Abstract
Intracellular trafficking underlies cellular functions ranging from membrane remodeling to receptor activation. During multicellular organ development, these basic cell biological functions are required as both passive machinery and active signaling regulators. Exocytosis, endocytosis, and recycling of several key signaling receptors have long been known to actively regulate morphogenesis and pattern formation during Drosophila eye development. Hence, intracellular membrane trafficking not only sets the cell biological stage for receptor-mediated signaling but also actively controls signaling through spatiotemporally regulated receptor localization. In contrast to eye development, the role of intracellular trafficking for the establishment of the eye-to-brain connectivity map has only recently received more attention. It is still poorly understood how guidance receptors are spatiotemporally regulated to serve as meaningful synapse formation signals. Yet, the Drosophila visual system provides some of the most striking examples for the regulatory role of intracellular trafficking during multicellular organ development. In this review we will first highlight the experimental and conceptual advances that motivate the study of intracellular trafficking during Drosophila visual system development. We will then illuminate the development of the eye, the eye-to-brain connectivity map and the optic lobe from the perspective of cell biological dynamics. Finally, we provide a conceptual framework that seeks to explain how the interplay of simple genetically encoded intracellular trafficking events governs the seemingly complex cellular behaviors, which in turn determine the developmental product.
Keywords: receptor sorting, degradation, signaling, neurogenetics, optic lobe
INTRODUCTION
The Drosophila visual system comprises of the eyes and optic lobes. The eye has been a powerful model system to study basic questions of tissue morphogenesis, pattern formation, and underlying signaling mechanisms for decades (Meinertzhagen and Hanson, 1993; Wolff and Ready, 1993). This is partly due to the beauty and crystalline organization of both the eye and the underlying brain regions, which greatly facilitate the discovery and study of pattern formation. Maybe even more importantly, the Drosophila eye has very practical experimental advantages: the visual system is not required for viability under laboratory conditions, and it is easily amenable to genetic and other experimental manipulations. Consequently, many discoveries of basic developmental mechanisms were made possible by studies of Drosophila visual system development, including the interplay of transcriptional regulation and cell biology during eye development (see Kumar, 2011; Rister and Desplan, 2011), and the discovery and characterization of key proteins in several signal transduction pathways [reviewed in (Dickson and Hafen, 1993)].
The optic lobe comprises roughly half of the Drosophila brain. It receives direct input from the photoreceptors in the first optic ganglion, the lamina. The layer-specific projections of photoreceptor axons in the optic lobe and the formation of a visual map in the lamina in particular have yielded important insights into the problem of brain wiring and synaptic specification (Clandinin and Feldheim, 2009; Sanes and Zipursky, 2010). While the role of intracellular trafficking in the signaling events that lead to pattern formation in the eye has been studied for a long time, the investigation of the role of intracellular trafficking as part of the brain wiring program has only recently become a research focus. This is certainly at least partly due to newly developed techniques that have only been developed or become widely available in recent years. The developments in two areas are most important and will be discussed below: first, molecular genetic manipulation of the visual system in vivo and second, the imaging of the intact visual system in vivo.
The classical advantage of the visual system as a model for genetic manipulation has received a major boost with the development of techniques to render mutations homozygous only in photoreceptors and visual interneurons in otherwise heterozygous animals (Stowers and Schwarz, 1999; Newsome et al., 2000; Chotard et al., 2005). In combination with other tricks from the Drosophila tool box, these methods allow the easy generation of transgenic flies in which a subset of photoreceptor cells can be rendered mutant and negatively or positively labeled with any combination of fluorescent proteins. In addition, the mutant and/or control cells can be engineered to express additional transgenes for experiments ranging from simple genetic rescue, to genetic interaction and structure function studies of individual proteins—all in otherwise wild type (heterozygous) flies. Several genetics screens in Drosophila have been performed to identify novel components that regulate intracellular trafficking in the eye. Abnormal eye pigmentation can serve as readout for genes that regulate protein delivery to lysosomes (Lloyd et al., 1998). For example, acinus (dacn) has been identified as a regulator of endosomal transport and autophagosomal maturation (Haberman et al., 2010). Genetic modifier screens in the eye have also led to unbiased discoveries of trafficking components for general trafficking machinery (Simonsen et al., 2007), as well as for developmental signaling pathways, such as epidermal growth factor receptor (EGFR) and Notch (Eun et al., 2007; Iyadurai et al., 2008).
The second major technological improvement derives from dramatic advances of imaging technology in recent years. In light of the ability to non-invasively monitor and “virtually dissect” intact specimens, the small size of Drosophila has become a considerable advantage. The Drosophila visual system in particular is amenable to the most advanced modern imaging in toto, i.e., a complete eye–brain complex from a developing fly fits under high-resolution laser scanning and other live imaging microscopes (Williamson and Hiesinger, 2010). In combination with the genetic techniques mentioned above, almost any transgenic animal can be generated where individual cells, intracellular compartments, or proteins are labeled with any kind of fluorophore to image cellular and subcellular biology in vivo in a developing eye–brain complex. It is arguably this combination of technological advantages that allows the study of intracellular trafficking in the development and function of specialized neurons in their in vivo and in situ context in ways that were previously only possible in cell culture. In light of these two major technological advances, it is maybe of little surprise that the last few years have yielded rich new insights into the cell biology, and membrane trafficking in particular, of the developing visual system.
In addition to technical challenges, the role of intracellular trafficking has long posed a conceptual challenge for the study of neural circuit formation and synapse specification. While it is clear that membrane trafficking and other cell biological machinery are required for specific recognition and signaling events (i.e., play a permissive role in the developmental program), it is more difficult to envision an active role of intracellular trafficking in the actual synapse specification process (i.e., an instructive role). A similar debate has accompanied the study of cytoskeletal dynamics in axon pathfinding for many years (Dickson, 2001). It is maybe a little bit surprising that so little attention has been given to intracellular trafficking as part of the “brain making program,” given that examples for key roles of receptor trafficking in signaling events underlying developmental processes abound. Here, we will not provide a complete review of all components of intracellular trafficking and their roles in signaling in general; this topic is covered in comprehensive and highly recommended reviews elsewhere (Polo and Di Fiore, 2006; von Zastrow and Sorkin, 2007; Sorkin and von Zastrow, 2009). Instead, we will focus on two topics: first, a more specialized review of the recent literature on the regulation of signaling by intracellular trafficking in visual system development, with emphasis on selected studies in the fly eye and optic lobe. There are several instances where signaling regulation through intracellular trafficking has been characterized in various tissues for receptors that have key roles in visual system development. We highlight such examples with the idea that lessons may be learned from other systems that may prove useful for visual system development. However, we will not systematically discuss everything that is known about the trafficking regulation of the discussed signaling pathways; instead, we try to focus on those cases through which key lessons about the discussed processes have been learned. Second, we provide a conceptual approach to understanding the role of intracellular trafficking in the larger developmental context of eye and brain development. For this second topic, the Drosophila visual system is uniquely qualified, as the fly eye is arguably one of the best characterized developmental model organs and fly eye–brain wiring has become a popular model to unravel the brain making program. The Drosophila visual system thereby combines both an accessible system for the study of cell biology as well as an in vivo system to study cell biology in the context of a multicellular organism.
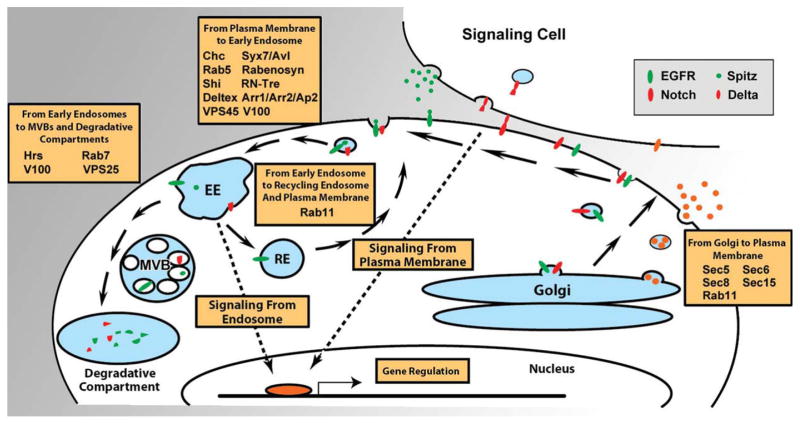
We want to conclude this introduction by offering a conceptual framework for the role of intracellular trafficking for signaling in general. In principle, the intersection of intracellular trafficking and signaling is based on regulatory mechanisms of receptor signaling from one of two places: signaling from the plasma membrane or signaling-competent endosomes. Figure 1 shows the known mechanisms by which signaling and intracellular trafficking regulate each other from the perspective of a signaling receptor (black arrows). For each of the mechanisms, excellent examples can be found in Drosophila visual system development how trafficking regulates signaling. These will be highlighted throughout the review. Less is known about direct effects of developmental signaling on the trafficking itself in this system (red arrows). We speculate that such mechanisms exist from the observation of highly coordinated intracellular and cellular behaviors. This meeting point of signaling and trafficking may provide a key to understanding the seemingly more complicated multicellular behaviors that govern pattern formation in the visual system.
Figure 1.
Feedback between intracellular trafficking and signaling from the viewpoint of a signaling receptor. Intracellular trafficking pathways are shown with black arrows; possible signaling effects with red arrows. Note that intracellular trafficking can directly regulate signaling via sorting, endocytosis, and exocytosis of receptors and signaling molecules. Furthermore, the signaling events themselves can regulate the intracellular trafficking of the receptor, leading to more complicated cellular behaviors by the interplay of simple mechanisms. See Introduction and Conclusion sections for details.
INTRACELLULAR TRAFFICKING IN EYE DEVELOPMENT
Drosophila eye development originates from ~20 progenitor cells of the eye primordia in the embryonic blastoderm under control of “master” transcription factors, most prominently eyeless/Pax6 (Halder et al., 1995; Gehring and Ikeo, 1999). These progenitor cells give rise to an eye imaginal disc that develops during larval stages and pupation. The cells of the undifferentiated eye imaginal disc are specified and patterned in late larval and early pupal stages. Drosophila eye development is reviewed in great detail in many excellent reviews and book chapters, including reviews by Kumar (2011) and Xia and Ready (2011). Briefly, the pattern formation of the eye starts with the morphogenetic furrow, a temporal wave of cell differentiation that progress from posterior to anterior. The morphogenetic furrow leaves a crystalline-like pattern of differentiating cells in its wake that transforms into around 800 single unit eyes, called ommatidia. This feat of pattern formation requires both coordinated biophysical properties through intracellular membrane and cytoskeletal regulation as well as spatiotemporally regulated signaling. Here, we will focus on the role of intracellular trafficking in the regulation of signaling.
Early Eye Development: From the Morphogenetic Furrow to Eye Patterning
The anterior progression of the morphogenetic furrow is regulated by a concerted effort of signaling cascades including the Hedgehog (Hh), Wingless (Wg), BMP, JAK/STAT, EGFR, and Notch pathways. Notch and EGFR act upstream of Hh, which functions together with Decapentaplegic (Dpp) to drive furrow initiation and progression, whereas Wg inhibits the progression (Treisman and Rubin, 1995; Kumar and Moses, 2001; Baker, 2007; Roignant and Treisman, 2009). One common theme of these pathways is that the ligands from the sending cells, either transmembrane (Delta and Serrate) or secreted (Hh and Wg), have to interact with transmembrane receptors on the receiving cells. Membrane trafficking regulates the release and transport of the four secreted signaling molecules that control eye development: Unpaired (Upd), Wg, Dpp, and Hh. The trafficking machinery regulates the secretion of ligands, transportation to the site of action (either neighboring or several cell diameters away), and the activation of intracellular signaling components upon ligand binding at the receiving cell, as shown in Figure 2.
Figure 2.
Intracellular trafficking implicated in Drosophila visual system development. Shown are the canonical intracellular trafficking routes. Notch and EGF receptors (red and green ovals, respectively) and their ligands Delta and Spitz (small red oval, green circle) serve as example receptor–ligand pairs. Many other receptors are discussed in the text. Orange circles represent secreted morphogens (Hh, Wg, Dpp, Upd). Text boxes highlight trafficking proteins that are discussed for their role in visual system development.
During eye development, numerous intracellular trafficking mechanisms have been characterized that are required to regulate signaling by sorting these ligands or ligand–receptor complexes. For example, the novel adaptor protein Phyllopod regulates the trafficking of components of both the Wingless and Notch signaling pathways in early endocytic vesicles (Nagaraj and Banerjee, 2009). The loss of phyllopod causes accumulations of functional receptors in intracellular compartments, which in turn causes gain-of-function phenotypes for both Notch and Wingless. Interestingly, phyllopod is a transcriptional target of EGFR signaling during eye development (Nagaraj and Banerjee, 2009). In a similar fashion, Unpaired, the secreted ligand of the JAK/STAT pathway, is a Notch target gene. The intracellular transport of Upd is regulated by Erupted, a Tsg101 homolog that functions in endosomal sorting and multivesicular body formation. Defects in erupted result in elevated JAK/ STAT activity (Moberg et al., 2005; Gilbert et al., 2009). These findings exemplify a common theme of sorting and degradation in the downregulation of signaling molecules intracellularly. Similar mechanisms are observed for several intracellular trafficking or degradation mutants during both eye development and eye–brain wiring, as discussed below.
In the adult eye, each ommatidium contains eight photoreceptors, R1–R8, which are specified by a combination of many signaling pathways. During development the R8 cell is the first differentiated photoreceptor. The transcription factor atonal determines R8 cell fate behind the morphogenetic furrow, with negative and positive regulation from Notch and EGFR pathways, respectively (Frankfort and Mardon, 2002; Hsiung and Moses, 2002). The Notch signaling pathway plays key roles in cell-fate specification in the eye (Greenwald, 1998). It functions by enhancing atonal expression [“proneural enhancement,” (Baker and Yu, 1997)] and by inhibiting the neighboring cells from adopting the pro-neuronal fate [“lateral inhibition,” (Meir et al., 2002)]. Signaling through the Notch receptor is triggered by its interaction with either of the two transmembrane ligands, Delta, or Serrate from the signaling cells. On binding its ligand, Notch is sequentially cleaved, and the intracellular domain translocates into the nucleus and functions as a transcriptional co-activator of the target genes (Mumm and Kopan, 2000; Weinmaster, 2000). These cleavages also release the extracellular domain, which is “transendocytosed” into Delta-expressing cells as a prerequisite for signaling (Sun and Artavanis-Tsakonas, 1996, 1997; Seugnet et al., 1997; Parks et al., 2000). During wild type development, Notch and Delta colocalize in endocytic vesicles, and this colocalization is disrupted when endocytosis is acutely blocked and prevents the internalization of Notch in the Delta-expressing cells (Parks et al., 2000). Why is the endocytosis of Delta and Notch required for signaling? Two prominent models have been proposed [for review see (Le Borgne et al., 2005)]. The first model suggests that after binding to Notch, endocytosis of the ligand exerts mechanical force required for cleavage and release of the Notch intracellular domain (Parks et al., 2000); the second model suggests that to be activated (i.e., signaling-competent), Delta must be internalized and processed in endosomes, and then recycled back to the plasma membrane (Wang and Struhl, 2004). During eye development, the endocytic epsin liquid facets (lqf) is required for correct levels of Delta internalization. The enzyme Fat Facets (faf) deubiquitinates Lqf to induce this internalization, which in turn leads to increased Delta signaling (Overstreet et al., 2004). The ubiquitin-dependent endocytosis of Delta is further regulated by auxilin, a J-domain-containing protein known to cooperate with Hsc70 in the disassembly of clathrin coats from clathrin-coated vesicles (Hagedorn et al., 2006). These examples highlight the importance of Delta endocytosis in the signaling cell for Notch activation in the receiving cells.
Notch signaling is further regulated by activation on the early endosomes in the receiving cells (Tien et al., 2009). Several of the positive signaling regulators/facilitators identified in recent years, including Deltex, Syx7/Avl, Rabenosyn-5, and Vps45, are trafficking components that may bring Notch to the endosomes (Hori et al., 2004; Lu and Bilder, 2005; Morrison et al., 2008). Deltex actively promotes the relocalization and stabilization of Notch in the late endosomes, from where Notch signaling can occur (Hori et al., 2004). Rab5, Rabenosyn-5, Vps45, and Syx7/Avl control entry into early endosomes; downregulation of these genes results in loss of Notch activity (Hori et al., 2004; Lu and Bilder, 2005; Morrison et al., 2008). Since these genes are required for general endocytic transport, it is likely that they function non-selectively to regulate signaling pathways that need to enter endosomes (see Fig. 2).
Recent studies also implicate the v-ATPase, a proton pump that acidifies intracellular compartments, in the regulation of Notch signaling (Nelson, 2003; Forgac, 2007; Jefferies et al., 2008). While v-ATPase acidification of lysosomes is required for the degradation of all endocytosed receptors, acidification of early endosomes was recently shown to be required specifically for Notch signaling (Yan et al., 2009; Vaccari et al., 2010). Disruption of the v-ATPase regulator Rabconnectin-3 as well as several v-ATPase subunits (vha55, vha68-2, vhaAC39, and vhaSFD) impairs acidification of endosomal compartments required for Notch activation. Interestingly, v-ATPase-dependent acidification is required before the so-called S3 cleavage, which releases the Notch intracellular domain that translocates to the nucleus to regulate transcription. This implies that S3 cleavage occurs on endosomal compartments and thereby endosomes actively regulate Notch signaling (Yan et al., 2009; Vaccari et al., 2010).
Membrane transport downstream of early endosomes controls the inactivation and degradation of internalized Notch. Disruption of endosomal sorting proteins of the ESCRT-II complex cause intracellular Notch accumulations in photoreceptors. In mutants for vps36 these accumulations do not correlate with increase Notch activity. This phenotype is similar to mutations in car and dor, which possibly function downstream of the ESCRT-II complex and are components required for trafficking to lysosomes (Sevrioukov et al., 1999; Akbar et al., 2009). These results suggest that Notch accumulates in signaling-inactive, late endosomal, or degradative compartments in these mutants (Vaccari et al., 2008). In contrast to vps36, the two other ESCRT-II proteins vps22 and vps25 are required to maintain a controlled level of Notch signaling in the eye (Thompson et al., 2005; Herz et al., 2009). Blockage of endosomal trafficking in vps25 results in endosomal accumulation of Notch receptor. Similar to phyllopod mutant, these receptor accumulations lead to increased Notch activity, which in turn leads to ectopic expression of Upd and non-autonomous cell proliferation (Thompson et al., 2005; Vaccari and Bilder, 2005). These results show that several endosomal sorting mechanisms exist that regulate the timing of Notch signaling from signaling-competent compartments. Taken together, a clear role for endocytic regulation of Notch signaling has been established for Drosophila visual system development. Many more intracellular trafficking genes have been identified by screening for Notch signaling regulators in the eye (Shalaby et al., 2009). The numerous examples from other systems suggest that the underlying cell biological mechanisms are shared with other cells and signaling processes, and that the specific effects on eye development are context-dependent.
Similar insights on the role of endosomal regulation of receptor-mediated signaling come from the studies of EGFR signaling in the Drosophila visual system. EGFR is a transmembrane receptor tyrosine kinase that dimerizes and transactivates itself on binding of its ligand Spitz. In the developing eye, the EGFR pathway regulates cell-fate determination (R8 cell maintenance), cell cycle progression, ommatidial rotation, and cell survival (Freeman, 1996; Shilo, 2003). In photoreceptors, polarized secretion of the EGFR ligand Spitz is required for proper development of the signal-receiving cells. Spitz is secreted from the cell bodies to trigger neurogenesis in nearby cells (Tio and Moses, 1997), as well as from the axon terminal for lamina patterning and will be discussed in this context in Section “the Eye–Brain Connection” (Huang et al., 1998). Spitz requires activation through intramembrane proteases of the rhomboid class from a precursor protein (Freeman, 2009). Different Rhomboid proteases reside in different subcellular compartments and regulate spatiotemporal activation of Spitz and consequently EGFR signaling in the receiving cells. Specifically, Rhomboid-1 resides in a late compartment of the secretory pathway in the photoreceptor cell body, where Spitz secretion is required for cell type specification and eye patterning. In contrast, Spitz secretion from axons relies on Rhomboid-3, which resides in extensions of the endoplasmic reticulum that extend all the way to the synaptic terminal (Yogev et al., 2008, 2010). These findings highlight an astounding level of cell biological regulation of ligand secretion in space and time as a means to regulate patterning of first the eye and subsequently the axon target area with the same ligand–receptor pair. It is furthermore a beautiful example for specificity as a function of several simpler mechanisms: First, photoreceptors generate the ligand precursor without the amount of synthesis being a rate-limiting step; second, the ligand precursor traffics through the ER and secretory pathway where it encounters differentially compartmentalized Rhomboids that activate the ligand in a spatiotemporally restricted manner. Differentially compartmentalized Rhomboids therefore fulfill the requirements for playing “instructive” roles in regulating spatiotemporally regulated EGFR signaling. The next question of course is how the different Rhomboids are differentially localized. We are tempted to speculate that simple, context-dependent trafficking mechanisms ultimately underlie the observed spatiotemporal specificity.
Similar to its ligand the EGF receptor is directly regulated by endocytic trafficking during eye development. For example, the phospholipid membrane composition affects both EGFR and Notch signaling through altered endocytosis (Weber et al., 2003). The E3-ubiquitin ligase cbl plays a role for the ligand-dependent inactivation of the EGFR through endocytosis and lysosomal degradation. Loss of cbl causes overgrowth, differentiation defects, and increased ommatidial spacing, consistent with its role in negatively regulating EGFR signaling (Wang et al., 2008). Similarly, the tyrosine phosphatase Myopic promotes EGFR signaling on Rab5-positive endosomal compartments (Miura et al., 2008). These findings are consistent with the earlier finding that signaling from endosomes is directly controlled by regulated progression to late endosomal compartments and degradation (Lloyd et al., 2002).
Lessons From Intracellular Trafficking in Other Tissues
Secretion of morphogens is thought to rely on the canonical secretory pathway. Morphogens reach their targets either by diffusion through extracellular space or transcytosis through cells (Freeman, 2002). Examples for both mechanisms of morphogen transport have been described for the developing wing disc and embryo. An effective means to distinguish between the diffusion and transcytosis model is the acute blockage of endocytosis using the temperature-sensitive dynamin mutant shibirets. In the wing, blocking of endocytosis by shibirets mutant clones between signal sending cells and receiving cells do not block movement of Dpp, Hh, and Wg, indicating that endocytosis is not required for the movement of these morphogens between cells (Strigini and Cohen, 1999; Belenkaya et al., 2004; Han et al., 2004). On the other hand, endocytosis of Wg has been shown to be a strong modulator of Wg signaling in the wing (Seto and Bellen, 2006). In shibirets mutant embryos, Wg dispersal is limited, favoring a role for planar transcytosis in Wg signaling (Bejsovec and Wieschaus, 1995; Moline et al., 1999). In contrast, in clathrin heavy chain (chc) mutants where clathrin-dependent endocytosis is blocked, Wg spreads further from the signaling cells, favoring the extracellular diffusion model (Dubois et al., 2001). In addition, receptor-mediated endocytosis, followed by lysosomal degradation, also plays an important role in shaping the gradients by removing morphogen from extracellular space, therefore downregulates the signaling (Dubois et al., 2001; Han et al., 2004; Torroja et al., 2004; Piddini et al., 2005). Since no comparable studies have to our knowledge been performed in the eye disc, the precise role of morphogen endocytosis during visual system development is currently unresolved.
Late Eye Development: Apoptosis, Photoreceptor Positioning, and Rhabdomere Development
After ommatidial assembly, excess interommatidial cells are removed by apoptosis, which is controlled by two antagonizing pathways: Notch provides pro-death signals; whereas EGFR provides survival signals (Cagan and Ready, 1989; Miller and Cagan, 1998; Kurada and White, 1999; Yu et al., 2002). Notch-induced programmed cell death in the interommatidial pigment cells is inhibited by chc hypomorphic alleles, indicating the requirement of clathrin-dependent endocytosis for down-regulation of Notch signaling (Peralta et al., 2009). The regulation of EGFR by intracellular trafficking in interommatidial cells has to our knowledge not been studied specifically in this cell type, but may have similarities with EGFR signaling in other cells, as discussed above.
Intracellular trafficking also regulates photoreceptor positioning. Posterior to the morphogenetic furrow, as each photoreceptor differentiates, the nuclei migrate apically while the axons extend basally into the brain. Nuclear translocation is required during eye development, as misregulation of the translocation causes abnormal eye morphology (Fan and Ready, 1997). Moreover, misregulation of nucleus positioning may be responsible for neurological disorders such as lissencephaly in mammals (Olson and Walsh, 2002; Vallee and Tsai, 2006). Recent findings suggest a role of intracellular transport in regulating photoreceptor positioning. The RabGAP RN-Tre functions together with the endosomal GTPases Rab5 and Rab11, and is required for maintaining apical localization of photoreceptor nuclei. Loss of rab5 or rab11, as well as misexpression of RN-Tre causes R-cell nuclei mis-localization (Houalla et al., 2010). However, whether the vesicular transport machinery actively carries a specific protein to position the nuclei or whether the endocytic pathway is passively required is still unclear. Previously, it has been shown that microtubule motors Dynein and Kinesin regulate nuclei positioning by controlling microtubule movement (Whited et al., 2004). One attractive model would be that a Rab5-Shi-Rab11 endocytic-recycling pathway targets specific proteins, possibly microtubule motors or regulators, to regulate photo-receptor positioning.
To develop a functional photoreceptor, the light-sensing membrane domain, the rhabdomere, must be established in the apical membrane. The biogenesis and maintenance of this subdomain is highly regulated by intracellular trafficking, particularly by targeted exocytosis for the transport of proteins to the polarized membrane (Schuck and Simons, 2004). Rhabdomere morphogenesis takes place during pupation, and rhodopsin and the phototransduction machinery are recruited in massive intracellular trafficking processes. In the functioning photoreceptors, Rhodopsin is converted into meta-rhodopsin by light, subsequently phosphorylated by GPRK1 and becomes bound to arrestins which quench its activity. Interaction between Rhodopsin and arrestins leads to the endocytosis and lysosomal degradation of Rhodopsin. There are several mechanisms that control Rhodopsin activity, deregulation of which may lead to retinal degeneration [for review see (Wang and Montell, 2007)]. Rhodopsin transport and maturation require Rab1 and Rab6 for Golgi-ER trafficking (Satoh et al., 1997; Shetty et al., 1998). Rab11 functions in post-Golgi transport of rhodopsin and transient receptor potential (TRP) to the photosensitive organelles, independent of its previously known function in recycling endosomes (Satoh et al., 2005). Rab11 functions together with its linker protein dRip11 and the myosin motor MyoV; it further utilizes the exocyst complex as an effector for apical exocytosis via the association with Sec6 through Sec5 (Beronja et al., 2005; Li et al., 2007). The turnover of Rhodopsin is tightly regulated by endocytosis. Purturbation of the endocytic pathway by mutations in Arr1 (Satoh and Ready, 2005), Arr2 (Cronin et al., 2006), and AP2 (Orem et al., 2006) result in retinal degeneration and photoreceptor cell death. The toxicity caused by loss of Arr2 is decreased through increasing endocytosis by Ceramidase (Acharya et al., 2008). A recent study indicates a cross-talk between the autophagy and endocytic pathways in Rhodopsin degradation. Decreased autophagy increases Rhodopsin accumulation in late endosomes, thereby causing retinal degeneration, which can be suppressed by rab7 overexpression (Midorikawa et al., 2010). Although a previous study indicates that inhibition of autophagy does not affect eye development (Wang et al., 2009), this finding sheds light on the protective roles for autophagy in retinal degeneration (Midorikawa et al., 2010). In summary, the biogenesis, function, and maintenance of the rhabdomere are actively regulated by several aspects of intracellular trafficking. Because the level of functional rhodopsin is critical for photoreceptor function and survival, intracellular trafficking may be utilized as an active means to remove damaged rhodopsin and replenish the site of function with newly synthesized rhodopsin.
INTRACELLULAR TRAFFICKING IN OPTIC LOBE DEVELOPMENT
Compared with the development of the eye and the photoreceptors, less is known about the development of the many cell types that characterize the optic lobes. While photoreceptors are part of the peripheral nervous system, all optic lobe neurons belong to the central nervous system (CNS). Many of the mechanisms that regulate signaling in the eye can be expected to be similarly employed during optic lobe development. However, two key features differentiate neuronal development in the optic lobe from the eye disc: First, CNS neurons develop over a longer period of time, giving rise to more cell types and a more complicated cellular environment. Second, a larger number of neuronal and glial cells play a more important role in the patterning of brain compartments as well as individual neuronal development. Taken together these differences cause the optic lobe to be seemingly even more complex than the eye, both in structure and cellular makeup (Fischbach and Hiesinger, 2008).
The optic lobe receives direct input from the photoreceptors to processes motion, color and other visual features (Ting and Lee, 2007). Four major neuropils contain the synaptic connections: the lamina, medulla, lobula, and the lobula plate. The optic lobe progenitor cells arise during embryonic development and neuronal differentiation continues throughout the larval stages (Goodman and Doe, 1993; Truman et al., 1993). Onset of neuronal differentiation in the CNS therefore precedes photoreceptor differentiation during eye development. Consequently, differentiation in the optic lobe anlagen mostly commences several days before brain wiring begins, with the exception of the induction of lamina neuron specification by ingrowing photoreceptors, as discussed in the Section “the Eye–Brain Connection.” In contrast to most optic lobe neurons, photoreceptor axons select postsynaptic targets little more than two days after differentiation. This simple temporal difference in development has profound effects on the requirements for intracellular trafficking and degradation machinery, as discussed below. In the following sections, we will review the role of intracellular trafficking on early optic lobe development (differentiation) and late optic lobe development (brain wiring) separately.
Early Optic Lobe Development: Cell Type Specification and Optic Anlagen Patterning
Neural differentiation depends on a plethora of signaling pathways that regulate transcription factors that in turn direct transcriptional programs underlying cell fate. Similar to the developing eye, numerous signaling pathways intersect and the spatiotemporal dynamics of signaling molecules is highly regulated by cell biological machinery. Again the EGFR and Notch pathways serve as key opposing patterning forces. They work in concert to regulate the proneural wave, which is a progression of neuroblast differentiation in the outer optic anlagen. This wave initially occurs in the medial neuroepithelium and moves laterally in the region of the outer optic anlagen that becomes the medulla (Daniel et al., 1999; Egger et al., 2010; Yasugi et al., 2010). The role of EGFR signaling is to promote neurogenesis in the proneural wave (Yasugi et al., 2008, 2010) while Notch signaling represses neuroblast differentiation and favors keeping progenitors in their neuroepithelial state (Egger et al., 2010; Yasugi et al., 2010). Regulation of Notch and EGFR signaling by intracellular trafficking has been studied in much greater detail in the eye than the optic lobe and is discussed in the previous section on eye development.
Glial cells are of special importance in optic lobe development and brain development in general. The role of glia in eye development is reviewed in detail in this issue (Yuva-Aydemir and Klämbt, 2011). Developmental glial cell migration requires cell–cell interactions between glia and neurons. A molecule identified to be important for glial migration in the lamina is the axonal scaffolding protein Dachsous (Ds). Ds mutants cause both axonal guidance defects and migration defects of glia in the optic lobe (Dearborn and Kunes, 2004). How Ds may be regulated by intracellular trafficking is not known. However, glial cell migration along motor axons during embryo development is mediated by cell adhesion molecules such as the guidance receptor Fasciclin 2, which is found in specific cells of the optic lobe, including lamina monopolar neurons (Schneider et al., 1995; Silies and Klambt, 2010). There are neuronal- and glial-specific isoforms of Fasciclin 2 that interact to direct glial migration. At points where neuronal-Fasciclin 2 concentrations are low in motor neuron axons, the glia continues to migrate down the axon. However, at axonal segments with higher concentrations of neuronal-Fasciclin 2, the glia stops migrating. Fasciclin 2 partially co-localizes with endocytic markers Rab4, Rab5, and Rab11. Expression of shibirets or a dominant negative form of Rab4 in neurons causes a disruption of the gradient in stage 16 embryo (Silies and Klambt, 2010). For more details, see the chapter by Yuva-Aydemir and Klämbt (2011) in this issue. As discussed later, Fasciclin 2 is affected by trafficking mutants, such as sec15 and v100, in the optic lobe and photoreceptors (Mehta et al., 2005; Williamson et al., 2010b). How these mutants affect glia migration in the optic lobe has not been characterized. Glia also provides important trophic support necessary for neuronal survival. Studies in which glia are genetically ablated by mutating drop dead or repo leads to the loss of neurons in the optic lobe (Buchanan and Benzer, 1993; Xiong and Montell, 1995). What trophic factors are provided by glial cells in the optic lobe is unknown.
Several pathways, including Notch, JAK/STAT, Dpp, and Hh have been implicated glial development. Dpp functions upstream of the transcription factors gcm and gcm2, which are found in glial cells in the optic lobe. Cells mutant for dpp signaling in the visual system cause a loss of glial differentiation in lamina (Yoshida et al., 2005). Gain-of-function of either gcm or gcm2 in the developing optic lobe can lead to ectopic gliogenesis in the medulla (Chotard et al., 2005). As discussed in the eye section, mutations in the ESCRT-II protein vps25 cause accumulations of Notch and Dpp (Vaccari and Bilder, 2005). Since to our knowledge there are no corresponding trafficking studies specifically for glia development in the optic lobe, we refer to our discussion of these mechanisms in Section “Intracellular Trafficking in Eye Development.” In summary, there is a dearth of studies specifically studying intracellular trafficking during early optic lobe development. However, several of the signaling receptors and neuronal and glial regulators involved in early optic lobe development have been studied in other systems, as highlighted below.
Lessons From Intracellular Trafficking in Other Tissues
The proneural wave during optic lobe development is negatively regulated by JAK/STAT signaling through the transmembrane receptor domeless (dome) and one of three ligands Unpaired1, 2, 3 (Upd1, Upd2, Upd3) (Yasugi et al., 2008; Flaherty et al., 2009). JAK/STAT signaling is initially located in the far lateral neuroepithelium cells of the outer optic anlage, in the region that will eventually become the lamina. Loss of JAK/STAT signaling causes premature neuroblast differentiation from the neuroepithelium at the expense of other cell types (including glia) and affects optic lobe patterning, similar to notch mutants (Yasugi et al., 2008; Ngo et al., 2010). To our knowledge, there are no studies on the intracellular regulation of JAK/STAT specifically in the optic lobe. However, detailed studies in Drosophila follicle cells of the egg chamber, the larval wing imaginal disc and cell culture reveal key aspects of the regulation of JAK/STAT signaling by endocytic trafficking (Devergne et al., 2007; Vidal et al., 2010). On Upd binding to Dome, the receptor–ligand complex undergoes clathrin-dependent endocytosis. Dome co-localizes with the early endosome markers 2xFYVE and Rab5, and the late endosomal marker Rab7 but not the recycling endosome marker Rab11. In the earlier study, numerous mutants of the endolysosomal pathway including chc rab5, hrs, and deep orange (but not rab11) are reported to disrupt JAK/STAT signaling (Devergne et al., 2007). This is in contrast to a recent re-investigation of the relationship of JAK/STAT signaling and endocytic regulation using a genome-wide RNAi screen approach (Vidal et al., 2010). In contrast to the earlier study, the authors find evidence for a negative regulatory function of endocytosis. Whereas Devergne et al., (2007) suggest that endocytosis is required for signaling (consistent with signaling on endosomes), the later findings suggest that endocytosis turns signaling off, consistent with signaling from the plasma membrane (comp. Fig. 1). However, the observations by Devergne et al. (2007) that mutations in components of early endocytosis (rab5, chc), endosome maturation (hrs) as well as late endosomes/lysosomes (deep orange) all lead to loss of signaling is unusual and not easily explained with signaling on endosomes. In this scenario, loss of rab5 function should indeed impair signaling, but not hrs, a protein required for the maturation of signaling endosomes that functions in turning signaling off (Lloyd et al., 2002). If Upd/Dome signaling is actively regulated from early endosomal compartments, hrs mutants would be expected to exhibit increased signaling. Finally, deep orange has been shown to affect downstream degradation of lysosomes or lysosome-related organelles. Vidal et al. (2010) suggested that the difference between their work and the previously published results in the study by Devergne et al. (2007) could be due to an alteration of cell fate, but the two sets of data remain to be fully reconciled.
Late Optic Lobe Development: Axon Pathfinding and Synaptic Specification
The study of intracellular trafficking in the cells of the optic lobe is technically more difficult than subcellular resolution studies in the eye or wing discs. Consequently, few studies exist that directly test intracellular trafficking in CNS neurons of the optic lobe. However, an important experimental advantage for the study of optic lobe neurons comes from genetic tools. Most importantly, the original eyFLP systems (Stowers and Schwarz, 1999; Newsome et al., 2000) render thousands of optic lobe neurons mutant that are dispensible for viability under laboratory conditions (Chotard et al., 2005; Mehta et al., 2005; Williamson et al., 2010b). This allows the genetic study for mutants that affect any stage of optic lobe development without causing early lethality. Similarly, at least one Gal4 driver line (Mz1369-Gal4) has been described that strongly and selectively expresses in the developing optic lobes during early development and expresses in other CNS neurons only during pupation (Hiesinger et al., 1999). Therefore, it is possible to express transgenes specifically in the developing optic lobe. Together, these tools allow for tissue-specific loss-of-function and gain-of-function studies of this brain region during development.
Using the optic lobe and photoreceptor-specific eyFLP technique, the exocyst component sec15 was identified to function in both photoreceptors and optic lobe neurons (Mehta et al., 2005). The exocyst complex was originally identified in yeast where it functions in polarized secretion (Hsu et al., 2004). In Drosophila, different exocyst components have strikingly different phenotypes in the developing visual system. Specifically, mutations in the core subunits sec5 and sec6 cause a complete ablation of the eye (Murthy et al., 2003; Beronja et al., 2005). In contrast, sec15 is only required for polarized trafficking of a subset of cargo vesicles and its loss leads to axonal accumulations of receptors and signaling molecules in developing photo-receptors and optic lobe neurons (Mehta et al., 2005). Interestingly, Sec15 is an effector of Rab11, the defining marker of recycling endosomes (Jafar-Nejad et al., 2005; Wu et al., 2005). However, Rab11 has numerous functions, not all of which can be ascribed to a canonical recycling endosome, e.g., Rhodopsin trafficking as described above. The precise function of Sec15/Rab11-mediated polarized secretion remains unclear.
Similar to mutations in sec15, loss of the synaptic vesicle SNARE n-syb in optic lobe neurons causes defects only after cell type differentiation and axon pathfinding (Hiesinger et al., 1999). Furthermore, both sec15 and n-syb mutants lead to intracellular guidance receptor accumulations, albeit for different receptors. The onset of receptor accumulations in n-syb mutants precedes synapse formation, making it unlikely that loss of neurotransmitter release is responsible for the developmental defects. This conclusion is corroborated by the characterization of a sensitive time period before synapse formation during which n-syb function is developmentally required (Rister and Heisenberg, 2006). The observation that n-Syb functions as a neuron-specific vesicle SNARE in intracellular membrane fusion suggests that n-syb functions in intraneuronal receptor trafficking in addition to synaptic vesicle exocytosis.
The vesicular ATPase has a role in Notch-dependent early development, as discussed in Section “Intracellular Trafficking in Eye Development” (Yan et al., 2009; Vaccari et al., 2010). These defects are presumably due to complete loss of v-ATPase function. In contrast, loss of the neuron-specific subunit a1 (v100) only causes neuron-specific phenotypes. In photoreceptors, loss of v100 first causes a loss of neurotransmitter release (Hiesinger et al., 2005) and then, through a different mechanism, adult-onset degeneration (Williamson et al., 2010a). Curiously, loss of v100 does not cause any developmental phenotypes in photoreceptors, yet results in dramatic axonal mistargeting in the optic lobes (Williamson et al., 2010b). V100 underlies a neuronal sort-and-degrade mechanism that increases neuronal degradative capacity. Loss of the v100-dependent degradation mechanism only becomes developmentally detrimental during the longer developmental time period of CNS neuronal development in the optic lobe, but not in photoreceptors. Specific manipulations of the v100-dependent degradation pathway can be utilized to sort presumably all actively sorted receptors in the endolysosomal system into degradation-incompetent compartments. This may serve as a means to spatiotemporally characterize actively sorted receptors in different neuronal compartments during development and function (Williamson et al., 2010b). The v100-dependent degradation mechanism is also an example of a permissive or passive cell biological mechanism that, in combination with other such mechanisms, can lead to highly specific intracellular sorting, as discussed in Section “the Eye–Brain Connection.”
The axons of photoreceptors R1–R6 which form the primary visual map in the lamina proceed to target selection between two layers of glia in the distal optic lobe. The theme of boundary or compartment formation as a function of glia is persistent throughout much of brain development. Such boundaries separate specific brain regions from one another, for example, the separation between the lobula and lamina (Edwards and Meinertzhagen, 2010). Lamina glia secretes the ligand Slit where it acts to prevent cell mixing during development. Similarly, cells of the lobula cortex specifically express multiple Roundabout (Robo) family receptors, effectively compartmentalizing the optic lobe (Tayler et al., 2004). Unfortunately, there are to our knowledge no studies on the intracellular trafficking of Slit or Robo specifically in cells of the developing optic lobe. However, intracellular sorting that tightly regulates the spatio-temporal localization of Robo has been described in detail for axonal midline crossing in the Drosophila embryo (Keleman et al., 2002; Keleman et al., 2005). Here, during a brief time window, Robo is removed from the membrane to allow axonal midline crossing once. The time window is kept short by the limited expression of the intracellular sorting receptor Commissureless (Comm). Indeed, the sorting of Robo by Comm is one of the best examples of a highly specific active role of intracellular sorting in a very distinct axon pathfinding choice.
THE EYE–BRAIN CONNECTION
Photoreceptor neurons extend axons into the developing larval brain soon after cell type specification. Of the eight subtypes of photoreceptors, R1–R6 terminate in the first optic ganglion, the lamina, whereas R7 and R8 project through the lamina and terminate in the second optic neuropil, the medulla. The R1–R6 photoreceptor terminals undergo an elaborate lateral reorganization that yields as many synaptic cartridges in the visual map in the brain as there are ommatidia in the adult eye. These cartridges contain each one R1–R6 terminal from the six photoreceptors that “see” the same point in space. Strikingly, the cell bodies of these six photoreceptors reside in six different ommatidia in the eye due to the anatomical feature of separate light-sensitive elements under the same lens. The wiring principle of combining axons from photoreceptors that “see” the same point in space in a single synaptic ensemble is called neural superposition. The developmental program that underlies this principle has received much attention as a feat of synapse-specific wiring (Clandinin and Zipursky, 2000; Hadjieconomou et al., 2010). Similar to R1–R6 targeting and sorting in the lamina, the layer-specific sorting of R7 and R8 terminals in the medulla has become a powerful model to unravel the molecules and signaling that orchestrate neural circuit formation (Melnattur and Lee, 2011).
The formation of this retinotopic map in the brain is guided by a temporal wave of differentiation of the photoreceptor neurons in the developing eye disc that translates into a wave of axon terminal arrivals in the brain. The process by which the terminals of R1–R6 correctly sort into their cognate cartridges is still poorly understood. However, key features of this developmental program have been determined, including the idea that visual map formation does not require visual input or activity (Hiesinger et al., 2006). The evidence against a role of activity in visual system development is two-fold: First, mutations in genes that exclusively function in the generation and conduction of electrical potentials as well as neurotransmitter release do not affect visual system development or synaptic connectivity. This includes the precise number of synapses formed by each individual photoreceptor terminal in the lamina. Second, mutations identified in a large scale screen for synapse development and function provided a clear dissection of genetically separable parts of the developmental program. Specifically, the program for the generation of a fixed and precise number of synapses is cell-autonomous in photoreceptors and independent of the earlier sorting of photoreceptors into correct cartridges, as described above. Hence, each photoreceptor autonomously generates a fixed and precise number of synapses that is independent of the nature of the synaptic contacts or their activity (Hiesinger et al., 2006). These findings establish the primary visual map in the fly as a genetically encoded system.
What is the role of cell biological machinery as part of this genetic program? Wg and Dpp secretion is required in the target field for proper neuronal differentiation (Kaphingst and Kunes, 1994). The development of lamina neurons is induced by secretion of the signaling proteins Hh and Spitz from the axons of the photoreceptors (Huang and Kunes, 1996; Huang et al., 1998). Secretion of Spitz is spatiotemporally regulated by a cell biological mechanism based on differential compartmentalization of intramembrane proteases of the rhomboid class, as described in the section on eye development. Interestingly, a specific rhomboid (Rho-3) is spatiotemporally localized in extensions of the endoplasmic reticulum at the axon termini to specifically generate Spitz for patterning of postsynaptic targets in the lamina (Yogev et al., 2008, 2010). The intracellular trafficking pathway required for secretion is thought to be the canonical secretory pathway.
Intracellular trafficking can regulate the spatiotemporal localization of receptors, as discussed below, or complete organelles. The latter phenomenon is maybe best demonstrated by the identification of the mitochondrial adaptor protein Milton in photoreceptors (Stowers et al., 2002; Glater et al., 2006). Milton acts by recruiting the kinesin heavy chain to mitochondria and its loss of function leads to accumulation of mitochondria in the cell body and absence of mitochondria at synapses. Mutations in a different kinesin (kinesin-3) have revealed transport vesicles that carry components for synapse maturation (Pack-Chung et al., 2007). In both cases, the transport of organelles is required for the further development and function of synapses and represents a simple and highly specific trafficking mechanism as part of the developmental program.
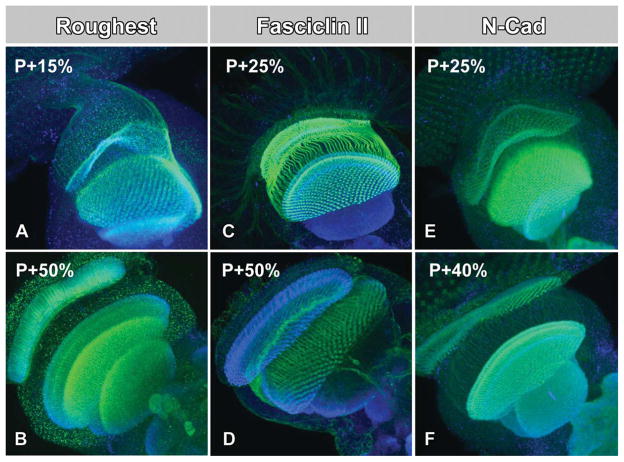
The key proteins thought to orchestrate axon path-finding, visual map formation, and optic lobe development are guidance cues that serve as attractive or repellent cell–cell or cell–matrix recognition signals. Guidance cues are either transmembrane receptors or secreted proteins that, similar to morphogens, bind to receptors at varying distances in the developing organ. Guidance receptors are membrane-bound transmembrane proteins that typically act as adhesion proteins via their extracellular domain and contain adaptor or direct signaling activity on their intracellular domain. A hallmark of guidance receptor expression during brain wiring is spatiotemporal dynamics. Figure 3 shows examples for the guidance receptors Roughest, Fasciclin 2, and N-Cadherin (Ramos et al., 1993; Lin and Goodman, 1994; Lee et al., 2001). Indeed, several mutants have recently been identified that affect the dynamics of spatiotemporal guidance receptor localization and that consequently lead to photoreceptor wiring defects. In the study by Hiesinger et al. 2006, the synapses in more than 50 mutants were analyzed with transmission electron microscopy; 75% of these mutants exhibited defects in synaptic partner selection, but not synapse formation itself. Interestingly, almost all of the developmental mutants also exhibited clear ultrastructural defects of their intracellular vesicle composition. Several of these mutants have already been characterized, including the exocyst component sec15 (Mehta et al., 2005) and the vesicular ATPase component v100 (Hiesinger et al., 2005), both of which are described in more detail in the previous section on optic lobe development. These mutants are similar to the earlier description of the vesicle SNARE neuronal synaptobrevin (n-syb): in all cases intracellular trafficking defects affect the spatiotemporal localization of guidance receptors. None of these mutants affects photoreceptor differentiation, early development or axon pathfinding in photoreceptors, yet have severe effects on optic lobe development (Hiesinger et al., 1999; Hiesinger et al., 2005; Mehta et al., 2005; Williamson et al., 2010a,b). At least for V100 it is now clear that this difference is not due to different functions in the different cell types, but rather due to the context-dependency of the same function in different neurons. Specifically, as described in the previous section, photoreceptor development proceeds faster than CNS neuronal development, changing the requirement for intracellular trafficking machinery, such as degradation machinery (Williamson et al., 2010b). In addition, each of these genes is either neuron-specific or strongly enriched in neurons. Taken together, these observations suggest that neurons employ specialized intracellular trafficking machinery for the establishment of synaptic connectivity downstream of axon pathfinding.
Figure 3.
Three guidance receptors implicated in visual system development. (A, B) Roughest resides in a punctate pattern in axons during axon pathfinding and visual map formation and becomes restricted to synaptic neuropils during synapse formation. (C, D) Fasciclin 2 resides evenly on photoreceptor axons throughout visual map formation. (E, F) N-Cadherin is enriched in areas of future and ongoing synapse formation. Guidance Receptors are labeled in green; synapses labeled for Synaptotagmin in blue.
What have we learned so far about specific functions of intracellular trafficking for eye-brain wiring from trafficking mutant like sec15, n-syb, and v100? None of these trafficking regulators has pinpointed an “instructive” signal that controls a specific localization or signaling event for a specific guidance receptor. Instead, all three exert simple cell biological functions that make sense only in the light of many interconnected cell biological processes. Specifically, sec15 is required for the axonal trafficking of a subset of receptors and signaling components during visual map formation, but there is no evidence that it actively regulates the localization of a certain receptor during a specific developmental time window. Loss of either n-syb or v100 leads to slow developmental intracellular protein accumulations that again are not specific to a distinct guidance receptor. Instead, v100 is part of a neuron-specific endolysosomal degradation mechanism that does not affect photoreceptor development within the short time frame of eye development, but causes guidance receptor signaling defects during the longer time frame of CNS neuron development in the optic lobe. However, loss of v100 in photoreceptors reveals a different strategy employed to achieve specificity: only receptors that undergo active turnover in the endolysosomal system in a spatiotemporally specific manner are subject to v100-dependent accumulation. This is best demonstrated with a mutant version of v100 that re-routes all actively trafficked receptors into degradation-incompetent compartments (Williamson et al., 2010a,b). Interestingly, only receptors that are synthesized and transported to either the cell body or the synapse are subject to V100-dependent sorting and degradation at the respective places, e.g., Rst during eye patterning only in the cell bodies and the proto-cadherin Flamingo only at synapses during visual map formation (Williamson et al., 2010b). It can easily be envisioned that these receptors are also subject to another simple cell biological mechanism that regulates their synthesis and trafficking. The specificity for the precise spatiotemporal localization would then be a function of the intersection of these simple cell biological mechanisms. In other words, complicated spatiotemporal dynamics of a single receptor are likely to be the result of the dynamic interplay of several simple intracellular trafficking mechanisms, none of which is intrinsically “instructive.”
The dynamics of guidance receptors provides insight into what intracellular mechanisms may underlie their seemingly complicated spatiotemporal trafficking. For the examples shown in Figure 3, Rst and Fasciclin 2 show highly dynamic changes in their cellular and subcellular localization profiles, whereas N-Cadherin always appears synaptic. Indeed, Rst and Fas2 require active downregulation at distinct time points of axon pathfinding and target recognition (Schneider et al., 1995). Correspondingly, overexpression of either receptor leads to pathfinding defects that are worse than the null mutants. In contrast, overexpression of N-Cadherin causes no eye–brain wiring defects (Williamson et al., 2010b). A class of guidance receptors that has received a lot of recent attention for the ability to generate an extraordinary amount of isoforms is Dscam1 (Hattori et al., 2008; Schmucker and Chen, 2009). Curiously, the generation of many isoforms in the same neuron, rather than solving the brain wiring puzzle, would provide a formidable challenge for intracellular trafficking dynamics to regulate different spatiotemporal dynamics. Indeed, recent work has established that the principle mode of action for these receptors is the expression of cell-specific isoforms for the purpose of self-recognition and avoidance. This function is a beautiful example for a simple mechanism that, only in concert with other (possibly equally simple) mechanisms, leads to complicated cellular behaviors and ultimately wiring patterns, as recently shown for the precise multi-partner synapses formed by photoreceptors in the lamina (Millard et al., 2010). However, the functions as a constant self-avoidance receptor may not require tightly regulated spatiotemporal dynamics, similar to N-Cadherin. A picture emerges wherein spatiotemporally controlled turnover rates of specific guidance receptors inform us about their utilization and cell biological regulation during brain wiring.
In summary, the functions so far identified for intracellular trafficking machinery suggest simple cell biological mechanisms or “sub-programs” during late photoreceptor and brain development as outlined in Figure 1. For example, the neuronal intracellular sorting and degradation mechanism discussed above is defined by only a few proteins. During early development, this mechanism is not critical for receptor sorting during cell differentiation. In contrast, the necessity to sort large numbers of different receptors during the establishment of synaptic connectivity requires constant receptor turnover for other sorting mechanisms to function. Hence, the same genetically encoded cell biological mechanism only becomes part of the developmental program for brain wiring, but not cell differentiation. Conceptually, this idea is consistent with the more general observation that visual map formation can be genetically dissected into simple developmental sub-programs. We propose that the appearance of complexity in the developmental product is the outcome of a concatenation of simple processes, as discussed below.
ON THE ROLE OF INTRACELLULAR TRAFFICKING IN VISUAL SYSTEM DEVELOPMENT OR HOW SIMPLE MECHANISMS ENCODE COMPLICATED PATTERNS
We have reviewed many examples how cell biological machinery, and intracellular trafficking in particular, govern the development of the eye and its connection to the brain. A common theme of these examples is the simplicity of the underlying cell biological mechanisms. No single intracellular trafficking process determines pattern formation in the eye or the synaptic specificity in the brain. Yet, we know that tightly regulated intracellular trafficking is required for the dynamic and precise endo-/exo-cytosis of receptors and signaling events that determine the developmental product.
The most straightforward and widely assumed functions of intracellular trafficking during pattern formation in general, and brain wiring in particular, are simple mechanisms that are typically described as “permissive.” The idea is that basic machinery, including energy from ATP as well as membranes and cytoskeleton, must be present but plays no active or “information-containing” role. Mutations that cause loss of energy, membrane, or cytoskeletal dynamics will certainly affect pattern formation and brain wiring. However, little may be learned from such mutants about how developmental processes are encoded. This is because they themselves are just machinery or substrates, but contain no information about the developmental program. In contrast, “instructive” mechanisms actively direct developmental processes through signaling or biophysical mechanisms in changing cell shape etc. This distinction has been extensively discussed for the role of cytoskeletal dynamics during axon pathfinding (Dickson, 2001). Since then the quest for cell biological mechanisms that instruct pattern formation, axon pathfinding and synaptic partner choices has become somewhat of a holy grail.
The individual cell biological mechanisms reviewed here are simple and uncover little in the direction of instructive cell biological mechanism for eye patterning or synapse-specific wiring. Yet, the same cell biological mechanisms reveal intracellular spatiotemporal trafficking dynamics that determine the often instructive regulation of signaling receptors. Indeed, the discussion of an instructive role of attractive or repellent guidance receptor signaling is only intelligible in light of tightly regulated spatiotemporal localization. The transcription and translation of a guidance receptor is not any more “instructive” than the cell biological machinery that governs its spatio-temporal dynamics. Information about the developmental program is not encoded in either sets of genes, but in the interplay of intracellular trafficking and receptor signaling, as suggested in Figure 1. More generally, we therefore propose that “instructive” mechanisms can arise from the dynamic interplay of “permissive” machinery. The study of molecular mechanisms at the level of vesicle trafficking, sorting, or degradation may reveal little about how the eye or the brain is put together. Similarly, the study of molecular mechanisms of single receptors and their signaling is more often described as “instructive,” but really does not reveal where the information for the developmental product comes from any more than the cell biological machinery. We therefore argue that the concatenation of simple genetically encoded processes and the ensuing dynamic interplay of these processes are sufficient to give rise to the seeming complexity of the developmental product, including the synaptic specificity of genetically encoded neural circuits. The Drosophila visual system, including its hard-wired eye-brain connection, may come to serve as a key model to decipher this dynamic interplay and developmental program.
Acknowledgments
We thank Dr. Adam Haberman and all members of the Hiesinger laboratory for discussion. We apologize to all of our colleagues whose work was not discussed because of space constraints or our shortcomings.
Contract grant sponsor: Welch Foundation; contract grant number: I-1657.
Contract grant sponsor: Cancer Prevention Research Institute of Texas (CPRIT).
Contract grant sponsor: National Institute of Health (NEI/NIH); contract grant number: RO1018884.
References
- Acharya JK, Dasgupta U, Rawat SS, Yuan C, Sanxaridis PD, Yonamine I, Karim P, et al. Cell-nonautonomous function of ceramidase in photoreceptor homeostasis. Neuron. 2008;57:69–79. doi: 10.1016/j.neuron.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar MA, Ray S, Kramer H. The SM protein Car/ Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles. Mol Biol Cell. 2009;20:1705–1714. doi: 10.1091/mbc.E08-03-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE. Patterning signals and proliferation in Drosophila imaginal discs. Curr Opin Genet Dev. 2007;17:287–293. doi: 10.1016/j.gde.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Baker NE, Yu SY. Proneural function of neurogenic genes in the developing Drosophila eye. Curr Biol. 1997;7:122–132. doi: 10.1016/s0960-9822(06)00056-x. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Wieschaus E. Signaling activities of the Drosophila wingless gene are separately mutable and appear to be transduced at the cell surface. Genetics. 1995;139:309–320. doi: 10.1093/genetics/139.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–244. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U. Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol. 2005;169:635–646. doi: 10.1083/jcb.200410081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RL, Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron. 1993;10:839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989;3:1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Chotard C, Leung W, Salecker I. Glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron. 2005;48:237–251. doi: 10.1016/j.neuron.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Feldheim DA. Making a visual map: Mechanisms and molecules. Curr Opin Neurobiol. 2009;19:174–180. doi: 10.1016/j.conb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000;28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Cronin MA, Lieu MH, Tsunoda S. Two stages of light-dependent TRPL-channel translocation in Drosophila photoreceptors. J Cell Sci. 2006;119:2935–2944. doi: 10.1242/jcs.03049. [DOI] [PubMed] [Google Scholar]
- Daniel A, Dumstrei K, Lengyel JA, Hartenstein V. The control of cell fate in the embryonic visual system by atonal, tailless and EGFR signaling. Development. 1999;126:2945–2954. doi: 10.1242/dev.126.13.2945. [DOI] [PubMed] [Google Scholar]
- Dearborn R, Jr, Kunes S. An axon scaffold induced by retinal axons directs glia to destinations in the Drosophila optic lobe. Development. 2004;131:2291–2303. doi: 10.1242/dev.01111. [DOI] [PubMed] [Google Scholar]
- Devergne O, Ghiglione C, Noselli S. The endocytic control of JAK/STAT signalling in Drosophila. J Cell Sci. 2007;120:3457–3464. doi: 10.1242/jcs.005926. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Rho GTPases in growth cone guidance. Curr Opin Neurobiol. 2001;11:103–110. doi: 10.1016/s0959-4388(00)00180-x. [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Hafen E. Genetic dissection of eye development in Drosophila. In: Bate M, Martinez Arias A, editors. The Development of Drosophila Melanogaster. II. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1993. pp. 1327–1362. [Google Scholar]
- Dubois L, Lecourtois M, Alexandre C, Hirst E, Vincent JP. Regulated endocytic routing modulates wingless signaling in Drosophila embryos. Cell. 2001;105:613–624. doi: 10.1016/s0092-8674(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 2010;90:471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Gold KS, Brand AH. Notch regulates the switch from symmetric to asymmetric neural stem cell division in the Drosophila optic lobe. Development. 2010;137:2981–2987. doi: 10.1242/dev.051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun SH, Lea K, Overstreet E, Stevens S, Lee JH, Fischer JA. Identification of genes that interact with Drosophila liquid facets. Genetics. 2007;175:1163–1174. doi: 10.1534/genetics.106.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SS, Ready DF. Glued participates in distinct microtubule-based activities in Drosophila eye development. Development. 1997;124:1497–1507. doi: 10.1242/dev.124.8.1497. [DOI] [PubMed] [Google Scholar]
- Fischbach KF, Hiesinger PR. Optic lobe development. Adv Exp Med Biol. 2008;628:115–136. doi: 10.1007/978-0-387-78261-4_8. [DOI] [PubMed] [Google Scholar]
- Flaherty MS, Zavadil J, Ekas LA, Bach EA. Genome-wide expression profiling in the Drosophila eye reveals unexpected repression of notch signaling by the JAK/STAT pathway. Dev Dyn. 2009;238:2235–2253. doi: 10.1002/dvdy.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgac M. Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- Frankfort BJ, Mardon G. R8 development in the Drosophila eye: A paradigm for neural selection and differentiation. Development. 2002;129:1295–1306. doi: 10.1242/dev.129.6.1295. [DOI] [PubMed] [Google Scholar]
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- Freeman M. Morphogen gradients, in theory. Dev Cell. 2002;2:689–690. doi: 10.1016/s1534-5807(02)00193-4. [DOI] [PubMed] [Google Scholar]
- Freeman M. Rhomboids: 7 years of a new protease family. Semin Cell Dev Biol. 2009;20:231–239. doi: 10.1016/j.semcdb.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. Pax 6: Mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Gilbert MM, Beam CK, Robinson BS, Moberg KH. Genetic interactions between the Drosophila tumor suppressor gene ept and the stat92E transcription factor. PLoS One. 2009;4:e7083. doi: 10.1371/journal.pone.0007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CS, Doe CQ. Embryonic development of the Drosophila central nervous system. In: Bate M, Martinez Arias A, editors. The Development of Drosophila Melanogaster. II. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1993. pp. 1131–1206. [Google Scholar]
- Greenwald I. LIN-12/Notch signaling: Lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- Hadjieconomou D, Timofeev K, Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol. 2010;21:76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Hagedorn EJ, Bayraktar JL, Kandachar VR, Bai T, Englert DM, Chang HC. Drosophila melanogaster auxilin regulates the internalization of Delta to control activity of the Notch signaling pathway. J Cell Biol. 2006;173:443–452. doi: 10.1083/jcb.200602054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development. 2004;131:601–611. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz HM, Woodfield SE, Chen Z, Bolduc C, Bergmann A. Common and distinct genetic properties of ESCRT-II components in Drosophila. PLoS One. 2009;4:e4165. doi: 10.1371/journal.pone.0004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesinger PR, Reiter C, Schau H, Fischbach KF. Neuropil pattern formation and regulation of cell adhesion molecules in Drosophila optic lobe development depend on synaptobrevin. J Neurosci. 1999;19:7548–7556. doi: 10.1523/JNEUROSCI.19-17-07548.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesinger PR, Fayyazuddin A, Mehta SQ, Rosenmund T, Schulze KL, Zhai RG, Verstreken P, et al. The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell. 2005;121:607–620. doi: 10.1016/j.cell.2005.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, et al. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Fostier M, Ito M, Fuwa TJ, Go MJ, Okano H, Baron M, Matsuno K. Drosophila deltex mediates suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development. 2004;131:5527–5537. doi: 10.1242/dev.01448. [DOI] [PubMed] [Google Scholar]
- Houalla T, Shi L, van Meyel DJ, Rao Y. Rab-mediated vesicular transport is required for neuronal positioning in the developing Drosophila visual system. Mol Brain. 2010;3:19. doi: 10.1186/1756-6606-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung F, Moses K. Retinal development in Drosophila: Specifying the first neuron. Hum Mol Genet. 2002;11:1207–1214. doi: 10.1093/hmg/11.10.1207. [DOI] [PubMed] [Google Scholar]
- Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. Int Rev Cytol. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- Huang Z, Shilo BZ, Kunes S. A retinal axon fascicle uses spitz, an EGF receptor ligand, to construct a synaptic cartridge in the brain of Drosophila. Cell. 1998;95:693–703. doi: 10.1016/s0092-8674(00)81639-6. [DOI] [PubMed] [Google Scholar]
- Iyadurai SJ, Robinson JT, Ma L, He Y, Mische S, Li MG, Brown W, Guichard A, Bier E, Hays TS. Dynein and Star interact in EGFR signaling and ligand trafficking. J Cell Sci. 2008;121:2643–2651. doi: 10.1242/jcs.027144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H, Andrews HK, Acar M, Bayat V, Wirtz-Peitz F, Mehta SQ, Knoblich JA, Bellen HJ. Sec15, a component of the exocyst, promotes notch signaling during the asymmetric division of Drosophila sensory organ precursors. Dev Cell. 2005;9:351–363. doi: 10.1016/j.devcel.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Jefferies KC, Cipriano DJ, Forgac M. Function, structure and regulation of the vacuolar (H+)-ATPases. Arch Biochem Biophys. 2008;476:33–42. doi: 10.1016/j.abb.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst K, Kunes S. Pattern formation in the visual centers of the Drosophila brain: Wingless acts via decapentaplegic to specify the dorsoventral axis. Cell. 1994;78:437–448. doi: 10.1016/0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Ribeiro C, Dickson BJ. Comm function in commissural axon guidance: Cell-autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- Kumar JP. My what big eyes you have: How the Drosophila retina grows. Dev Neurobiol. 2011;71:1133–1152. doi: 10.1002/dneu.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar JP, Moses K. The EGF receptor and notch signaling pathways control the initiation of the morpho-genetic furrow during Drosophila eye development. Development. 2001;128:2689–2697. doi: 10.1242/dev.128.14.2689. [DOI] [PubMed] [Google Scholar]
- Kurada P, White K. Epidermal growth factor receptor: Its role in Drosophila eye differentiation and cell survival. Apoptosis. 1999;4:239–243. doi: 10.1023/a:1009648724937. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Bardin A, Schweisguth F. The roles of receptor and ligand endocytosis in regulating Notch signaling. Development. 2005;132:1751–1762. doi: 10.1242/dev.01789. [DOI] [PubMed] [Google Scholar]
- Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Li BX, Satoh AK, Ready DF. Myosin V. Rab11, and d Rip11 direct apical secretion and cellular morphogenesis in developing Drosophila photoreceptors. J Cell Biol. 2007;177:659–669. doi: 10.1083/jcb.200610157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Lloyd V, Ramaswami M, Kramer H. Not just pretty eyes: Drosophila eye-colour mutations and lysosomal delivery. Trends Cell Biol. 1998;8:257–259. doi: 10.1016/s0962-8924(98)01270-7. [DOI] [PubMed] [Google Scholar]
- Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- Mehta SQ, Hiesinger PR, Beronja S, Zhai RG, Schulze KL, Verstreken P, Cao Y, et al. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 2005;46:219–232. doi: 10.1016/j.neuron.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Hanson TE. The development of the optic lobe. In: Bate M, Martinez Arias A, editors. The Development of Drosophila Melanogaster. II. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1993. pp. 1363–1491. [Google Scholar]
- Meir E, von Dassow G, Munro E, Odell GM. Robustness, flexibility, and the role of lateral inhibition in the neurogenic network. Curr Biol. 2002;12:778–786. doi: 10.1016/s0960-9822(02)00839-4. [DOI] [PubMed] [Google Scholar]
- Melnattur KV, Lee C-H. Visual circuit assembly in Drosophila. Dev Neurobiol. 2011;71:1286–1296. doi: 10.1002/dneu.20894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa R, Yamamoto-Hino M, Awano W, Hinohara Y, Suzuki E, Ueda R, Goto S. Autophagy-dependent rhodopsin degradation prevents retinal degeneration in Drosophila. J Neurosci. 2010;30:10703–10719. doi: 10.1523/JNEUROSCI.2061-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard SS, Lu Z, Zipursky SL, Meinertzhagen IA. Drosophila dscam proteins regulate postsynaptic specificity at multiple-contact synapses. Neuron. 2010;67:761–768. doi: 10.1016/j.neuron.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Cagan RL. Local induction of patterning and programmed cell death in the developing Drosophila retina. Development. 1998;125:2327–2335. doi: 10.1242/dev.125.12.2327. [DOI] [PubMed] [Google Scholar]
- Miura GI, Roignant JY, Wassef M, Treisman JE. Myopic acts in the endocytic pathway to enhance signaling by the Drosophila EGF receptor. Development. 2008;135:1913–1922. doi: 10.1242/dev.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Moline MM, Southern C, Bejsovec A. Directionality of wingless protein transport influences epidermal patterning in the Drosophila embryo. Development. 1999;126:4375–4384. doi: 10.1242/dev.126.19.4375. [DOI] [PubMed] [Google Scholar]
- Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, Pfeiffer BD, Celniker SE, Stenmark H, Bilder D. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell. 2008;19:4167–4176. doi: 10.1091/mbc.E08-07-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Kopan R. Notch signaling: From the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Nagaraj R, Banerjee U. Regulation of notch and wingless signalling by phyllopod, a transcriptional target of the EGFR pathway. EMBO J. 2009;28:337–346. doi: 10.1038/emboj.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. A journey from mammals to yeast with vacuolar H+-ATPase (V-ATPase) J Bioenerg Biomembr. 2003;35:281–289. doi: 10.1023/a:1025768529677. [DOI] [PubMed] [Google Scholar]
- Newsome TP, Asling B, Dickson BJ. Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development. 2000;127:851–860. doi: 10.1242/dev.127.4.851. [DOI] [PubMed] [Google Scholar]
- Ngo KT, Wang J, Junker M, Kriz S, Vo G, Asem B, Olson JM, Banerjee U, Hartenstein V. Concomitant requirement for Notch and Jak/Stat signaling during neuro-epithelial differentiation in the Drosophila optic lobe. Dev Biol. 2010;346:284–295. doi: 10.1016/j.ydbio.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EC, Walsh CA. Smooth, rough and upside-down neocortical development. Curr Opin Genet Dev. 2002;12:320–327. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- Orem NR, Xia L, Dolph PJ. An essential role for endocytosis of rhodopsin through interaction of visual arrestin with the AP-2 adaptor. J Cell Sci. 2006;119:3141–3148. doi: 10.1242/jcs.03052. [DOI] [PubMed] [Google Scholar]
- Overstreet E, Fitch E, Fischer JA. Fat facets and liquid facets promote delta endocytosis and delta signaling in the signaling cells. Development. 2004;131:5355–5366. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- Pack-Chung E, Kurshan PT, Dickman DK, Schwarz TL. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci. 2007;10:980–989. doi: 10.1038/nn1936. [DOI] [PubMed] [Google Scholar]
- Parks AL, Klueg KM, Stout JR, Muskavitch MA. Ligand endocytosis drives receptor dissociation and activation in the Notch pathway. Development. 2000;127:1373–1385. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- Peralta S, Gomez Y, Gonzalez-Gaitan MA, Moya F, Vinos J. Notch down-regulation by endocytosis is essential for pigment cell determination and survival in the Drosophila retina. Mech Dev. 2009;126:256–269. doi: 10.1016/j.mod.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Piddini E, Marshall F, Dubois L, Hirst E, Vincent JP. Arrow (LRP6) and Frizzled2 cooperate to degrade Wingless in Drosophila imaginal discs. Development. 2005;132:5479–5489. doi: 10.1242/dev.02145. [DOI] [PubMed] [Google Scholar]
- Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Ramos RG, Igloi GL, Lichte B, Baumann U, Maier D, Schneider T, Brandstatter JH, Frohlich A, Fischbach KF. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 1993;7:2533–2547. doi: 10.1101/gad.7.12b.2533. [DOI] [PubMed] [Google Scholar]
- Rister J, Desplan C. The retinal mosaics of opsin expression in invertebrates and vertebrates. Dev Neurobiol. 2011;71:1212–1226. doi: 10.1002/dneu.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J, Heisenberg M. Distinct functions of neuronal synaptobrevin in developing and mature fly photoreceptors. J Neurobiol. 2006;66:1271–1284. doi: 10.1002/neu.20284. [DOI] [PubMed] [Google Scholar]
- Roignant JY, Treisman JE. Pattern formation in the Drosophila eye disc. Int J Dev Biol. 2009;53:795–804. doi: 10.1387/ijdb.072483jr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol. 2005;15:1722–1733. doi: 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Satoh AK, O’Tousa JE, Ozaki K, Ready DF. Rab11 mediates post-Golgi trafficking of rhodopsin to the photosensitive apical membrane of Drosophila photoreceptors. Development. 2005;132:1487–1497. doi: 10.1242/dev.01704. [DOI] [PubMed] [Google Scholar]
- Satoh A, Tokunaga F, Kawamura S, Ozaki K. In situ inhibition of vesicle transport and protein processing in the dominant negative Rab1 mutant of Drosophila. J Cell Sci. 1997;110(Pt 23):2943–2953. doi: 10.1242/jcs.110.23.2943. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Chen B. Dscam and DSCAM: Complex genes in simple animals, complex animals yet simple genes. Genes Dev. 2009;23:147–156. doi: 10.1101/gad.1752909. [DOI] [PubMed] [Google Scholar]
- Schneider T, Reiter C, Eule E, Bader B, Lichte B, Nie Z, Schimansky T, Ramos RG, Fischbach KF. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron. 1995;15:259–271. doi: 10.1016/0896-6273(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Schuck S, Simons K. Polarized sorting in epithelial cells: Raft clustering and the biogenesis of the apical membrane. J Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- Seto ES, Bellen HJ. Internalization is required for proper Wingless signaling in Drosophila melanogaster. J Cell Biol. 2006;173:95–106. doi: 10.1083/jcb.200510123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during Notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–598. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- Sevrioukov EA, He JP, Moghrabi N, Sunio A, Kramer H. A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol Cell. 1999;4:479–486. doi: 10.1016/s1097-2765(00)80199-9. [DOI] [PubMed] [Google Scholar]
- Shalaby NA, Parks AL, Morreale EJ, Osswalt MC, Pfau KM, Pierce EL, Muskavitch MA. A screen for modifiers of notch signaling uncovers Amun, a protein with a critical role in sensory organ development. Genetics. 2009;182:1061–1076. doi: 10.1534/genetics.108.099986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty KM, Kurada P, O’Tousa JE. Rab6 regulation of rhodopsin transport in Drosophila. J Biol Chem. 1998;273:20425–20430. doi: 10.1074/jbc.273.32.20425. [DOI] [PubMed] [Google Scholar]
- Shilo BZ. Signaling by the Drosophila epidermal growth factor receptor pathway during development. Exp Cell Res. 2003;284:140–149. doi: 10.1016/s0014-4827(02)00094-0. [DOI] [PubMed] [Google Scholar]
- Silies M, Klambt C. APC/C(Fzr/Cdh1)-dependent regulation of cell adhesion controls glial migration in the Drosophila PNS. Nat Neurosci. 2010;13:1357–1364. doi: 10.1038/nn.2656. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Cumming RC, Lindmo K, Galaviz V, Cheng S, Rusten TE, Finley KD. Genetic modifiers of the Drosophila blue cheese gene link defects in lysosomal transport with decreased life span and altered ubiquitinated-protein profiles. Genetics. 2007;176:1283–1297. doi: 10.1534/genetics.106.065011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Schwarz TL. A genetic method for generating Drosophila eyes composed exclusively of mitotic clones of a single genotype. Genetics. 1999;152:1631–1639. doi: 10.1093/genetics/152.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigini M, Cohen SM. Formation of morphogen gradients in the Drosophila wing. Semin Cell Dev Biol. 1999;10:335–344. doi: 10.1006/scdb.1999.0293. [DOI] [PubMed] [Google Scholar]
- Sun X, Artavanis-Tsakonas S. The intracellular deletions of delta and serrate define dominant negative forms of the Drosophila notch ligands. Development. 1996;122:2465–2474. doi: 10.1242/dev.122.8.2465. [DOI] [PubMed] [Google Scholar]
- Sun X, Artavanis-Tsakonas S. Secreted forms of DELTA and SERRATE define antagonists of Notch signaling in Drosophila. Development. 1997;124:3439–3448. doi: 10.1242/dev.124.17.3439. [DOI] [PubMed] [Google Scholar]
- Tayler TD, Robichaux MB, Garrity PA. Compartmentalization of visual centers in the Drosophila brain requires Slit and Robo proteins. Development. 2004;131:5935–5945. doi: 10.1242/dev.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Tien AC, Rajan A, Bellen HJ. A notch updated. J Cell Biol. 2009;184:621–629. doi: 10.1083/jcb.200811141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CY, Lee CH. Visual circuit development in Drosophila. Curr Opin Neurobiol. 2007;17:65–72. doi: 10.1016/j.conb.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Tio M, Moses K. The Drosophila TGF alpha homolog Spitz acts in photoreceptor recruitment in the developing retina. Development. 1997;124:343–351. doi: 10.1242/dev.124.2.343. [DOI] [PubMed] [Google Scholar]
- Torroja C, Gorfinkiel N, Guerrero I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development. 2004;131:2395–2408. doi: 10.1242/dev.01102. [DOI] [PubMed] [Google Scholar]
- Treisman JE, Rubin GM. Wingless inhibits morpho-genetic furrow movement in the Drosophila eye disc. Development. 1995;121:3519–3527. doi: 10.1242/dev.121.11.3519. [DOI] [PubMed] [Google Scholar]
- Truman JW, Taylor BJ, Awad TA. Formation of the adult nervous system. In: Bate M, Martinez Arias A, editors. The Development of Drosophila Melanogaster. II. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1993. pp. 1245–1275. [Google Scholar]
- Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development. 2010;137:1825–1832. doi: 10.1242/dev.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- Vidal OM, Stec W, Bausek N, Smythe E, Zeidler MP. Negative regulation of Drosophila JAK-STAT signalling by endocytic trafficking. J Cell Sci. 2010;123:3457–3466. doi: 10.1242/jcs.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Lao U, Edgar BA. TOR-mediated autophagy regulates cell death in Drosophila neurodegenerative disease. J Cell Biol. 2009;186:703–711. doi: 10.1083/jcb.200904090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Montell C. Phototransduction and retinal degeneration in Drosophila. Pflugers Arch. 2007;454:821–847. doi: 10.1007/s00424-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Wang W, Struhl G. Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development. 2004;131:5367–5380. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- Wang Y, Werz C, Xu D, Chen Z, Li Y, Hafen E, Bergmann A. Drosophila cbl is essential for control of cell death and cell differentiation during eye development. PLoS One. 2008;3:e1447. doi: 10.1371/journal.pone.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber U, Eroglu C, Mlodzik M. Phospholipid membrane composition affects EGF receptor and Notch signaling through effects on endocytosis during Drosophila development. Dev Cell. 2003;5:559–570. doi: 10.1016/s1534-5807(03)00273-9. [DOI] [PubMed] [Google Scholar]
- Weinmaster G. Notch signal transduction: A real rip and more. Curr Opin Genet Dev. 2000;10:363–369. doi: 10.1016/s0959-437x(00)00097-6. [DOI] [PubMed] [Google Scholar]
- Whited JL, Cassell A, Brouillette M, Garrity PA. Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development. 2004;131:4677–4686. doi: 10.1242/dev.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson WR, Hiesinger PR. Preparation of developing and adult Drosophila brains and retinae for live imaging. J Vis Exp. 2010;15:936. doi: 10.3791/1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson WR, Wang D, Haberman AS, Hiesinger PR. A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J Cell Biol. 2010a;189:885–899. doi: 10.1083/jcb.201003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson WR, Yang T, Terman JR, Hiesinger PR. Guidance receptor degradation is required for neuronal connectivity in the Drosophila nervous system. PLoS Biol. 2010b;8:e1000553. doi: 10.1371/journal.pbio.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern ormation in the Drosophila retina. In: Bate M, Martinez Arias A, editors. The Development of Drosophila Melanogaster. II. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1325. [Google Scholar]
- Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–885. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- Xia H, Ready DF. Ectoplasm, ghost in the R cell machine? Dev Neurobiol. 2011;71:1246–1257. doi: 10.1002/dneu.20898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong WC, Montell C. Defective glia induce neuronal apoptosis in the repo visual system of Drosophila. Neuron. 1995;14:581–590. doi: 10.1016/0896-6273(95)90314-3. [DOI] [PubMed] [Google Scholar]
- Yan Y, Denef N, Schupbach T. The vacuolar proton pump. V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell. 2009;17:387–402. doi: 10.1016/j.devcel.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasugi T, Sugie A, Umetsu D, Tabata T. Coordinated sequential action of EGFR and notch signaling pathways regulates proneural wave progression in the Drosophila optic lobe. Development. 2010;137:3193–3203. doi: 10.1242/dev.048058. [DOI] [PubMed] [Google Scholar]
- Yasugi T, Umetsu D, Murakami S, Sato M, Tabata T. Drosophila optic lobe neuroblasts triggered by a wave of proneural gene expression that is negatively regulated by JAK/STAT. Development. 2008;135:1471–1480. doi: 10.1242/dev.019117. [DOI] [PubMed] [Google Scholar]
- Yogev S, Schejter ED, Shilo BZ. Drosophila EGFR signalling is modulated by differential compartmentalization of Rhomboid intramembrane proteases. EMBO J. 2008;27:1219–1230. doi: 10.1038/emboj.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev S, Schejter ED, Shilo BZ. Polarized secretion of Drosophila EGFR ligand from photoreceptor neurons is controlled by ER localization of the ligand-processing machinery. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Soustelle L, Giangrande A, Umetsu D, Murakami S, Yasugi T, Awasaki T, Ito K, Sato M, Tabata T. DPP signaling controls development of the lamina glia required for retinal axon targeting in the visual system of Drosophila. Development. 2005;132:4587–4598. doi: 10.1242/dev.02040. [DOI] [PubMed] [Google Scholar]
- Yu SY, Yoo SJ, Yang L, Zapata C, Srinivasan A, Hay BA, Baker NE. A pathway of signals regulating effector and initiator caspases in the developing Drosophila eye. Development. 2002;129:3269–3278. doi: 10.1242/dev.129.13.3269. [DOI] [PubMed] [Google Scholar]
- Yuva-Aydemir Y, Klämbt C. Long-range signaling systems controlling glial migration in the Drosophila eye. Dev Neurobiol. 2011;71:1310–1316. doi: 10.1002/dneu.20893. [DOI] [PubMed] [Google Scholar]