Abstract
Regulation of tone, blood pressure, and blood flow in the cerebral vasculature is of vital importance, particularly in the developing infant. We tested the hypothesis that, in addition to accretion of smooth muscle cells (SMCs) in cell layers with vessel thickening, significant changes in smooth muscle structure, as well as phenotype, extracellular matrix, and membrane proteins, in the media of cerebral arteries (CAs) during the course of late fetal development account for associated changes in contractility. Using transmission electron, confocal, wide-field epifluorescence, and light microscopy, we examined the structure and ultrastructure of CAs. Also, we utilized wire myography, Western immunoblotting, and real-time quantitative PCR to examine several other features of these arteries. We compared the main branch ovine middle CAs of 95- and 140-gestational day (GD) fetuses with those of adults (n = 5 for each experimental group). We observed a graded increase in phenylephrine- and KCl-induced contractile responses with development. Structurally, lumen diameter, media thickness, and media cross-sectional area increased dramatically from one age group to the next. With maturation, the cross-sectional profiles of CA SMCs changed from flattened bands in the 95-GD fetus to irregular ovoid-shaped fascicles in the 140-GD fetus and adult. We also observed a change in the type of collagen, specific integrin molecules, and several other parameters of SMC morphology with maturation. Ovine CAs at 95 GD appeared morphologically immature and poorly equipped to respond to major hemodynamic adjustments with maturation.
Keywords: vascular smooth muscle, fascicle, fetus
in the united states and other countries, severe neurological impairment is far too common. More than 10,000 (2–3 per 1,000) of the ∼4.5 million infants born each year can be expected to have severe neurological disability. Among very preterm (<32 wk gestation) births, the prevalence of brain damage is particularly high (32, 39). Dysregulation of cerebral blood flow (CBF) with intracerebral hemorrhage is a major pathogenetic factor in such disorders. Thus, maintenance of well-regulated cerebral vascular tone and blood flow is essential to the fetus and the adult. During the past several decades, we have shown significantly different action of certain signal transduction pathways in the fetus compared with the adult. For instance, we have reported that cerebrovascular reactivity, including that of α-adrenergic mechanisms, changes dramatically with maturational development (15, 27, 29). Importantly, we have shown that, in fetal cerebral arteries (CAs), Ca2+-dependent pathways, including the α1B- and α1D-adrenergic receptors (ARs), are not associated with contractile mechanisms but, rather, play a major role in gene regulation for the developing vasculature. With development, there is a significant increase in the abundance of each of the α1-AR subtypes, and the α1D-AR-mediated contractile response increases (15). In addition, we have shown that, in fetal, but not adult, CAs, the RhoA/Rho kinase pathway plays a critical role in the PKC-mediated contractile response. Also, caldesmon and CPI-17 are involved in PKC-mediated CA contractility in the adult, but not in the fetus (14).
These development-associated responses are only part of the picture, however, and a critical issue is that many other factors and enzymes interact to complicate this scenario. Importantly, our previous studies give evidence that the Ca2+-sensitization mechanisms in many instances are attenuated or absent in the developing fetus and newborn. These differences in signal transduction may be responsible for the increased vulnerability of the premature and near-term fetus to CBF dysregulation compared with the adult. However, apart from the differences in signal transduction mechanisms, there is a significant difference in the gross morphological appearance of these vessels with maturational development. In a previous report, we demonstrated that expression levels of α-actin, a thin-filament protein involved in contraction, remained relatively constant during the course of development, as did the effects of inhibition of its polymerization on contractility. In contrast, α-tubulin, important in intracellular protein trafficking, showed a significant age-related decrease in expression and played a relatively minor role in contractility (41).
Nonetheless, fetal arteries are in the process of vasculo- and angiogenesis; they are also thin and smaller in diameter. Of critical importance is the scarcity of data correlating structural and functional changes in these arteries with developmental maturation. To fill the gaps of existing knowledge and to gain a more complete understanding of the biological basis for the regulation of CBF and its dysregulation, we tested the hypothesis that, in addition to accretion of smooth muscle cells (SMCs) in cell layers with vessel thickening, significant changes in smooth muscle structure, as well as smooth muscle phenotype, occur in the media of CAs during the course of late fetal development that account for associated changes in contractility. Here we report quantitative and qualitative differences, as visualized by transmission electron microscopy (TEM), confocal microscopy, wide-field epifluorescence microscopy, wire myography, ELISA, and real-time quantitative PCR in the CAs of 95-gestational day (GD) fetuses, 140-GD (near-term) fetuses, and adult sheep.
MATERIALS AND METHODS
All experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the American Physiological Society “Guiding Principles for Research Involving Animals and Human Beings.” The experiments were approved by the Animal Care and Use Committee of Loma Linda University. We obtained pregnant and nonpregnant ewes (≥2 yr old) from Nebeker Ranch (Lancaster, CA); the pregnant ewes were carrying one or two fetuses of either sex at ∼95 ± 2 or ∼140 GD (n = 5 for each experimental group).
Tissue collection and processing for electron microscopy.
Initially, nonpregnant and pregnant ewes (and, thus, their fetuses) were anesthetized by an injection of pentobarbital sodium (10 mg/kg iv) and then by endotracheal delivery of a constant flow of isoflurane (1–2% in O2). Each fetus was delivered through a midline abdominal incision and supported on the belly of the anesthetized ewe, with umbilical vessels intact. The fetal heart was rapidly exposed through a midline thoracotomy, and a glass cannula prefilled with normal saline was inserted through the left ventricle and passed into the root of the aorta. The attached tubing was placed in a peristaltic pump (model 1215, Harvard Apparatus, Holliston, MA) and into an open reservoir containing ∼300 ml of physiological saline. In rapid succession, the peristaltic pump was activated, the descending aorta and umbilical cord were clamped, and the right ventricle was incised for outflow. Saline was delivered at a normal mean arterial blood pressure of ∼45 mmHg. Once the venous effluent was clear, the perfusate was changed to primary fixative (2.5% glutaraldehyde, 2% paraformaldehyde, 0.4% tannic acid, 1% dextrose, and 0.1 M sodium cacodylate) and 500–1,000 ml total volume. Then fetuses were weighed, and the brains, with CAs attached, were removed and stored in primary fixative until dissection later that day. Similarly, in nonpregnant ewes (n = 5), we performed a midline thoracotomy and provided mechanical ventilation until perfusion of fixative at a mean arterial pressure of ∼70 mmHg was completed. Main branch middle CAs (MCAs) were carefully stripped of their connective tissue investments and meningeal attachments under a dissecting microscope and stored overnight in primary fixative. On the following morning, each artery was submerged in a small pool of fixative on a dental wax plate and sliced with a razor blade under a dissecting microscope into ∼1-mm-thick transverse segments (rings). Slicing of arteries into segmental blocks revealed the relative fragility of the 95-GD arteries compared with 140-GD arteries in fetuses and adults. Arterial segments were placed in vials of fixative for an additional 2 h; then they were immersed for 5 min in 0.1 M sodium cacodylate buffer and, subsequently, for 2 h in 1% osmium tetroxide in the same buffer. After a brief rinse in distilled water, vessel rings were dehydrated through a graded ethanol series (70%, 85%, 95%, and 100%), passed through two changes of propylene oxide, and gradually infiltrated with epoxy resin (PolyBed 812, Polysciences, Warrington, PA) overnight in 1:1 resin-propylene oxide, for 2–6 h in 2:1 resin-propylene oxide, and for 30–60 min in 100% resin. Segments were then embedded in flat molds (Pelco 105, Ted Pella, Redding, CA) containing fresh resin and polymerized for 16–24 h in an oven at 60–70°C.
Tissue collection for confocal imaging, light microscopy, wide-field epifluorescence microscopy, Western immunoblotting, real-time PCR, and wire myography.
For each experiment, five or more animals were used; in the case of fetal twins, n was counted as one. Pregnant and nonpregnant ewes were anesthetized as described above. After the fetus was delivered by hysterotomy, the fetuses and ewes were killed with an overdose of the proprietary euthanasia solution Euthasol [pentobarbital sodium (100 mg/kg) and phenytoin sodium (10 mg/kg); Virbac, Ft. Worth, TX]. Studies were performed in isolated cerebral vessels cleaned of adipose and connective tissue. To avoid the complications of endothelial-mediated effects, we removed the endothelium by carefully inserting a small wire three times, as previously described (13, 14, 26).
Real-time PCR.
The real-time PCR method is described in detail elsewhere (11, 12). Briefly, we used an Allprep DNA/RNA mini kit (catalog no. 80204, Qiagen, Valencia, CA) according to the manufacturer's instructions to isolate and quantify RNA. Isolated mRNA was analyzed using a Nanodrop 2000c (Thermo Scientific, Wilmington, DE) at 260/280-nm wavelength to check for quality and quantity. A 260- to 280-nm ratio of 1.8–2 was accepted for quantification with real-time PCR. The mRNA was reverse-transcribed using the Quantitect RT kit, which includes a DNase treatment step before making cDNA (Qiagen). Real-time PCR was performed on a LightCycler (model 1.5, Roche, Indianapolis, IN) using the SYBR Green method. Primers were designed using Primer3, a web-based software, and Quantitect SYBR Green PCR Master Mix (Qiagen). Cycles required (based on initial mRNA of a particular gene) to reach a threshold detection limit by the real-time PCR were recorded and normalized to a housekeeping gene (GAPDH). We determined the mRNA fold changes with the cycle threshold (ΔΔCT) method after performing validation curves.
Confocal and epifluorescence imaging studies.
Using a laser scanning confocal imaging workstation (Olympus, Center Valley, PA) with an inverted microscope and standard techniques, we examined the expression of collagen, laminin, elastin, integrin, and several other proteins in CA segments. Briefly, a cryostat (Leica Microsystems, Buffalo Grove, IL) was used to slice arterial segments into 100-μm sections, which were sealed on microscope slides with Vectashield mounting medium containing 4′,6-diaminido-2-phenylindole (catalog no. H-1200, Vector Labs, Burlingame, CA). Images were recorded serially through the whole tissue thickness in ∼0.3-μm increments (1,024 × 1,024 pixels, 12-bit resolution) using a ×63 oil immersion Plan Apochromat (numerical aperture 1.4) objective. A pinhole size corresponding to an imaging depth of ∼0.6 μm helped us overlap the images through the z-axis. Images were analyzed using Image-Pro software (Media Cybernetics, Bethesda, MD), and protein expression was measured as fluorescence intensity per unit area normalized to fluorescence intensity per unit area of the α-smooth muscle actin control. All the antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Using epifluorescence microscopy (Carl Zeiss, Thornwood, NY), we examined smoothelin and proliferating cell nuclear antigen (PCNA) expression in 10-μm slices of arterial segments prepared with a cryostat (Leica Microsystems) with fluorophore-tagged antibodies (Invitrogen, Carlsbad, CA).
TEM observations and measurements.
Thin (90- to 100-nm) sections adjacent to the serial sections used for stereology (see below) were made with a diamond knife on an ultramicrotome (Sorvall MT-600, DuPont, Wilmington, DE), mounted on uncoated 300-mesh copper grids or on formvar-coated slot grids, stained for 10 min with uranyl acetate and for 3 min with lead citrate, and then observed on an electron microscope (model 10C, Carl Zeiss, Jena, Germany). For qualitative observations, most of the sections passed through the artery's axis, yielding transverse profiles of the circumferentially oriented SMCs, facilitating evaluation of cellular and extracellular details. For study of cellular connectivity at the ends of SMCs, blocks were oriented perpendicular to the vessel axis to provide sections with longitudinal cell profiles. Images of representative regions of the media in axial sections were digitized with a scanner (Duoscan T1200, Agfa-Gevaert, Mortsel, Belgium). The images were observed and measured using the Java version of NIH Image (ImageJ, Research Services Branch, National Institute of Mental Health, Bethesda, MD; http://rsb.info.nih.gov/ij/). We determined the following morphometric parameters on 25 SMC profiles per animal: nuclear cross-sectional area (CSA) as percentage of SMC CSA, nuclear heterochromatin CSA as percentage of nuclear CSA, total dense body profile CSA as percentage of SMC CSA, and basal lamina thickness. Using the particle-measuring capability of the ImageJ program, we determined percentage of extracellular matrix (ECM) CSA occupied by electron-dense structures (collagen fibrils, basal lamina material, and elastin) in five low-magnification fields per animal. Direct measurements were performed on digital enlargements of electron micrographs with original magnifications of ×1,600–20,000.
TEM stereology.
The “disector” principle, an unbiased stereological method for estimating the number and size of discrete objects, such as cells, in a random volume, has been applied to determine cell number and size in vascular smooth muscle (38). Our extension of this principle to TEM sections of developing main branch MCAs was a modification of a light microscopy method (31). Briefly, using an ocular reticule on a light microscope, we measured the minimum and maximum diameters of the vessel lumen optically on the five blocks selected randomly and averaged the results to obtain vessel mean lumen diameter. Each block was rotated around the vessel axis, and a series of axial serial sections were cut on an ultramicrotome. The first (top) section and the last (bottom) section of the series were cut at a thickness of 100 nm, mounted on a formvar-coated slot grid, and stained. Intervening 100- or 200-nm-thick sections had a combined thickness of 8–10 μm. We displayed electron micrographs of the vessel wall in the top and bottom sections of the series at a calibrated enlargement on a 21-inch monitor using an image editor (Photoshop version 6.0, Adobe Systems, San Jose, CA) and measured media thickness at the calibrated magnification. Then, while toggling between the two images, we digitally aligned the images by superimposing identifiable cell-association patterns. An unbiased rectangular counting frame (22), which fit entirely within the media of the displayed vessel wall, was digitally drawn on each of the aligned sections. Thereby, two disectors were defined as the volumes created by projections of the counting frame from the top section to the bottom section of the series and from the bottom section to the top section, respectively. SMC nuclei that appeared within counting frames on either section were counted as nuclear ends if they did not appear in the digitally aligned opposite section. Because SMCs have a single nucleus (31), we calculated the mean number of SMCs per unit volume of media (i.e., the SMC numerical density) by dividing half the total number of nuclear ends by the total disector volume. We calculated the number of SMCs per unit vessel length by multiplying the SMC numerical density by the media CSA. We then determined the proportion of the media (i.e., volume fraction) occupied by SMCs by point counting in the first section. From the above measurements and calculated results, we calculated average cell volume. In addition, counting cell profiles in the top section and the number of nuclear profiles in the top and bottom sections permitted us to calculate nucleus length, cell length, cell CSA, and the number of cell layers. The equations used for these calculations are shown in Table 1.
Table 1.
Stereology parameters and formulas
| Parameter | Symbol | Formula | Units |
|---|---|---|---|
| Media thickness | m1 | μm | |
| Media CSA | al | m2 | |
| Cell CSA | ac | m2 | |
| No. of cell layers | Nc | — | |
| SMC numerical density | Nv | m−3 × 10−3 | |
| SMC no. per unit arterial length | Nl | mm−1 | |
| Cell length | lc | m | |
| Cell volume | vc | m3 | |
| Average nucleus length | ln | m |
SMC, smooth muscle cell; CSA, cross-sectional area; ct, number of cells in top section of disector ends; ll, lumen diameter (m) of disector; hd, height of disector; nb, no. of nuclei in bottom section of disector media; nd, downward-pointing nuclear end; nt, no. of nuclei in top section; nu, upward-pointing nuclear end; Vd, disector volume (m−3); Vv, volume fraction of SMCs in media. Equations are from Refs. 20 and 30.
Wire myography.
CA rings (4 mm long) were suspended in organ baths (Radnoti Glass Instruments, Monrovia, CA) that contained 5 or 10 ml of modified Krebs-Henseleit buffer maintained at 37°C and aerated with 95% O2-5% CO2 (pH 7.4). Each ring was suspended between two tungsten wires passed through the lumen: one wire was anchored to the glass hook at the bottom of the organ chamber, and the other was connected to a tissue hook attached to a low-compliance force transducer (Radnoti Glass Instruments) for the measurement of isometric force (14). The transducers were connected to an analog-to-digital data interface (Powerlab 16/30, ADInstruments, Colorado Springs, CO) attached to a computer, and the data were stored on magnetic or optical media for later analysis. At the beginning of each experiment, vessels were equilibrated without tension for 0.5–1 h, as described previously (13–15, 28, 29).
Statistics.
Values are means ± SE. Differences between groups were evaluated for significance using one-way ANOVA and Newman-Keuls post hoc analysis. P < 0.05 was considered significant. For each experiment, five animals were included in each group.
RESULTS
Contractile response to 10 μM phenylephrine and 122 mM KCl.
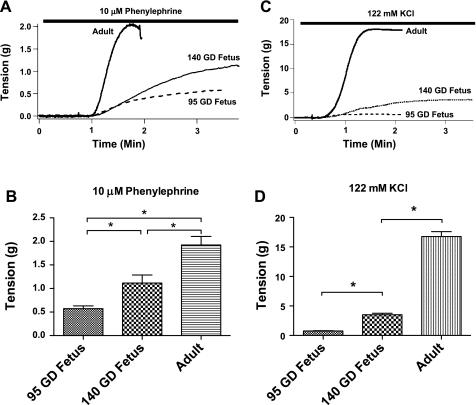
We observed a significant increase in contractile response with both agonists with developmental maturation (Fig. 1).
Fig. 1.
A and C: contractile responses to 10 μM phenylephrine and 122 mM KCl. Representative traces show phenylephrine and KCl stimulation of cerebral arteries from 95- and 140-gestational day (95 GD and 140 GD) fetuses and adults. B and D: tension generated by each group of arteries after stimulation with phenylephrine and KCl. Values are means ± SE; n = 5 in each group. *Significant difference by ANOVA and post hoc Newman-Keuls analysis.
Ultrastructure of the vessel wall.
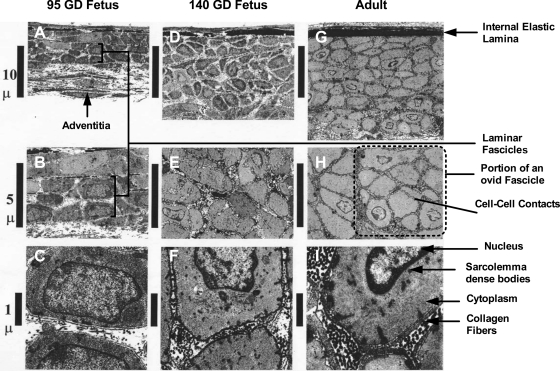
By TEM of axial longitudinal sections of full vessel wall thickness, we compared cell-to-cell associations, cell-to-ECM associations, and cell architecture of medial smooth muscle myocytes at the two fetal stages (95 and 140 GD) and in the adult. As demonstrated in Fig. 2, in the longitudinal plane, medial SMCs were cut perpendicular to their long axes, which course circumferentially around the vessel. Thus they appear as transverse profiles at various levels along their length (Fig. 2). As shown in Fig. 2, the tunica adventitia was less dense in the 95-GD than the 140-GD fetus (compare Fig. 2A with Fig. 2D). In the adult arteries, the tunica adventitia (only a small amount of which appears in Fig. 2G) was thickened still further, and its longitudinal collagen fibrils were denser (i.e., less cellular component). In the 95-GD fetus, the tunica media comprised concentric layers of somewhat radially flattened SMCs (Fig. 2, A and B) and was two-thirds the thickness of the that in the 140-GD fetus (Fig. 2D) and only one-half the thickness of that in the adult (Fig. 2G). By 140 GD, the SMC arrangement had changed from concentric layers to a mosaic of ovoid cell clusters (fascicles in 3 dimensions) of variable axial and radial thicknesses and variable numbers of SMCs (Fig. 2, D and E). This mosaic arrangement of ovoid fascicles also was seen in the media of the adult vessels (Fig. 2, G and H). However, these fascicle boundaries were made somewhat less distinct by increased accumulation of ECM components between and within the fascicles (Fig. 2I). In the 95-GD fetus, the internal elastic lamina (IEL) of vessels, densely stained by the tannic acid component of the primary fixative, was approximately one-half the thickness of the IEL of vessels in the 140-GD fetus (Fig. 2D) or the adult (Fig. 2G). The tunica intima of the adult arteries was distinguished from that of both fetal groups by the presence of an irregular layer of obliquely oriented intimal SMCs between the endothelium and the IEL (Fig. 2G).
Fig. 2.
Transmission electron microscopy images of arterial walls of main branch middle cerebral arteries in axial longitudinal section. Original magnification: ×1,600 (A, D, and G), ×8,000 (B, E, and H), and ×10,000 (C, F, and I).
At intermediate magnification (Fig. 2; ×8,000 on the microscope, scale bar 5 μm), the entire thickness of the media of vessels from 95-GD fetuses could be visualized (Fig. 2B); however, only half of the thickness of the media of vessels from 140-GD fetuses (Fig. 2E) or adults (Fig. 2H) was contained in a single field of view. At this magnification, by 95 GD, rather than an aggregate of single cellular units, the media consisted of bundles or fascicles of SMCs (Fig. 2B). Each fascicle is ensheathed by a basal lamina. Within the fascicle in the 95-GD fetus, we frequently observed contacts between cells (Fig. 2, A and B). These were less commonly seen at 140 GD (Fig. 2, D and E) and were not observed in the adult (Fig. 2, G and H). We observed no cell contacts between fascicles in any age group. In all three groups, the contacts among medial SMCs were the simple apposition type, defined as sites at which the plasma membranes of closely opposed cells run parallel (21, 37). Adult media (Fig. 2, G and H) also comprised fascicles, but, because of the greater accumulation of collagen and strands of basal lamina material in the ECM, their boundaries were more difficult to discern. In addition, sarcolemma-associated dense bodies were obvious in the transversely sectioned SMCs of 140-GD fetuses and adults (Fig. 2, F and I). Although they were also present in interphase SMCs at 95 GD, these sarcolemma-associated dense bodies were fewer and smaller. These dense bodies also were more numerous in 140-GD fetus and adult than 95-GD fetus SMCs. In Fig. 2, C, F, and I, the ECM located between CA SMCs is compared in a 95-GD fetus, a 140-GD fetus, and an adult sheep. In vessels from the 95-GD fetus, collagen fibrils are sparse and appear to insert into the fragmentary basal lamina. In contrast, in the adult vessel (Fig. 2I), collagen fibrils are considerably denser and appear to link adjacent SMCs via the basal lamina.
Moreover, in the 95-GD fetal MCA, the CA SMC is dominated by the nucleus, which occupies a large proportion of the cell (Fig. 2C). At this age, the surrounding cytoplasmic rim contains a relatively large amount of synthetic and secretory machinery. In contrast, in the 140-GD fetus and adult (Fig. 2, F and I), SMCs have a smaller, more heterochromatic nucleus and more tightly packed myofilaments. Greater amounts of collagen fibrils and basal lamina material are contained in the intrafascicular ECM of the 140-GD fetus and adult than the 95-GD fetus.
Morphometric results.
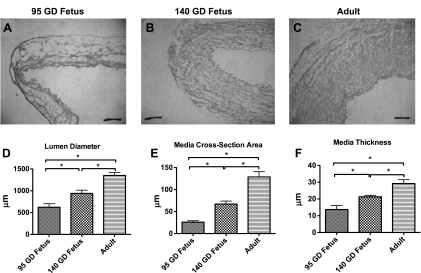
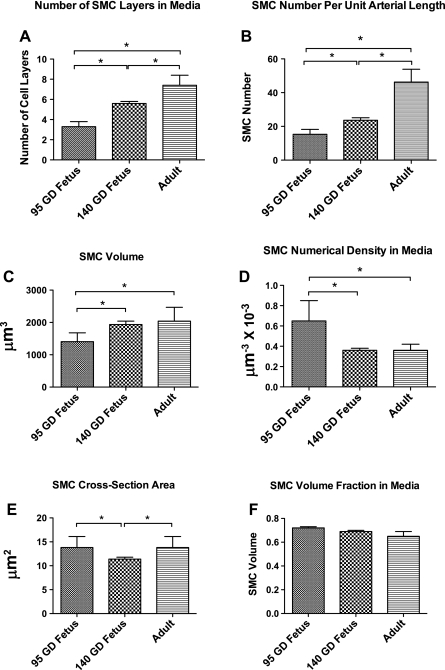
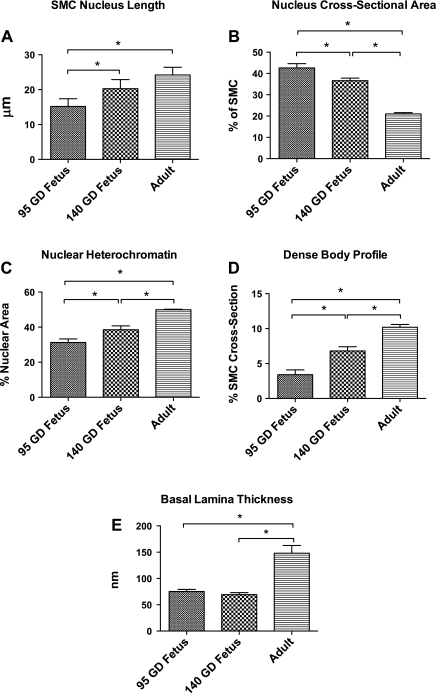
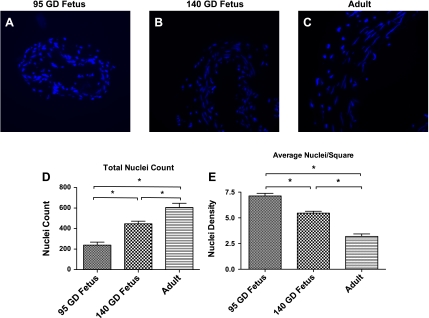
Table 2 and Fig. 3 demonstrate the results of light-microscopic measurement of artery lumen diameter, media thickness, and media CSA. Also, Table 2 and Figs. 4 and 5 demonstrate the measurements of SMC and ECM profiles by digitized electron micrographs, and Fig. 6 depicts the measurements of nuclear density and overall nuclear counts in a SMC cross section by wide-field epifluorescence microscopy. Maturational development was associated with an increase in lumen diameter (Fig. 3D), media CSA (Fig. 3E), media thickness (Fig. 3, A–C and F), and the number of SMC layers in the media (Fig. 4A), as well as the number of SMCs per unit arterial length (Fig. 4B). With development, SMC density decreased (Fig. 4D); however, total SMC volume increased (Fig. 4C). Moreover, SMC CSA was reduced in the 140-GD fetus compared with the 95-GD fetus and adult (Fig. 4E), whereas the fraction of SMC volume in total media remained constant (Fig. 4F). There was also an increase in SMC nucleus length with age (Fig. 5A), but the nuclear CSA was reduced significantly with development (Fig. 5B). Nucleus heterochromatin as percentage of nuclear area was increased with age (Fig. 5C). We also observed an increase in total nuclei in the arterial wall with age (Fig. 6, A–D). However, average nuclear density was reduced significantly with development from the 95-GD fetus to the 140-GD fetus to the adult (Fig. 6E). As also noted in Table 2, the fraction of the media ECM profile area occupied by electron-dense ECM components (including elastin, fibrillar collagen, and basal lamina material) increased by 38% between the 95- and 140-GD fetus and by an additional 22% between the 140-GD fetus and the adult. Finally, measurements of basal lamina thickness in the three groups showed a significant increase in thickness between fetal and adult CAs; nonetheless, at the earlier age, the basal laminae appeared patchy and incomplete; measurements are provided in Table 2.
Table 2.
Transmission electron microscopy measurements and stereological analysis of media, medial SMCs, and medial ECM components of ovine middle cerebral artery
| Fetus |
|||
|---|---|---|---|
| 95 GD | 140 GD | Adult | |
| Lumen | |||
| Diameter, μm | 623 ± 80a,e | 941 ± 73b (52) | 1,351 ± 68 (44) |
| Vessel wall | |||
| Media thickness, μm | 13.7 ± 2.3a,f | 21.3 ± 0.9c (55) | 29.2 ± 2.3 (37) |
| Media CSA, μm2 × 103 | 26.0 ± 3.2a,d | 67.1 ± 6.8b (158) | 128.7 ± 12.0 (92) |
| No. of SMC layers in media | 3.3 ± 0.5b,d | 5.6 ± 0.2 (70) | 7.4 ± 1.0 (32) |
| SMC volume fraction in media | 0.72 ± 0.01 | 0.69 ± 0.01 (−4) | 0.65 ± 0.04 (−5) |
| SMC numerical density in media, μm−3 × 10−3 | 0.65 ± 0.20 | 0.36 ± 0.02c (−45) | 0.36 ± 0.06 |
| SMC no. per unit arterial length, μm−1 | 15.3 ± 2.9b,f | 23.6 ± 1.5c (54) | 46.3 ± 7.6 (96) |
| SMC | |||
| Length, μm | 102 ± 16c,e | 171 ± 14 (68) | 151 ± 15 (−12) |
| CSA, μm2 | 13.8 ± 2.3 | 11.4 ± 0.4 (−17) | 13.8 ± 2.3 (21) |
| Volume, μm3 | 1,407 ± 271 | 1,937 ± 105 (38) | 2,042 ± 427 (5) |
| Nucleus length, μm | 15.2 ± 2.2c | 20.3 ± 2.6 (34) | 24.2 ± 2.2 (19) |
| Nucleus as %SMC (midlength) CSA | 42.6 ± 2.0a,f | 36.6 ± 1.2a (−14) | 21.0 ± 0.6 (−43) |
| Nuclear heterochromatin as %nuclear area | 31.29 ± 2.0a,f | 38.5 ± 2.2b (24) | 49.9 ± 0.3 (30) |
| Dense body profiles as %SMC CSA | 3.4 ± 0.7a,d | 6.8 ± 0.6b (100) | 10.2 ± 0.4 (50) |
| Basal lamina thickness, nm | 75.3 ± 4.0b | 69.2 ± 3.8b (−8) | 148.0 ± 14.8 (114) |
| ECM | |||
| Electron-dense structures as %ECM CSA | 44.7 ± 3.9a,e | 61.8 ± 2.5b (38) | 75.4 ± 2.5 (22) |
Values are means ± SE of 5 in each group; percent change from 95-gestational day (GD) fetus is shown in parentheses. Electron-dense structures include collagen fibrils, basal lamina material and elastin. ECM, extracellular matrix. Significantly different from adult:
P < 0.001;
P < 0.01;
P < 0.05. Significantly different from 140 GD:
P < 0.001;
P < 0.01;
P < 0.05.
Fig. 3.
A–C: light microscopy images of cerebral arteries from 95-GD fetus, 140-GD fetus, and adult. D–F: lumen diameter, media cross-sectional area, and media thickness of cerebral arteries from 95-GD fetus, 140-GD fetus, and adult. Values are means ± SE; n = 5 in each group. Exact values and percent differences are given in Table 2. *Significant difference (P < 0.05) by 1-way ANOVA and post hoc Newman-Keuls analysis.
Fig. 4.
Smooth muscle cell (SMC) profile by digitized electron micrographs. Values are means ± SE; n = 5 in each group. Exact values and percent differences are given in Table 2. *Significant difference (P < 0.05) by 1-way ANOVA and post hoc Newman-Keuls analysis.
Fig. 5.
Nuclear profile (A–C), dense bodies measurement (D), and basal lamina thickness measurement (E) by digitized electron microscopy. Values are means ± SE; n = 5 in each group. Exact values and percent differences are given in Table 2. *Significant difference (P < 0.05) by 1-way ANOVA and post hoc Newman-Keuls analysis.
Fig. 6.
Nuclear count and density per 18-mm2 area examined by wide-field epifluorescence microscopy. A–C: exemplary images. D: total nuclei count in a cross-sectional area. E: nuclei per 18-mm2 area. Values are means ± SE; n = 5 in each group. *Significant difference (P < 0.05) by 1-way ANOVA and post hoc Newman-Keuls analysis.
Phenotypes of SMC.
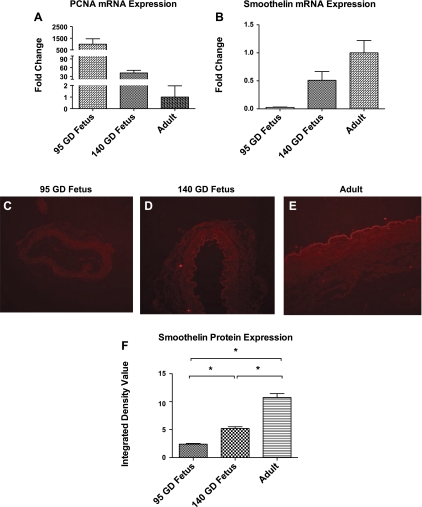
The present study demonstrates a significant decrease in mRNA expression of PCNA, a marker of the proliferative SMC phenotype, with maturation (Fig. 7A). Moreover, a significant increase in mRNA expression of the contractile phenotypic marker smoothelin was observed with maturation, as measured by real-time quantitative PCR (Fig. 7B). In a previous study, we demonstrated a similar significant reduction in PCNA protein expression by Western immunoblot analysis (41). Also, we observed a significant increase in smoothelin protein levels with maturation by wide-field epifluorescence microscopy and Western immunoblotting (Fig. 7, C–F).
Fig. 7.
A and B: fold change in smoothelin (a marker of contractile phenotype) and proliferating cell nuclear antigen (PCNA; a marker of synthetic SMC phenotype) mRNA expression examined by quantitative real-time PCR in 95- and 140-GD fetus compared with adult. C–E: exemplary wide-field epifluorescence microscopy images of smoothelin expression. F: relative integrated density (arbitrary unit) of smoothelin expression.
ECM of vessel wall.
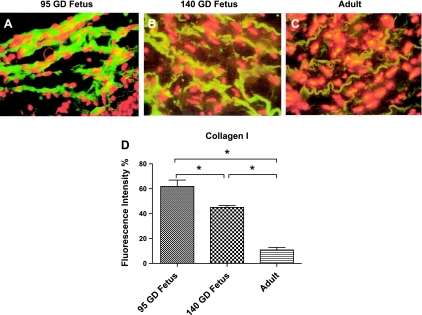
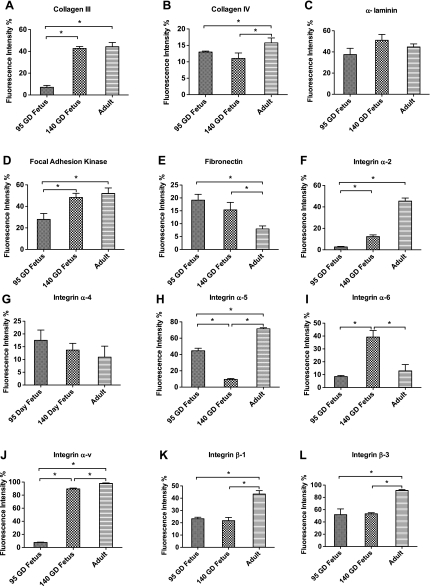
Using laser scanning confocal microscopy, we examined the expression of different collagens and integrins in the vessel wall from a 95-GD fetus, a 140-GD fetus, and an adult. Collagen I (Fig. 8) and fibronectin (Fig. 9E) decreased significantly with development. In contrast, expression of collagen III (Fig. 9A) and focal adhesion kinase (Fig. 9D) was significantly higher in the 140-GD fetus and adult than the 95-GD fetus. Moreover, collagen IV expression (Fig. 9B) was significantly higher in the adult than both fetal age groups. Integrin-α2 (Fig. 9F) and integrin-αv (Fig. 9J) expression was increased with age. Integrin-α5 (Fig. 9H) expression was significantly lower in the 140-GD day fetus than the 95-GD fetus and adult. In contrast, integrin-α6 expression was significantly higher in the 140-GD fetus than the 95-GD fetus and adult (Fig. 9I). Also, integrin-β1 (Fig. 9K) and integrin-β3 (Fig. 9L) expression was significantly increased in the adult compared with the 95- and 140-GD fetus.
Fig. 8.
A–C: exemplary confocal microscopy images showing expression of collagen III (green) in cerebral arterial segments from 95-GD fetus, 140-GD fetus, and adult. D: fluorescence intensity as percentage of control. Values are means ± SE; n = 5 in each group. *Significant difference (P < 0.05) by 1-way ANOVA and post hoc Newman-Keuls analysis.
Fig. 9.
Extracellular matrix and integrin profiles from confocal microcopy in 95-GD fetus, 140-GD fetus, and adult. Values are means ± SE; n = 5 in each group. *Significant difference (P < 0.05) by 1-way ANOVA and post hoc Newman-Keuls analysis.
DISCUSSION
Premature infants are at severalfold higher risk of germinal matrix hemorrhage and stroke, with development of cerebral palsy and other disorders, than full-term neonates. Failure of the major regulators of the CBF, such as anterior CAs, MCAs, and posterior CAs, has been implicated in these disorders. Moreover, being the major artery of the cerebral circulation, the MCA is more commonly involved in stroke. Additionally, the MCA also is commonly involved in cerebral aneurysms in the pediatric age group (8). The structural immaturity may be an important player in such aneurysms. Also, evidence supports the notion that blood flow velocity of the MCA is a predictor of cerebral palsy in infants (7). Other than signaling pathways, higher fragility of the cerebral vasculature of the preterm infants is an important factor in the genesis of these disorders. In previous studies, we reported changes in several important signaling molecules, as well as cytoskeletal proteins (14, 15, 26, 41). In the present studies, we extend our findings and report important structural differences in ECM, cell adhesion molecules, and SMC phenotypes, as well as cerebral vessel ultrastructure and related changes in contractility. Physiologically, we demonstrate a graded increase in phenylephrine- as well as KCl-induced contractile response with development from premature fetus to near-term fetus to adult (Fig. 1). In a previous study, we demonstrated a similar increase in contractile response induced by phenylephrine, serotonin, and the PKC agonist phorbol 12,13-dibutyrate with maturation from fetus to adult (13–16, 27, 34, 41). However, the major aim of the present studies was to identify the structural basis of the functional differences in the CAs with development as demonstrated by others and us (13–15, 23, 29).
The reduced contractile ability of premature fetal vessels to adrenergic stimulation may be a factor in increased intracerebral bleeding after rupture/injury of cerebral vessels. Also, we observed significantly greater fragility in vessels of the premature fetus than the near-term fetus and adult. Increased fragility of the premature fetal vessels may be a consequence of reduced collagen III (Fig. 9A) and focal adhesion molecules (Fig. 9D) in the premature fetus compared with the near-term fetus and adult. We also observed a reduced expression of integrin-α2, -α6, and -αv in the premature fetus compared with the near-term fetus and adult. Integrins play a crucial role in cell adhesion as well as stabilization. Furthermore, we found increased expression of collagen I (Fig. 8) and fibronectin (Fig. 9E) in the premature fetus compared with the near-term fetus and adult. The specific roles of these vital extracellular moieties in premature fetal CAs are not known and require further investigation. Nonetheless, these molecules are known to play a crucial role in providing tensile strength and cell-to-cell adhesion, which may contribute to increased fragility of 95-GD fetal vessels compared with 140-GD fetal and adult vessels.
In terms of overall arterial structure, in the change from 95- to 140-GD ovine fetus (equivalent to ∼27 and 40 wk gestation, respectively, in humans) to adult, the increase in media thickness, media CSA, and number of SMC layers, while perhaps not unexpected, was quite dramatic (Figs. 3 and 4; Table 2). Also, in terms of media structure, the significant increase with maturation in SMC number per unit arterial length was large. In the present study, although SMC length, CSA, and volume increased slightly from the fetus to the adult, these changes were not significant (Table 2). The results on media thickness are in agreement with a report of an ∼22% increase in overall MCA thickness from the 140-GD ovine fetus to the adult (35). Nonetheless, they differ significantly from another report of almost a doubling of SMC length and width in fresh unstressed segments of ovine MCA by light microscopy (5). The significant decrease in the ratio of nucleus to cell size, the increase in dense body profiles, and the marked thickening and more uniform basal lamina also were most striking. Of particular interest was the significant decrease of relative nuclear size in relation to cell volume: 14% from 95 to 140 GD and ∼43% from full-term fetus to adult (Table 2). This was associated with a marked increase in myofilaments and, from 95 to 140 GD, a 100% increase in the fraction of the SMC CSA occupied by dense body profiles, with a further 50% increase in the adult (Table 2). In rat aortas, the basal lamina is relatively scant at birth (4, 17). Basal lamina thickening has also been noted in developing guinea pig tracheal muscle (5, 9). In the present study, SMC basal lamina thickness increased 114% from 140 GD to the adult (Table 2). Particularly striking in this regard was the change from an “incomplete and patchy” basal lamina at 95 GD to a more complete uniform appearance by 140 GD. Much of this increase was probably due to accretion of collagen type III, proteoglycan, lamin-1, entactin/nidogen, fibronectin, and probably other proteins (30, 40).
In addition, and potentially of considerable biological importance, was the age-related increase in ECM, particularly in regard to the fraction of ECM occupied by collagen fibrils and basal lamina-like material (Figs. 8 and 9; Table 2). In muscular arteries such as the MCA of the adult, the vascular extracellular space averages ∼20% of smooth muscle volume (24). We are unaware of other studies on changes in the ECM with development. The present study suggests that while the volume of extracellular space per unit media CSA did not change dramatically, the fraction occupied by electron-dense structures, including collagen type III fibrils and basal lamina material, increased 38% from 95 to 140 GD and increased again by 22% in the adult (Table 2).
Also of note was the arrangement of CA SMCs into fascicles, which presumably play a critical role in coordinated maintenance of myogenic tone (10) and contraction. Such fascicles appear as transversely transected, closely packed aggregates of commonly oriented SMCs, each ensheathed by a basal lamina. Importantly, from the 95- to the 140-GD fetus, there was a change in arrangements of SMC fascicles from concentric/lamellar bands to ovoid bundles, suggesting that these represent sequential forms of fascicular development. The specific collagen fibers and integrin also probably play a critical role in specific arrangements of SMCs during early fetal life. Several decades ago, Clark and Glagov (4) described musculoelastic fascicles in vascular media of elastic and muscular arteries, but this structural feature has received little attention. As observed by these authors, the organization of these fascicles suggests their role in the response to tensile stress by groups of SMCs (4, 17). Without doubt, such maturational morphological changes are of vital importance to the ability of these vessels to maintain vascular tone and to regulate blood pressure to withstand major blood pressure surges and other hemodynamic stress.
Several workers have described the architecture of the arterial wall (3), as well as the ultrastructure of SMCs per se (9). Nonetheless, surprisingly few reports have described the ontogeny of SMC development in a particular vascular bed. The present studies provide evidence of an increase in “contractile” cells compared with the “synthetic” phenotype with maturation. Other studies suggest that, with maturation, synthetic SMCs have the capability to switch to the contractile phenotype (6, 36). In ovine thoracic and abdominal aortas, extensive vessel wall remodeling has been shown to occur during postnatal life (25). Similar to the present study, in ovine carotid arteries, Hutanu and colleagues (23) reported increased contractility associated with soluble protein, actin and myosin, filamin, adult smooth muscle myosin heavy chain (MHC) type 2 (SM2) and medial wall thickness and reciprocal decreases in nonmuscle MHC-B, PCNA, and medial cellular density with development from the 88-GD to full-term fetus, newborn, and adult. In the same study, they reported reduced non-receptor- and receptor-mediated contractions in midgestation and an increase thereafter, paralleling the transition from synthetic to contractile vascular smooth muscle phenotype. The present study further provides a structural and morphological basis for such physiological changes. Also, in near-term ovine fetus, Arens and co-workers (1) demonstrated biochemical and functional immaturity of the aorta and femoral artery compared with umbilical and adult vessels. In the female fetus and newborn lamb, these investigators also demonstrated significant differences in biochemical maturation between the aorta SMCs and bladder SMCs (2). These and the present studies underscore the heterogeneity among various smooth muscle types. In ovine CAs, we and others also described other aspects of developmental-associated changes in composition and function (27, 35, 41).
The question arises: to what extent are these changes with development physiologically relevant in terms of structure-function relations? In several studies during the past decade, Harris, Koehler, and colleagues (18–20) reported on the regulation of CBF in 93-GD (0.6 gestation) and 133-GD (0.9 gestation) fetal sheep. Over this developmental time span, CBF measured with radioactive-labeled microspheres increased from 59 ± 6 to 148 ± 23 ml·min−1·100 g−1, while cerebral O2 consumption increased from 53 ± 4 to 150 ± 13 μmol·min−1·100 g−1, each function increasing almost threefold (18). Nitric oxide synthase activity increased to a similar degree, from 0.6 to 0.9 gestation, presumably exerting a basal vasodilatory effect through the production of nitric oxide (33). One might postulate that the structural changes reported in the present study, i.e., increase in lumen diameter, media thickness, CSA, and number of cell layers, more dense ECM, and so forth, are important components in association with the increase in CBF.
Perspectives and Significance
To our knowledge, the present studies are the first to quantify many of the structural and ultrastructural features of CAs, including their fascicles of SMCs, as they develop from the immature to the more mature fetus, and to the adult, and relate these to the increase in contractility. These reflect a progression from more synthetic and poorly connected SMCs at 95 GD to more functionally linked cells at 140 GD and robustly linked cells in the adult. Undoubtedly, these CA structural differences are of vital importance to the development and integrity of the mature vessel and its role in the regulation of tone, blood pressure, and blood flow. From a clinical perspective, the morphological changes in the CA with maturation from the preterm to full-term fetus and on to the adult would appear to have considerable biological importance. Quite obviously, the present study raises a number of important questions. To what extent do these findings in the ovine cerebral vasculature apply to the human? What does SMC organization into fascicles contribute to vascular function? What role does the ECM play in arterial function? By what cellular and subcellular mechanisms is this maturational development of CAs regulated? These and other questions provide a challenge for investigators of the developing cerebral vasculature.
GRANTS
This study was supported by National Institute of Child Health and Human Development Grant HD-03807 (to L. D. Longo) and by funds from the Loma Linda University Department of Human Pathology and Anatomy (to D. A. Henderson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.G., D.A.H., N.C., and L.D.L. analyzed the data; R.G., D.A.H., N.C., and L.D.L. interpreted the results of the experiments; R.G., D.A.H., and N.C. prepared the figures; R.G. and L.D.L. drafted the manuscript; R.G. and L.D.L. edited and revised the manuscript; R.G., D.A.H., N.C., and L.D.L. approved the final version of the manuscript; D.A.H., N.C., and L.D.L. performed the experiments; L.D.L. is responsible for conception and design of the research.
ACKNOWLEDGMENTS
We thank Fred N. Lotgering for assistance in perfusing the initial animals used in these studies, Giovanni Longo and Dipali Goyal for technical assistance, and Brenda Kreutzer for assistance in preparing the manuscript.
REFERENCES
- 1. Arens Y, Chapados RA, Cox BE, Kamm KE, Rosenfeld CR. Differential development of umbilical and systemic arteries. II. Contractile proteins. Am J Physiol Regul Integr Comp Physiol 274: R1815–R1823, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Arens YH, Rosenfeld CR, Kamm KE. Maturational differences between vascular and bladder smooth muscle during ovine development. Am J Physiol Regul Integr Comp Physiol 278: R1305–R1313, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bohr D, Somlyo A. (Editors). Handbook of Physiology. The Cardiovascular System. Vascular Smooth Muscle. Bethesda, MD: Am. Physiol. Soc., 1980, sect. 2, vol. II [Google Scholar]
- 4. Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis 5: 19–34, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Elliott CF, Pearce WJ. Effects of maturation on cell water, protein, and DNA content in ovine cerebral arteries. J Appl Physiol 79: 831–837, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Frid MG, Moiseeva EP, Stenmark KR. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res 75: 669–681, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Fukuda S, Kato T, Kuwabara S, Kato I, Futamura M, Togari H. The ratio of flow velocities in the middle cerebral and internal carotid arteries for the prediction of cerebral palsy in term neonates. J Ultrasound Med 24: 149–153, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Fulkerson DH, Voorhies JM, Payner TD, Leipzig TJ, Horner TG, Redelman K, Cohen-Gadol AA. Middle cerebral artery aneurysms in children: case series and review. J Neurosurg Pediatr 8: 79–89, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Gabella G. Ultrastructure of the tracheal muscle in developing, adult and ageing guinea-pigs. Anat Embryol 183, 71–79, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Geary GG, Osol GJ, Longo LD. Development affects in vitro vascular tone and calcium sensitivity in ovine cerebral arteries. J Physiol 558: 883–896, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goyal R, Galffy A, Field SA, Gheorghe CP, Mittal A, Longo LD. Maternal protein deprivation: changes in systemic renin-angiotensin system of the mouse fetus. Reprod Sci 16: 894–904, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod Sci 17: 227–238, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Goyal R, Mittal A, Chu N, Arthur RA, Zhang L, Longo LD. Maturation and long-term hypoxia-induced acclimatization responses in PKC-mediated signaling pathways in ovine cerebral arterial contractility. Am J Physiol Regul Integr Comp Physiol 299: R1377–R1386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goyal R, Mittal A, Chu N, Shi L, Zhang L, Longo LD. Maturation and the role of PKC-mediated contractility in ovine cerebral arteries. Am J Physiol Heart Circ Physiol 297: H2242–H2252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goyal R, Mittal A, Chu N, Zhang L, Longo LD. α1-Adrenergic receptor subtype function in fetal and adult cerebral arteries. Am J Physiol Heart Circ Physiol 298: H1797–H1806, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyal R, Papamatheakis D, Loftin M, Vranchken K, Dawson A, Osman N, Blood AB, Pearce W, Longo L, Wilson S. Long-term maternal hypoxia: the role of extracellular Ca2+ entry during serotonin-mediated contractility in fetal ovine pulmonary arteries. Reprod Sci 18: 948–962, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyton JR, Lindsay KL, Dao DT. Comparison of aortic intima and inner media in young adult versus aging rats. Stereology in a polarized system. Am J Pathol 111: 234–246, 1983 [PMC free article] [PubMed] [Google Scholar]
- 18. Harris AP, Helou S, Gleason CA, Traystman RJ, Koehler RC. Fetal cerebral and peripheral circulatory responses to hypoxia after nitric oxide synthase inhibition. Am J Physiol Regul Integr Comp Physiol 281: R381–R390, 2001. 19. [DOI] [PubMed] [Google Scholar]
- 19. Harris AP, Koehler RC, Gleason CA, Jones MD, Traystman RJ. Cerebral and peripheral circulatory responses to intracranial hypertension in fetal sheep. Circ Res 64: 991–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Harris AP, Koehler RC, Nishijima MK, Traystman RJ, Jones MD. Circulatory dynamics during periodic intracranial hypertension in fetal sheep. Am J Physiol Regul Integr Comp Physiol 263: R95–R102, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Henderson RM. Types of cell contacts in arterial smooth muscle. Experientia 31: 103–105, 1975 [DOI] [PubMed] [Google Scholar]
- 22. Howard V. Unbiased Stereology: Three Dimensional Measurement in Microscopy. New York: Springer-Verlag, 2005 [Google Scholar]
- 23. Hutanu C, Cox BE, DeSpain K, Liu XT, Rosenfeld CR. Vascular development in early ovine gestation: carotid smooth muscle function, phenotype, and biochemical markers. Am J Physiol Regul Integr Comp Physiol 293: R323–R333, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kao C. Cellular Aspects of Smooth Muscle. Cambridge, UK: Cambridge University Press, 1997 [Google Scholar]
- 25. Langille B. Remodeling of developing and mature arteries: endothelium, smooth muscle, and matrix. J Cardiovasc Pharmacol 21: S11–S17, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Long W, Zhang L, Longo LD. Cerebral artery sarcoplasmic reticulum Ca2+ stores and contractility: changes with development. Am J Physiol Regul Integr Comp Physiol 279: R860–R873, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Longo LD, Ueno N, Zhao Y, Pearce WJ, Zhang L. Developmental changes in α1-adrenergic receptors, IP3 responses, and NE-induced contraction in cerebral arteries. Am J Physiol Heart Circ Physiol 271: H2313–H2319, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Longo LD, Ueno N, Zhao Y, Zhang L, Pearce WJ. NE-induced contraction, α1-adrenergic receptors, and Ins(1,4,5)P3 responses in cerebral arteries. Am J Physiol Heart Circ Physiol 270: H915–H923, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Longo LD, Zhao Y, Long W, Miguel C, Windemuth RS, Cantwell AM, Nanyonga AT, Saito T, Zhang L. Dual role of PKC in modulating pharmacomechanical coupling in fetal and adult cerebral arteries. Am J Physiol Regul Integr Comp Physiol 279: R1419–R1429, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Miosge N. The ultrastructural composition of basement membranes in vivo. Histol Histopathol 16: 1239–1248, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Mulvany MJ, Baandrup U, Gundersen HJ. Evidence for hyperplasia in mesenteric resistance vessels of spontaneously hypertensive rats using a three-dimensional disector. Circ Res 57: 794–800, 1985 [DOI] [PubMed] [Google Scholar]
- 32. Nelson KB. Can we prevent cerebral palsy? N Engl J Med 349: 1765–1769, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Northington FJ, Tobin JR, Harris AP, Traystman RJ, Koehler RC. Developmental and regional differences in nitric oxide synthase activity and blood flow in the sheep brain. J Cereb Blood Flow Metab 17: 109–115, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Papamatheakis DG, Vemulakonda S, Blood Q, Goyal R, Rubalcava M, Vrancken K, Bennett A, Dawson A, Osman NJ, Blood AB, Pearce WJ, Longo LD, Wilson SM. Preservation of serotonin-mediated contractility in adult sheep pulmonary arteries following long-term high-altitude hypoxia. High Alt Med Biol 12: 253–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pearce WJ, Hull AD, Long DM, Longo LD. Developmental changes in ovine cerebral artery composition and reactivity. Am J Physiol Regul Integr Comp Physiol 261: R458–R465, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Rensen SSM, Doevendans PAFM, van Eys GJJM. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15: 100–108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sosa-Melgarejo JA, Berry CL. Contact relationships between vascular smooth muscle cells: an in vivo and in vitro study. J Pathol 157: 213–217, 1989 [DOI] [PubMed] [Google Scholar]
- 38. Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134: 127–136, 1984 [DOI] [PubMed] [Google Scholar]
- 39. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics 110: 1220–1225, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Yurchenco P. Molecular architecture of basement membranes. FASEB J 4: 1577–1590, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Zhao Y, Xiao H, Long W, Pearce WJ, Longo LD. Expression of several cytoskeletal proteins in ovine cerebral arteries: developmental and functional considerations. J Physiol 558: 623–632, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]