiological events in CF lung disease; and (6) elucidating the initial airway inflammatory, remodeling, and repair mechanisms in CF lung disease.
Keywords: cystic fibrosis, airway disease, innate immunity, microbiology, genetics
At a Glance Commentary
Scientific Knowledge on the Subject
Emerging evidence suggests that lung disease begins very early in life in cystic fibrosis (CF), although it is initially “silent” without overt signs and symptoms of a progressive disease process. The nature of CF lung abnormalities in the first years of life remains poorly understood, and the possibility of preventing or delaying the onset of disease through early intervention has scarcely been explored. A new frontier in CF, made possible by the early diagnosis of CF with newborn screening, is to understand and characterize presymptomatic lung disease in infants and young children, leading to early interventions to mitigate disease progression at stages when therapeutic intervention or prevention may be most effective.
What This Study Adds to the Field
This review provides a summary of recommendations from an NHLBI workshop convened to review the progress and direction of CF research and to identify and prioritize the research opportunities that hold the most promise to provide new mechanistic insights into the genesis and evolution of early CF lung disease.
Cystic fibrosis (CF) is a life-shortening autosomal recessive disorder caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) (1, 2). CFTR is an anion channel that influences the composition and quantity of liquid on the surface of epithelia. Significant advances have increased understanding of CFTR structure and function and how mutations disrupt function (2). Survival and quality of life have improved, through better management of nutrition and respiratory infections, rather than through interventions that target the basic defect. Promising therapies directed to correcting dysfunctional CFTR (3, 4) are being tested in older children and adults with CF, but their effectiveness in infants and young children remains unexplored.
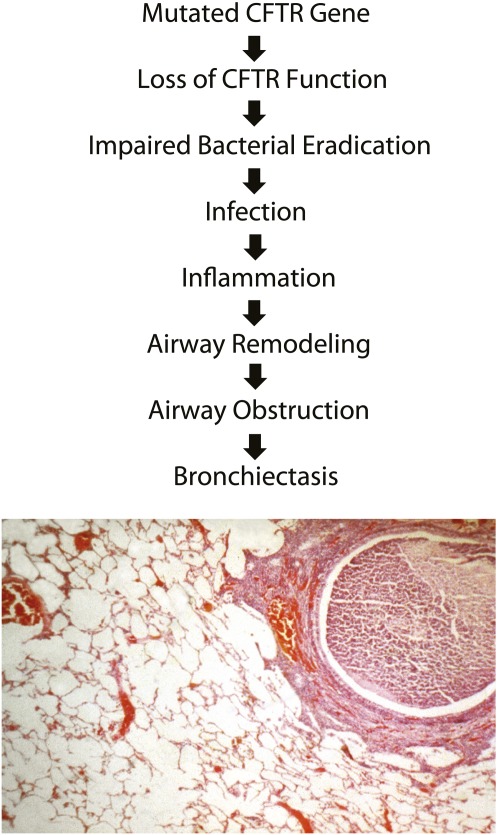
Because lung disease causes most CF morbidity and mortality, much research has focused on understanding the pathophysiological cascade of events progressing from CFTR mutations to irreversible lung damage (Figure 1). Impaired eradication of bacteria early in life induces a predominantly neutrophilic inflammatory response that injures the lung (5, 6). Airway remodeling and airway obstruction ensue (7). The temporal sequence from loss of CFTR function to initial bacterial airway infection, and the impact of environmental factors such as viruses on this process, are not clear.
Figure 1.
The steps hypothesized to be relevant to the progression from dysfunctional cystic fibrosis transmembrane conductance regulator (CFTR) to initial infection and inflammation and eventual development of airway structural damage are described. At each step, genetic and environmental modifiers can operate to alter outcome. The micrograph displays a bronchial airway from an individual with CF with the hallmark pathological findings including airway obstruction with mucus and inflammatory cells and peribronchial inflammatory response.
Since 2010, newborn screening for CF has been universal in the United States and many other countries (8), providing an unprecedented opportunity to longitudinally monitor disease progression (6, 9) and evaluate emerging therapeutic approaches from infancy (3, 4, 10). New CF animal models (11–14) provide a pulmonary phenotype for studying the origins of disease. In addition, researchers can employ improved and emerging technologies to measure airway physiology, structure, and disease progression (10, 15–22). These factors position the field to address questions about the initiating events of CF lung disease. With several promising therapies on the horizon, it is imperative that the pathogenesis of early lung disease be elucidated so that future interventions can prevent and not simply treat CF lung disease. For this reason, the National Heart, Lung, and Blood Institute (NHLBI, Bethesda, MD) convened a workshop on September 21–22, 2010 to review the progress of CF research and to prioritize research opportunities (Table 1) that promise to provide mechanistic insight into the genesis and evolution of early CF lung disease.
TABLE 1.
RESEARCH PRIORITIES FOR EARLY CF LUNG DISEASE
| 1. Explore pathogenic mechanisms of early CF lung disease |
| 2. Leverage newborn screening to elucidate the natural history and clinical manifestations of early CF lung disease |
| 3. Develop biomarkers of early lung disease that reflect CF pathophysiology, clinical outcome, and response to treatment |
| 4. Identify the role of genetics, genomics, and epigenetics in early CF disease pathogenesis |
| 5. Define early microbiological events in CF lung disease and how they lead to chronic infection |
| 6. Elucidate the initial airway inflammatory responses, innate and adaptive immune responses, and mechanisms of repair/remodeling in early CF lung disease |
Definition of abbreviation: CF = cystic fibrosis.
Priority 1: Explore Pathogenic Mechanisms of Early CF Lung Disease
CF lung disease is a multistep process. Immediately after birth, CF lungs may have a host defense defect that impairs bacterial eradication (14) and induces inflammation and airway remodeling. At some point, airways become chronically infected, and the cycle of infection, inflammation, and remodeling accelerates to obstruct and destroy airways. Loss of CFTR could also affect multiple individual processes along the pathway, leading toward structural damage. Elucidating discrete responsible mechanisms is also made difficult by the spatial and temporal heterogeneity of the disease (22).
The field is now positioned to begin elucidating the initial defects that trigger lung disease and define the directional connections among the many reported defects. Three animal models (11–13, 23) are available to study mechanisms—pigs, ferrets, and mice; each can generate in vitro models for dissecting molecular mechanisms and comparison with humans. Differences and similarities among CF models may inform investigators about mechanisms in early disease. Potential research opportunities include the following:
Elucidate the defect(s) that impairs host defense in the newborn lung.
Identify the sequence of events that causes progression of airway disease and learn how loss of CFTR alters the process.
Investigate cross-species comparisons to clarify early pathological events.
Define mechanisms underlying the heterogeneity of CF lung disease.
Priority 2: Leverage Newborn Screening to Elucidate the Natural History and Clinical Manifestations of Early CF Lung Disease
Study of presymptomatic CF infants identified by newborn screening will allow characterization of the earliest manifestations of the disease, including the relationship between lung infection and inflammation (6, 7, 9, 22), stratification by mutation subclass (2, 24), follow-up throughout the life span (6, 25), and intervention before irreversible disease develops (4, 10, 15, 24, 25). Technologies to elucidate features of CF physiology (airway surface liquid generation, mucus clearance, etc.) (16, 17) and disease biomarkers (9, 18–20) are in place. New CFTR modulators (4, 10, 24) and interventions that alter fluid and electrolyte transport (3, 4, 10, 16, 24, 26) will serve as valuable tools for better understanding early CF lung disease and establishing validated biomarkers and clinical end points in young, presymptomatic patients. Creating clinical sample repositories from subjects with early CF will enable future research. Potential research opportunities include the following:
Develop data and specimen (e.g., DNA, plasma) repositories beginning in the newborn period to expedite research.
Elucidate the natural history and clinical manifestations of early CF lung disease.
Develop new and improved technologies to monitor disease progression and outcome.
Determine the mechanisms responsible for pulmonary exacerbation in early CF.
Priority 3: Develop Biomarkers of Early Lung Disease That Reflect CF Pathophysiology, Clinical Outcome, and Response to Treatment
Characterization of lung disease in very young patients is challenging (15, 22, 25) because CF lung disease likely starts in the small airways (22), where early changes are physiologically and structurally difficult to measure. Early lung disease in CF is heterogeneous, with various degrees of air trapping and atelectasis (9, 21, 22, 27). Although imaging studies cannot visualize airways less than 1 mm in cross-section, the presence of structural changes in small airways can be visualized as trapped air even before any measurable changes in air flow (9, 21, 22). The use of bronchoalveolar lavage (BAL) has provided significant insight into early infection and inflammatory responses (5, 6, 21). Studies have assessed peripheral airway physiology in infants and preschool children by lung function testing, using the raised volume rapid thoracic compression (RVRTC) technique and the lung clearance index, which represent promising techniques that may become useful measures of small airway obstruction (6, 9, 15, 21, 28). Although promising, many of these procedures expose infants to ionizing radiation (computed tomography, CT) (29) or require sedation (BAL, CT, RVRTC), increasing cost/risk and reducing usefulness for therapeutic trials. Thus, there is a critical need for minimally invasive measures of early lung disease that reflect early CF pathophysiology, clinical outcomes, and responses to treatments. There is also a need to develop biomarkers that can discriminate levels of CFTR function and reflect its impact on ion transport and airway function (26). Potential research opportunities include the following:
Establish reliable clinical or physiological end points for evaluating the efficacy/safety of novel therapies.
Identify new and improved biomarkers of disease onset, progression, and severity that reflect clinical outcome or response to treatment.
Elucidate the connections between structural changes and physiological abnormalities, using new or improved technologies adapted for use in infants and young children.
Priority 4: Explore the Role of Genetics, Genomics, and Epigenetics in Early CF Lung Disease Pathogenesis
The relationship between CFTR genotype and pulmonary disease severity does not show a close correlation (30, 31). Several factors account for this complexity. First, many distinct CFTR mutations exist. Second, different mutations disrupt CFTR to variable extents. Third, transcriptional regulation of CFTR gene expression is spatially and temporally controlled and may vary as disease progresses. Fourth, several modifier genes and genomic regions influence lung disease severity (31, 32). Fifth, microRNAs (33) may play a significant role in disease modification, but have been little investigated in CF. Sixth, infection, inflammation, and other factors might produce epigenetic changes including histone acetylation, gene methylation, and other alterations (34, 35). Seventh, environmental factors account almost equally to genetic factors for variation in lung disease; unique exposures contribute the majority of the effect (36).
Several advances provide an opportunity to probe this complexity and decipher how these factors influence CF lung disease. These include next-generation sequencing technologies, genome-wide methods for assessing epigenetic modifications (35), and methodologies for understanding gene networks. The reservoir of naturally occurring mutations in CF centers and databases provides an important resource for study (2, 37), although efforts would benefit from expanding detailed genotypic and phenotypic resources and banked samples. Recently developed pig (14) and ferret (11) CF models and newer murine models (12, 13) provide powerful new opportunities for evaluating mechanisms of disease and therapies. Potential research opportunities include the following:
Expand knowledge of genetic heterogeneity and diversity of CFTR mutations linked to functional and phenotypic consequences.
Identify modifier genes.
Use genetic studies to gain mechanistic insights into CF biology.
Identify gene–gene and gene–environmental interactions in early lung pathogenesis.
Define epigenetic signatures in early life that predict disease status later in life.
Identify regions of the genome that regulate CFTR transcription and other relevant genes and determine their influence on disease severity.
Define microRNAs and small interfering RNAs influencing development and function of airway cells in early CF lung disease.
Priority 5: Define Microbiological Events in Early CF Lung Disease and How They Lead to Chronic Infection
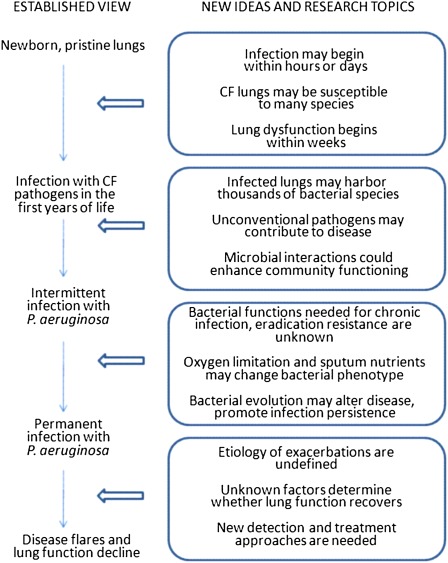
Bacterial airway infection and the resulting inflammation cause lung function decline (2, 6, 38). Antiinfective therapy has improved survival, but treatment remains inadequate: bacterial lineages persist despite countless antibiotic courses; antibiotics only reduce bacterial load, which rebounds off therapy (39); and antibiotic responses wane over time (39, 40). New ideas about CF microbiology (Figure 2) have come from the use of non–culture-based detection techniques, studies on microbial interactions, progress in understanding pathogen evolution, and studies on biofilms (15, 41, 42). Better understanding CF microbiology could improve outcomes in chronically infected patients.
Figure 2.
New ideas about each of the stages of cystic fibrosis infection suggest future research directions.
Defining how CFTR mutations cause infection susceptibility and identifying bacteria causing infection remain critical tasks. Research has focused on Pseudomonas aeruginosa (Pa) (38, 43) because Pa acquisition is associated with mortality and lung function decline (44, 45). However, non–culture-based techniques on upper airway samples have revealed a multitude of bacterial species (42). Identifying which species are actually present in CF lungs, and determining which contribute to disease, remain major questions. Other major challenges include identifying factors that promote the transition from early transient to chronic endobronchial infection, resistant to eradication. Identifying the nature and diversity of bacterial genetic adaptations, pivotal host–bacteria interactions, and microbiological changes that occur during pulmonary exacerbation (46) could suggest therapeutic strategies. Key research directions include the following:
Define how CFTR mutations cause susceptibility to infection, reservoirs of infecting bacteria, timing of infection onset, and organisms involved.
Investigate microbial diversity in the early CF lung and identify organisms causing disease.
Characterize key bacterial and host factors pivotal to the transition from acute to chronic infection.
Develop new agents with activity against bacteria in chronic infections, or that enhance the activity of existing drugs.
Identify mechanisms and biomarkers of exacerbations and develop new treatment approaches that improve patient management and outcomes.
Priority 6: Elucidate the Initial Airway Inflammatory Response, Innate and Adaptive Immune Responses, and Mechanisms of Repair/Remodeling in Early CF Lung Disease
Current understanding of how the CF airway changes over the first year(s) of life including composition of the microbiome, innate immunity, injury and repair remains rudimentary, but it is likely that inflammatory responses are central to the development of early airway disease (6, 7, 47). Recent advances in lung biology, including delineation of molecular programs underlying lung development and responses to injury and remodeling (48), recognition of the critical role of airway epithelial cells in inducing and regulating innate and adaptive immunity in the lung (49), and recognition of the fact that resolution of inflammation in the lung is an active, regulated process (50), provide novel tools for understanding early pathogenesis in the CF airway. Improved knowledge of inflammatory processes has obvious implications, both for insight into disease pathogenesis and for development of novel antiinflammatory therapeutics. Potential research opportunities include the following:
Identify the primary pathogenetic locus (or loci) that underlies the dysregulated inflammatory milieu of the CF airway.
Define molecular and cellular mechanisms responsible for developmental, inflammatory, innate and adaptive immunity, repair, and remodeling abnormalities of the early CF airways.
Develop validated biomarkers for assessing dysregulated airway inflammation, immunity, injury, and remodeling in early CF.
Conclusions
Emerging evidence suggests that CF lung disease begins in infancy and is initially “silent” without overt signs or symptoms. By the time symptoms appear, the disease process is established. Thus, understanding how loss of CFTR predisposes airways to infection and initiates the cascade of inflammation, remodeling, and airway obstruction is critical for developing new therapeutic and preventive strategies. New CF animal models and technologies for disease characterization are in place and can provide insights to inform future human studies of primary prevention and/or mitigation of CF lung disease in infancy and early childhood. The priority areas that this workshop identified provide a roadmap for better understanding the earliest stages of CF lung disease and preventing or delaying its onset.
Supplementary Material
Acknowledgments
Participants: Co-Chairs: Michael Welsh, M.D., University of Iowa, Iowa City, IA; Bonnie Ramsey, M.D., Seattle Children's Research Institute, Seattle, WA and University of Washington School of Medicine, Seattle, WA; Members- Group Leaders: Group 1, Eric Sorscher, M.D., University of Alabama, Birmingham, AL; Group 2, John Engelhardt, Ph.D., University of Iowa, Iowa City, IA; Group 3, Pradeep Singh, M.D., University of Washington, Seattle, WA; Group 4, Christopher Karp, M.D., Children's Hospital Medical Center, Cincinnati, OH; Group 5, Garry Cutting, M.D., Johns Hopkins University, Baltimore, MD; Group 6, Richard Boucher, M.D., University of North Carolina, Chapel Hill, NC; Group Members: Frank Accurso, M.D., University of Colorado School of Medicine, Denver, CO (Groups 1, 4, and 6); William Guggino, Ph.D., Johns Hopkins University, Baltimore, MD (Groups 2 and 6); Michael Knowles, M.D., University of North Carolina, Chapel Hill, NC (Group 5); Jay Kolls, M.D., Louisiana State University Health Science Center, New Orleans, LA (Groups 4 and 5); John Lipuma, M.D., University of Michigan, Ann Arbor, MI (Group 3); Susan Lynch, Ph.D., University of California at San Francisco School of Medicine, San Francisco, CA (Group 3); Paul McCray, M.D., University of Iowa, Iowa City, IA (Groups 4 and 5); Ronald Rubenstein, M.D., Ph.D., Children's Hospital of Philadelphia, and Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA (Groups 1, 2, and 6); NHLBI Staff: Susan Banks-Schlegel, Ph.D., Division of Lung Diseases, Bethesda, MD; James P. Kiley, Ph.D., Division of Lung Diseases, Bethesda, MD; Cystic Fibrosis Foundation Staff/Consultants: Drucy Borowitz, M.D., State University of New York at Buffalo, NY; Christopher Penland, Ph.D., Cystic Fibrosis Foundation, Bethesda, MD; Steven Rowe, M.D., MSPH, University of Alabama, Birmingham, AL.
Acknowledgments
The authors thank Laurel Feltz for assistance with manuscript preparation.
Footnotes
Supported by the Division of Lung Diseases, National Institutes of Health, of the U.S. Department of Health and Human Services.
Author Contributions: All authors contributed equally to this article.
Originally Published in Press as DOI: 10.1164/rccm.201111-2068WS on February 3, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–1080 [DOI] [PubMed] [Google Scholar]
- 2.Welsh MJ, Ramsey BW, Accurso F, Cutting G. Cystic fibrosis. : Scriver CR, Beaudet AL, Sly WS, Valle D, The metabolic and molecular basis of inherited diseases, 8th ed. New York: McGraw-Hill; 2001. pp. 5121–5188 [Google Scholar]
- 3.Kerem E, Hirawat S, Armoni S, Yaakov Y, Shoseyov D, Cohen M, Nissim-Rafinia M, Blau H, Rivlin J, Aviram M, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet 2008;372:719–727 [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutierrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 1997;156:1197–1204 [DOI] [PubMed] [Google Scholar]
- 6.Pillarisetti N, Williamson E, Linnane B, Skoric B, Robertson CF, Robinson P, Massie J, Hall GL, Sly P, Stick S, et al. Infection, inflammation, and lung function decline in infants with cystic fibrosis. Am J Respir Crit Care Med 2011;184:75–81 [DOI] [PubMed] [Google Scholar]
- 7.Chmiel JF, Berger M, Konstan MW. The role of inflammation in the pathophysiology of CF lung disease. Clin Rev Allergy Immunol 2002;23:5–27 [DOI] [PubMed] [Google Scholar]
- 8.Borowitz D, Robinson KA, Rosenfeld M, Davis SD, Sabadosa KA, Spear SL, Michel SH, Parad RB, White TB, Farrell PM, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr 2009;155:S73–S93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall GL, Logie KM, Parsons F, Schulzke SM, Nolan G, Murray C, Ranganathan S, Robinson P, Sly PD, Stick SM, et al. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS One 2011;6:e23932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goralski JL, Boucher RC, Button B. Osmolytes and ion transport modulators: new strategies for airway surface rehydration. Curr Opin Pharmacol 2010;10:294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, Sui H, Fischer JT, Yan Z, Liu X, Cho H-J, Joo NS, Zhang Y, Zhou W, Yi Y, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 2010;120:3149–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros 2011;10:S152–S171 [DOI] [PubMed] [Google Scholar]
- 13.Zhou Z, Duerr J, Johannesson B, Schubert SC, Treis D, Harm M, Graeber SY, Dalpke A, Schultz C, Mall MA. The ENAC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros 2011;10:S172–S182 [DOI] [PubMed] [Google Scholar]
- 14.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2010;2:29ra31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zemanick ET, Harris JK, Conway S, Konstan MW, Marshall B, Quittner AL, Retsch-Bogart G, Saiman L, Accurso FJ. Measuring and improving respiratory outcomes in cystic fibrosis lung disease: opportunities and challenges to therapy. J Cyst Fibros 2010;9:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson SH, Corcoran TE, Laube BL, Bennett WD. Mucociliary clearance as an outcome measure for cystic fibrosis clinical research. Proc Am Thorac Soc 2007;4:399–405 [DOI] [PubMed] [Google Scholar]
- 17.Harvey PR, Tarran R, Garoff S, Myerburg MM. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am J Respir Cell Mol Biol 2011;45:592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esther CR, Jr, Boysen G, Olsen BM, Collins LB, Ghio AJ, Swenberg JW, Boucher RC. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 2009;296:L987–L993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pattison SH, Elborn JS. Protein biomarkers in cystic fibrosis research: where next? Genome Med 2010;2:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ollero M, Astarita G, Guerrera IC, Sermet-Gaudelus I, Trudel S, Piomelli D, Edelman A. Plasma lipidomics reveals potential prognostic signatures within a cohort of cystic fibrosis patients. J Lipid Res 2011;52:1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis SD, Brody AS, Emond MJ, Brumback LC, Rosenfeld M. Endpoints for clinical trials in young children with cystic fibrosis. Proc Am Thorac Soc 2007;4:418–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiddens HA, Donaldson SH, Rosenfeld M, Pare PD. Cystic fibrosis lung disease starts in the small airways: can we treat it more effectively? Pediatr Pulmonol 2010;45:107–117 [DOI] [PubMed] [Google Scholar]
- 23.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Trokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008;321:1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasemann H, Ratjen F. Emerging therapies for cystic fibrosis lung disease. Expert Opin Emerg Drugs 2010;15:653–659 [DOI] [PubMed] [Google Scholar]
- 25.Stick SM, Sly PD. Exciting new clinical trials in cystic fibrosis: infants need not apply. Am J Respir Crit Care Med 2011;183:1577–1578 [DOI] [PubMed] [Google Scholar]
- 26.Rowe SM, Accurso F, Clancy JP. Detection of cystic fibrosis transmembrane conductance regulator activity in early-phase clinical trials. Proc Am Thorac Soc 2007;4:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long FR, Castle RG. Technique and clinical applications of full-inflation and end-exhalation controlled-ventilation chest CT in infants and young children. Pediatr Radiol 2001;31:413–422 [DOI] [PubMed] [Google Scholar]
- 28.Kieninger E, Singer F, Fuchs O, Abbas C, Frey U, Regamey N, Casaulta C, Latzin P. Long-term course of lung clearance index between infancy and school-age in cystic fibrosis subjects. J Cyst Fibros 2011;10:487–490 [DOI] [PubMed] [Google Scholar]
- 29.Donnelly LF, Frush DP. Pediatric multidetector body CT. Radiol Clin North Am 2003;41:637–655 [DOI] [PubMed] [Google Scholar]
- 30.Groman JD, Meyer M, Wilmott R, Zeitlin P, Cutting G. Variant cystic fibrosis phenotypes in the absence of CFTR mutations. N Engl J Med 2002;347:401–407 [DOI] [PubMed] [Google Scholar]
- 31.Cutting GR. Modifier genes in mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci 2010;1214:57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright FA, Strug LJ, Doshi VK, Commander CW, Blackman SM, Sun L, Berthiaume Y, Cutler D, Cojocaru A, Collaco JM, et al. Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat Genet 2011;43:539–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W, Hui C, Yu SS, Jing C, Chan HC. MicroRNAs and cystic fibrosis—an epigenetic perspective. Cell Biol Int 2011;35:463–466 [DOI] [PubMed] [Google Scholar]
- 34.Rodenhiser D, Mann M. Epigenetics and human disease: translating basic biology into clinical applications. CMAJ 2006;174:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol 2010;6:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J Pediatr 2010;157:802–807, e801–e803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sosnay PR, Castellani C, Corey M, Dorfman R, Zielenski J, Karchin R, Penland CM, Cutting GR. Evaluation of the disease liability of CFTR variants. Methods Mol Biol 2011;742:355–372 [DOI] [PubMed] [Google Scholar]
- 38.Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 2002;165:904–910 [DOI] [PubMed] [Google Scholar]
- 39.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 1999;340:23–30 [DOI] [PubMed] [Google Scholar]
- 40.Retsch-Bogart GZ, Quittner AL, Gibson RL, Oermann CM, McCoy KS, Montgomery AB, Cooper PJ. Efficacy and safety of inhaled aztreonam lysine for airway Pseudomonas in cystic fibrosis. Chest 2009;135:1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers GB, Hoffman LR, Döring G. Novel concepts in evaluating antimicrobial therapy for bacterial lung infections in patients with cystic fibrosis. J Cyst Fibros 2011;10:387–400 [DOI] [PubMed] [Google Scholar]
- 42.Zemanick ET, Sagel SD, Harris JK. The airway microbiome in cystic fibrosis and implications for treatment. Curr Opin Pediatr 2011;23:319–324 [DOI] [PubMed] [Google Scholar]
- 43.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kulich M, Kronmal R, Williams J, Ramsey BW; Early Pseudomonas Infection Control (EPIC) Investigators Comparative efficacy and safety of four randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med 2011;165:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol 1992;12:158–161 [DOI] [PubMed] [Google Scholar]
- 45.Parad RB, Gerard CJ, Zurakowski D, Nichols DP, Pier GB. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect Immun 1999;67:4744–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tunney MM, Klem ER, Fodor AA, Gilpin DF, Moriarty TF, McGrath SJ, Muhlebach MS, Boucher RC, Cardwell C, Doering G, et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax 2011;66:579–584 [DOI] [PubMed] [Google Scholar]
- 47.Sutanto EN, Kicic A, Foo CJ, Stevens PT, Mullane D, Knight DA, Stick SM; on behalf of the Australian Respiratory Early Surveillance Team for Cystic Fibrosis Innate inflammatory responses of pediatric cystic fibrosis airway epithelial cells: effects of nonviral and viral stimulation. Am J Respir Cell Mol Biol 2011;44:761–767 [DOI] [PubMed] [Google Scholar]
- 48.Whitsett JA, Haitchi HM, Maeda Y. Intersections between pulmonary development and disease. Am J Respir Crit Care Med 2011;184:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008;8:193–204 [DOI] [PubMed] [Google Scholar]
- 50.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005;6:1191–1197 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.