Abstract
Assembly of functioning testis and ovary requires a GATA4-FOG2 transcriptional complex. To define the separate roles for GATA4 and FOG2 proteins in sexual development of the testis we have ablated the corresponding genes in somatic gonadal cells. We have established that GATA4 is required for testis differentiation, for the expression of Dmrt1 gene, and for testis cord morphogenesis. While Sf1Cre-mediated excision of Gata4 permitted normal expression of most genes associated with embryonic testis development, gonadal loss of Fog2 resulted in an early partial block in male pathway and sex reversal. We have also determined that testis sexual differentiation is sensitive to the timing of GATA4 loss during embryogenesis. Our results now demonstrate that these two genes also have non-overlapping essential functions in testis development.
INTRODUCTION
Unlike other organs that can either develop normally or become malformed, the gonadal primordia are fully competent to embark on two natural developmental paths. This bipotential nature of the gonadal anlagen provides an unparalleled system to compare the two rival developmental mechanisms that culminate in outcomes that are dramatically divergent and, at the same time, completely predictable.
Transformation of the indifferent gonad into a testis is a prerequisite for male sex determination. The Sry gene is the initiator of testis development in eutherians (Swain and Lovell-Badge, 1999; Wilhelm et al., 2007) and many of the cellular and morphological events that occur downstream of Sry have been well characterized in mammals, particularly in mice. The critical morphogenetic event in embryonic testis development is testis cord formation (Combes et al., 2009; Coveney et al., 2008; Nel-Themaat et al., 2009). This profound reorganization of gonadal cells gives an embryonic testis its characteristic appearance and is required for normal male development. Shortly after the initiation of Sry expression at ~E10.5, there is a marked increase in the proliferation of coelomic epithelial cells in XY gonads. A fraction of the gonadal somatic cells differentiates to become Sertoli cells – the specialized cells that surround germ cells and form testis cords between E11.2 and E12.5. In the interstitial space between the cords reside the Leydig cells, which are responsible for testosterone production (Cool and Capel, 2009). SRY and subsequently the transcriptional regulator SOX9 are two key proteins required to initiate this distinctive structural arrangement. In contrast to the dramatic restructuring of embryonic testis, the mammalian ovary undergoes major morphological changes only after birth. Despite appearing almost dormant, embryonic ovaries initiate and maintain an active gene expression program that acts to suppress the male pathway of development and to promote meiosis (Brennan and Capel, 2004; Sekido and Lovell-Badge, 2009; Tevosian and Manuylov, 2008).
The GATA zinc-finger transcription factors (designated GATA1 to GATA6) bind the consensus target sequence WGATAR. These proteins play critical roles in various developmental processes, including hematopoietic and T cell differentiation, cardiac and coronary vasculature development, and liver, lung and gut morphogenesis (reviewed in (LaVoie, 2003; Patient and McGhee, 2002; Viger et al., 2008)). Gata4 appears to be the sole GATA family member active in somatic (and not germ) cells in the early developing gonad in mice (Anttonen et al., 2003; Heikinheimo et al., 1997; Lavoie et al., 2004; Viger et al., 1998). At E11.5, Gata4 is expressed in somatic cells of both XX and XY genital ridges (Heikinheimo et al., 1997; Ketola et al., 2000; Viger et al., 1998). At E13.5, Gata4 expression becomes sexually dimorphic: in XY gonads expression is upregulated in Sertoli cells and to some extent reduced in interstitial cells, whereas in XX gonads a strong-to-moderate expression is observed in all somatic cells (Anttonen et al., 2003). A similar pattern of ovarian Gata4 expression has been reported in the rat (Lavoie et al., 2004). Gata4 expression persists in the somatic cells of postnatal testes and in adult ovaries with predominant expression in granulosa cells (Anttonen et al., 2003; Heikinheimo et al., 1997; Viger et al., 1998).
The normal function of GATA proteins in vertebrates requires a physical interaction with multitype zinc-finger co-factors of the FOG (Friend of GATA) family (for reviews, see (Cantor and Orkin, 2005; Fossett et al., 2001)). The gonadal Fog2 expression generally parallels that of Gata4 between E11.0–E13.5 albeit Fog2 expression is skewed even stronger towards Sertoli cells; after E13.5 Fog2 becomes notably reduced in the testis and remains low during subsequent embryonic development ((Anttonen et al., 2003; Lu et al., 1999; Manuylov et al., 2007a; Svensson et al., 1999; Tevosian et al., 1999); Tevosian, unpublished).
Mouse fetuses homozygous for a null allele of Fog2 die at mid-gestation from cardiac defects (Tevosian et al., 2000). Because Fog2−/− embryos survive until ~E14.0, analysis of early gonad development in the absence of FOG2 was possible (Manuylov et al., 2008; Tevosian et al., 2002). In contrast, Gata4 null embryos die at E7.0–9.5 (Kuo et al., 1997; Molkentin et al., 1997), which precluded analysis of their gonadal differentiation. This problem was partially overcome by using a Gata4 knock-in allele (Gata4ki, a V217G amino acid substitution) that abrogates the interaction between GATA4 and FOG2 (or FOG1) (Crispino et al., 2001). Homozygous Gata4ki embryos survive to E13.0 when they die from cardiac abnormalities similar to those noted in Fog2−/− embryos (Crispino et al., 2001).
We demonstrated previously that GATA4 and FOG2 and their physical interaction are required for normal testis development (Tevosian et al., 2002). Fog2 null and Gata4ki/ki mutant XY gonads are able to initiate the expression of Sry (albeit at a substantially lower level compared to the wild-type controls), but not of several other genes crucial for normal Sertoli cell function (e.g., Sox9, Mis (Amh) and Dhh) (Tevosian et al., 2002). We have also reported that Fog2 haploinsufficiency prevents (suppresses) the dominant sex-reversal in Ods and Wt1-Sox9 XX mice (Manuylov et al., 2007a). Subsequently we have determined that ovarian development is profoundly affected by the loss of GATA4-FOG2 interaction (Manuylov et al., 2008). We have also identified the Dkk1 gene, which encodes a secreted inhibitor of canonical β-catenin signaling, as a target (direct or indirect) of GATA4-FOG2 repression in the gonad (Manuylov et al., 2008).
The Gata4ki allele allows unique insight into the importance of GATA4-FOG2 interaction in mammalian development. However, the specific function of GATA4 in gonadal development has not been examined separately from its FOG2 protein partner. To determine the distinct role of GATA4 in gonadal sex differentiation, we now used a conditional GATA4 loss-of-function approach. This circumvented early embryonic lethality of conventional Gata4 knockouts and permitted temporally and spatially restricted GATA4 inactivation. Outcomes of Gata4 gene loss in gonads were compared to conditional inactivation of Fog2. Our results demonstrated that the two proteins also have non-overlapping essential functions in the development of the testis.
MATERIALS and METHODS
Animals
Floxed strains
Gata4flox/flox mice and Gata4flap/flap were described previously (Ma et al., 2008; Zeisberg et al., 2005) and were maintained on a mixed 129xC56BL/6 genetic background. Briefly, the Gata4flox allele, which contains loxP sites flanking the translational start site and the region encoding the GATA4 N-terminal activation domains, expresses wild-type Gata4 (Zeisberg et al., 2005). Exposure of Gata4flox allele to a Cre recombinase removes exon 2. Due to aberrant splicing and an internal in frame start codon, the recombined allele synthesizes a truncated, transcriptionally inactive form of GATA4 (Bosse et al., 2006; Rivera-Feliciano et al., 2006). Mice were genotyped with primers Gata4for 5’-ccttgctttctgcctgctaacacac-3’ and Gata4rev 5’-tgtcattcttcgctggagccgc-3’ (all primers are shown 5’-to-3’). To ensure that the GATA4 loss phenotype is not influenced by the way Gata4 allele is modified (floxed), we have also used two other targeted alleles of Gata4, Gata4flap (Ma et al., 2008) (as described below) and Gata4loxP (Gata4tm1.1Sad/J (Watt et al., 2004)) (data not shown) with similar results. Gata4flap contains a loxP-Gata4 cDNA-transcriptional stop-loxP cassette followed by an alkaline phosphatase (AP) cDNA at the endogenous Gata4 start codon. In these animals, Gata4 cDNA completely replaced Gata4 expression from the endogenous gene. Cre recombinase deletes the Gata4 cDNA and the transcriptional stop signal with concomitant AP expression (Ma et al., 2008). In Gata4loxP animals Cre recombinase deletes exons 3–5 of the Gata4 gene that encode for both zinc finger DNA-binding domains and the nuclear localization signal that are essential for GATA4 function (Watt et al., 2004)). Fog2flox/flox mice have been previously described (Manuylov et al., 2007b) and were on pure C57BL/6 strain genetic background (a kind gift of Eva Eicher). Mice were genotyped with primers Fog2for ggtcttcgacatccatgtttcacagc and Fog2rev tgcgagaacaggtgttggtggaagtt (Manuylov et al., 2007b).
Cre strains
To produce mouse strains with gonad-specific deletions, targeted mice were crossed with a Sf1Cre line of animals (a kind gift of Dr. Keith Parker;(Bingham et al., 2006)) to obtain Sf1cre/+;Fog2flox/+ and Sf1cre/+;Gata4flox/+ males. Sf1Cre transgenic mice, in which a bacterial artificial chromosome (BAC) harboring Sf1 regulatory elements directs Cre expression to the somatic cells of the gonads (as well as to a limited number of extra-gonadal tissues), have been previously described (Bingham et al., 2006). Sf1Cre line was maintained on a mixed 129xC56BL/6 background. These males were mated to Gata4flox/flox females or Fog2flox/flox females to generate mutant animals with gonad-specific deletions, GATA4SF and FOG2SF. A Wt1CreERT2 line was previously described and maintained on a mixed 129xC56BL/6 background (Zhou et al., 2008). Wt1CreERT2 animals were used to obtain Gata4flox, Gata4flap and Gata4loxP mutants in a fashion similar to the one described above for Sf1Cre animals. Tamoxifen (Sigma T5648 emulsified in sesame oil) was injected intraperitoneally at 0.1 mg/g into pregnant females at E10.5, E11.5 or into adult mice to induce Cre activity. Animal sex was determined with Y chromosome specific primers: zfy5 gactagacatgtcttaacatctgtcc and zfy3 cctattgcatggactgcagcttatg. All animal work was approved by the Institutional Animal Care and Use Committees.
In Situ Hybridization
Whole mount in situ hybridization (WISH) was performed essentially as previously described (Manuylov et al., 2008). Embryos of various stages were dissected from the uterus and fixed with 4% paraformaldehyde in 1xPBS at 4°C overnight. To generate RNA in situ probes, cDNA fragments were produced from the embryonic total or embryonic gonadal RNA by RT-PCR. RNA in situ probes were generated by in vitro transcription of subcloned fragments. The following primers were used to generate Cst9 and Clu RNA probes: Cst9f, gcagtagctgtggagttctg and Cst9r, ttcagaccatggctctcctg; Cluf, actgttcaaccaacaatcctg and Clur, cttttcctgcggtattcctgt.
Immunofluorescence
Frozen sections were cut on the cryostat (Leica) and mounted on Vectabond (Vector Laboratories)-covered glass slides. Primary antibodies used for protein detection and their dilutions are described in Supp_Table 1. All primary antibodies were diluted in the antibody diluent solution (Dako); fluorescent secondary antibodies (all Alexa Fluor from Invitrogen) were used at 1:500 dilution. The slides were mounted in Vectashield with DAPI (40,6-Diamidino-2-phenylindole, Vector) and photographed. The confocal analysis was performed as previously described (Manuylov et al., 2007a).
TUNEL Assay
Testes and ovaries were dissected at different stages and apoptotic cells were detected by terminal deoxynucleotide UTP nick-end labeling (TUNEL) using an In Situ Cell Death Detection kit (Roche, Indianapolis, IN, USA). Nuclei were counterstained with Vectashield with DAPI (Vector, Burlingame, CA, USA). TUNEL-positive nuclei were counted in 10 random microscopic fields; three mice of each genotype (Gata4flox/flox, GATA4SF, Fog2flox/flox and FOG2SF) were analyzed.
Histology
Whole embryos or dissected gonads were fixed in Bouin’s solution, dehydrated through an ethanol series, and transferred to paraffin. Paraffin blocks were sectioned on an Olympus Cut 4060; the 7μ sections were collected onto Superfrost slides (Fisher) and dried overnight. Sections were stained with hematoxylin and eosin (H&E) and photographed.
RESULTS
Loss of GATA4 is Incompatible with Normal Testis Development
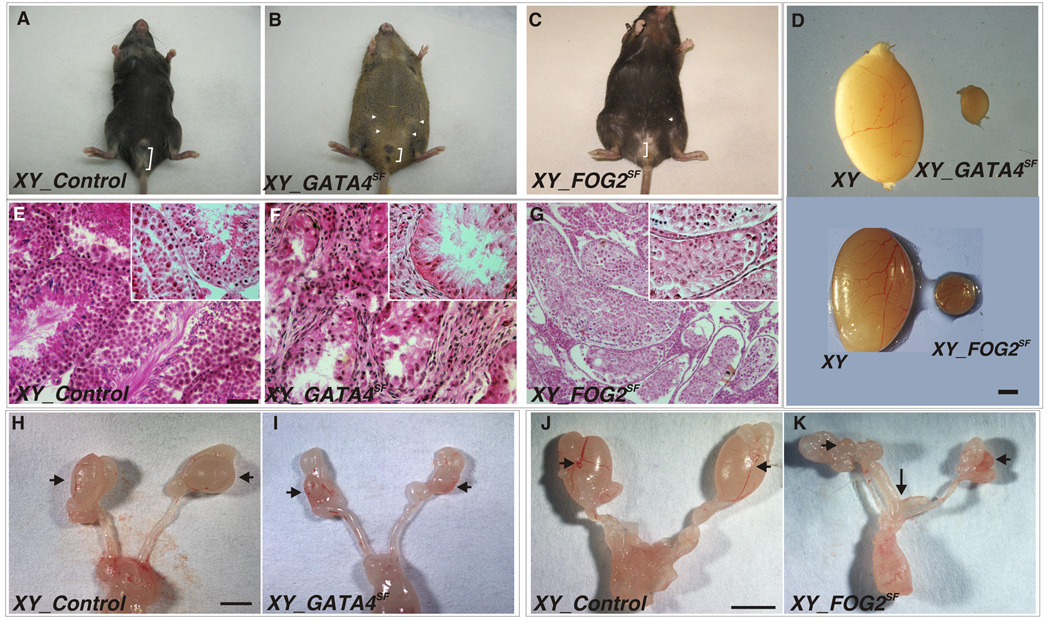
The crosses of Sf1Cre; Gata4flox/wt males with Gata4flox/flox females to generate experimental animals with the Sf1Cre; Gata4flox/flox genotype (abbreviated GATA4SF) yielded approximately equal numbers of XX and XY mice (52 and 48%, respectively; n = 6 litters). The genotype of all animals corresponded to the phenotype of the external genitalia. All (N=75) GATA4SF XY mice (Fig.1B) appeared homogenously as undervirilized males with a hypoplastic penis, mammary teats, and a relatively shorter ano-genital distance compared to that in the control males (Fig.1A). GATA4SF males (n=10) housed for 2–6 months with fertility-proven wild-type C57BL/6 females did not sire any progeny. The mean paired testis weight (±SEM) in heterozygous animals at 6 weeks of age was significantly greater than that of GATA4SF males of this age (84.7 ± 6.8 mg vs. 2.21 ± 0.43 mg, n = 6, P < 0.001; Fig.1D, GATA4SF). The testicle germinal epithelium in the mutants was disorganized and no germ (VASA+) cells or mature sperm were observed (Fig.1F; also Supp. Fig.1A–C). The abnormalities were already apparent at two weeks of age in live animals when GATA4SF testes appeared only partially descended and very small (Fig.1I; also Supp_Fig.1D–F); however, no female genital structures were ever observed.
Fig. 1.
A–C. Male development is arrested upon gonad-specific loss of GATA4 or FOG2. Ventral view of 6 week-old mice: normal male (A), XY GATA4SF (B) and XY FOG2SF(C). Mammary teats are evident in GATA4SF and FOG2SF animals (white arrowheads); the ano-genital distance (a white bracket) is shorter in both mutants. D. Isolated gonads from control males (left) and either GATASF (top) or FOG2SF (bottom) mice. Scale bar 1mm. E–G. Normal tubules contain mature sperm in control testis at 1.5 months (E) while the tubules in contemporaneous GATA4SF (F) and FOG2SF (G) gonads are malformed. No mature sperm is present in GATA4SF mutants at this stage (see also Supp_Fig.1) while rare mature sperm is present in FOG2SF mutants (Supp_Fig.2). Scale bar 100µ. Insets in (E–G) are at 400×. H–K. Isolated testes and associated reproductive organs from control (H, J) and either GATA4SF (I) or FOG2SF (K) XY mice. Scale bar 1mm. Note the reduced size of the gonads in both mutants (arrowheads) and presence of the uterus in the FOG2SF (arrow in K, see also Supp_Fig.2), but not GATA4SF animal.
Gonad-specific Loss of Fog2 Partially Blocks Testis Development and Leads to Sex Reversal
Conditionally targeted Fog2 animals have been previously used to evaluate the consequences of this gene loss in mammary gland and cardiac development (Manuylov et al., 2007b; Zhou et al., 2009). To directly compare the outcome of GATA4 loss to FOG2 loss we performed the gonad-specific deletion of Fog2 with the same Sf1Cre driver used above for GATA4SF mutants. Although external appearance of the XY Sf1Cre;Fog2flox/flox (abbreviated XY FOG2SF) mice (Fig.1C) was quite similar to that of GATA4SF mutants (Fig.1B), all XY FOG2SF animals presented as true hermaphrodites. The mutant gonads were in partially descended position and externally appeared either as small testes or ovotestes (Fig.1D, FOG2SF and data not shown). The majority of tubules in XY FOG2SF ovotestes appeared disintegrating and had a dramatically reduced epithelium width due to a severely decreased cellularity of spermatogenic epithelium; these tubules contained no spermatogenic cells (Fig.1G and Supp_Fig.2A,C). Upon close examination some tubules contained spermatogonia and rare mature sperm (Supp._Fig.2D). In approximately half of the cases the FOG2SF gonad morphologically resembled an ovary (Supp.Fig.2B; also described in more detail later). The reproductive tracts of these animals contained persistent Müllerian duct-derived structures (oviduct, uterus and vaginal tissues) in addition to the expected Wolffian-derived male organs (Fig.1K and Supp. Fig.2E). Similar to GATA4SF males, the XY FOG2SF animals were tested and found to never sired any progeny.
Efficient Inactivation of Gata4 and Fog2 in the SPCs of GATA4SF and FOG2SF Gonads
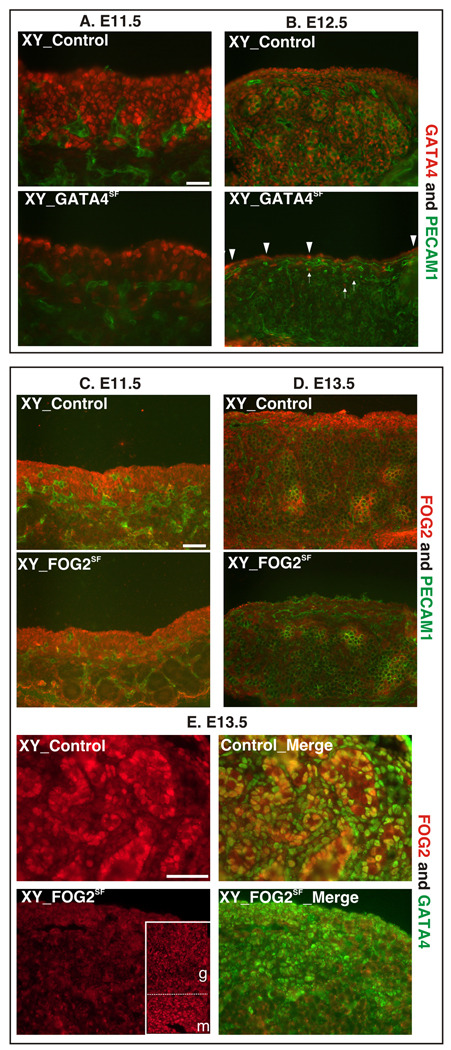
As has been shown previously by others and us, Sf1Cre excision is highly efficient in a subset of somatic progenitor cells (SPCs) within the gonad while most coelomic epithelial cells generally do not undergo efficient recombination in this setting (Bingham et al., 2006; Kim et al., 2007b; Manuylov et al., 2008). Recombination of the Gata4flox allele was highly effective during embryogenesis. The reduction in the amount of the GATA4-positive cells was already noticeable as early as E11.5 in both sexes, and at E12.5, GATA4 staining was practically absent from all the cells in gonad proper (Fig.2A–B; see also Fig.3A). In accordance with previous reports, Sf1Cre recombined inefficiently in coelomic epithelial cells, and many coelomic cells retained GATA4 expression even at E12.5 (e.g. arrowheads, Fig2B; Fig.3A). Sf1Cre-mediated excision of Fog2 was not apparent in gonads of either sex at E11.5 (Fig.2C). However at E12.5, FOG2 expression in SPCs was already notably diminished and it was greatly reduced by E13.5 (Fig.2D, E). Importantly, both proteins remain expressed as normal upon the partner protein loss (Fig.2E and Supp_Fig.3).
Fig. 2.
Efficient excision of Gata4/Fog2 genes in GATA4SF/FOG2SF gonads. Immunofluorescent staining of frozen gonadal sections from the control and GATA4SF (A–B) or FOG2SF (C–E) mice with anti-GATA4 (red, A and B; green, E), anti-FOG2 (red, C–E) antibodies. Embryonic germ cells and vasculature were stained with the anti-PECAM1 antibody (green) in A–D. Note that residual GATA4 staining in the E12.5 GATA4SF samples is confined to the coelomic epithelial (CE) area (arrowheads in B); a few rare GATA4 positive cells below CE are also indicated (arrows). Also note that GATA4 is expressed as normal upon Fog2 excision (E). Inset in (E) is a lower magnification of the same section which shows that while FOG2 staining is lost from the FOG2SF gonad (g), FOG2 staining in the mesonephros (m) appears intact as Sf1Cre is not active there. Scale bars 100µ.
Fig.3.
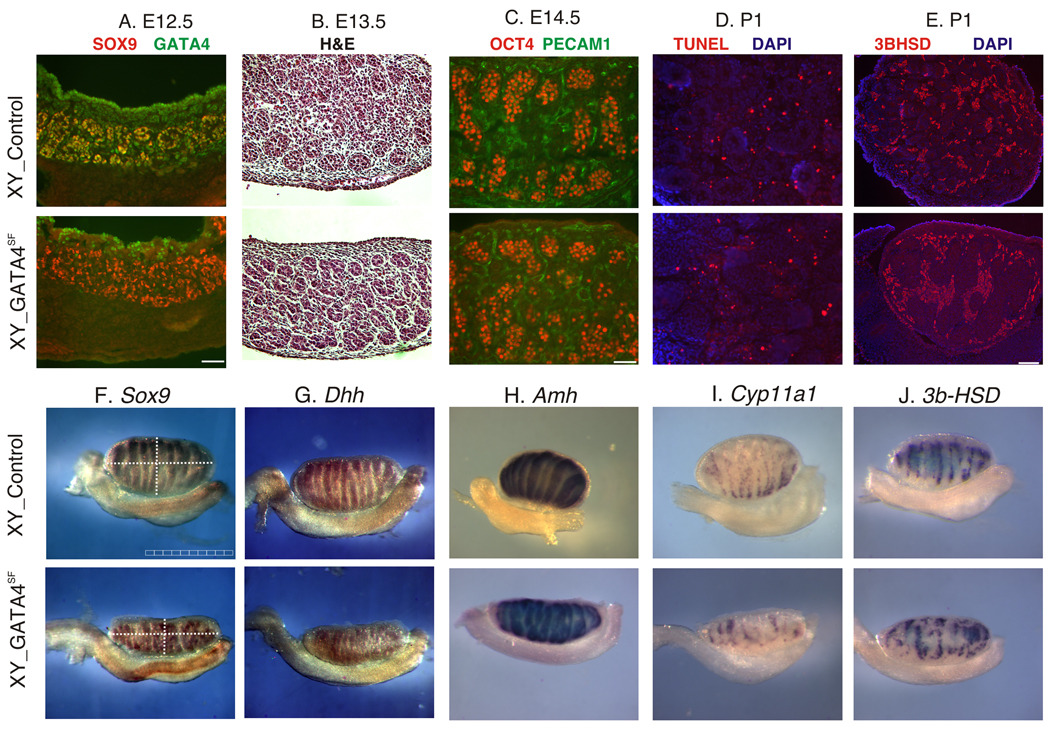
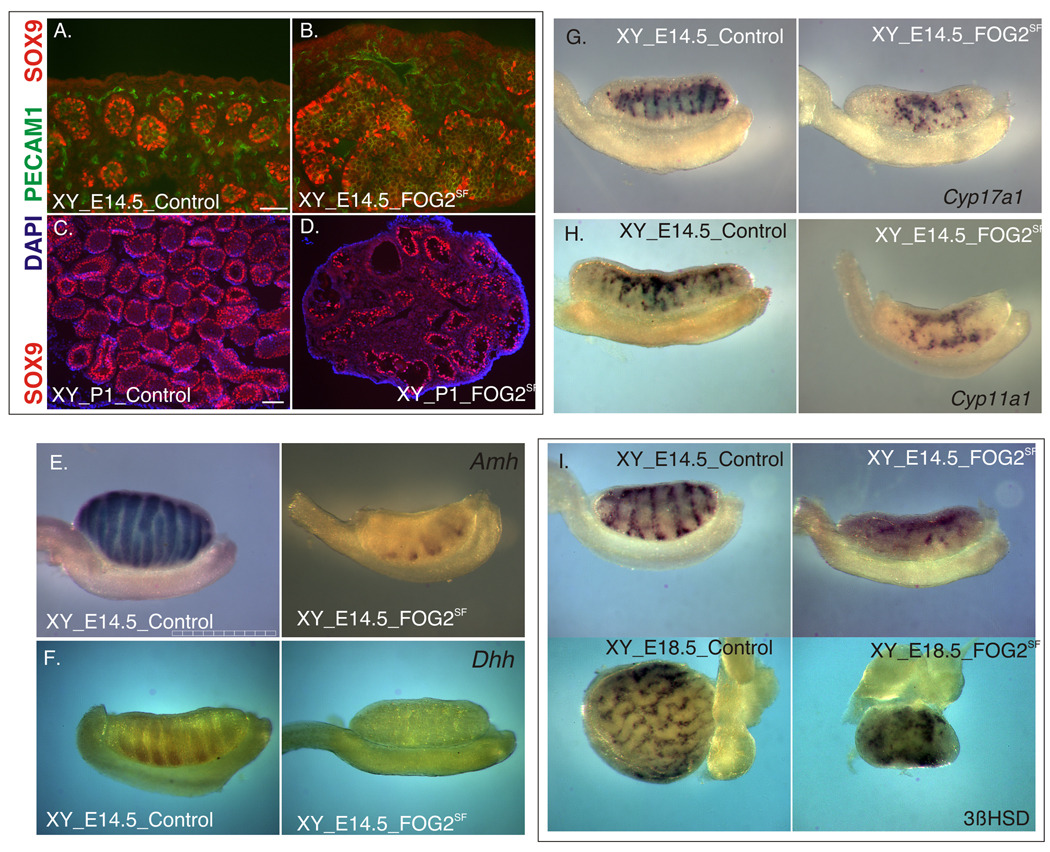
Normal expression of testis-associated genes in the GATA4SF males. A. Immunofluorescent staining of frozen gonadal sections from the control (top) and GATA4SF (bottom) E12.5 embryos with anti-SOX9 (red) and anti-GATA4 (green) antibodies. Note that, despite the loss of GATA4 expression, SOX9 was expressed normally in the mutant sample; also note residual GATA4 staining in the CE cells in the mutant. Scale bar 100µ. B. Histological staining of the control (top) and GATA4SF E13.5 testis sections. C. Immunofluorescent staining of gonadal sections from the control (top) and GATA4SF (bottom) E14.5 embryos with anti-OCT4 (red) and anti-PECAM1 (green) antibodies. Scale bar 100µ. D. TUNEL staining (red) of the frozen testis sections from the neonatal (P1) control (top) and GATA4SF mutant (bottom) animals. E. Immunofluorescent staining of frozen gonadal sections from the control (top) and GATA4SF (bottom) neonatal embryos with anti-3BHSD (red) antibody. Scale bar 200µ. F–J. Whole-mount ISH was performed on XY E14.5 control (top) or GATA4SF gonads (bottom) with indicated probes. Expression of Sertoli cell (A, F–H), germ cell (OCT4, C) and Leydig cell (E, I, J) markers did not change upon GATA4 loss; note that gonad thickness was slightly reduced in the mutant (e.g., F). The scale bar is 1mm.
Analysis of Male Gene Expression in Gonads with GATA4 Gene Loss
To evaluate the sex differentiation status of supporting cells in the GATA4SF testis we examined Sox9 gene expression. SOX9 is a transcription factor that is up-regulated by SRY in Sertoli cell precursors between E10.5 and E11.5 in the mouse (Barrionuevo et al., 2006; Chaboissier et al., 2004; Kent et al., 1996; Morais da Silva et al., 1996; Sekido and Lovell-Badge, 2008). Sox9 plays a central role in testis differentiation; it is sufficient for testis development (Qin and Bishop, 2005; Vidal et al., 2001) and, unlike SRY, SOX9 expression is easily detectable. Therefore, SOX9 is an informative early indicator of male development and Sertoli cell differentiation. Surprisingly, SOX9 expression appeared intact in GATA4SF E12.5 testis (Fig.3A).
This was unexpected, given that we have previously shown that the GATA4-FOG2 complex is required for normal development of Sertoli cells. Disruption of the interaction between these two protein partners led to an early block in testis development with concomitant loss of Sox9 expression (Tevosian et al., 2002). Testis development is traditionally broken into three stages: formation of the bipotential gonadal anlagen followed by a commitment to testis rather than ovary fate (sexual determination) and subsequent differentiation into a functional organ. To identify the stage at which testis development in the GATA4SF mutants became abnormal, we analyzed histology and gene expression patterns of mutant gonads. Before and at E13.5–E14.5 GATA4SF testes appeared grossly normal relative to testes from control littermates (Fig.3B) although the size of the mutant organ was consistently smaller (e.g. Fig.3F). Evaluation of gene expression associated with male development also did not identify any gross abnormalities (Fig.3C–J). We found no difference in the expression of genes normally associated with Sertoli-, Leydig- or germ cell development either at E14.5 (Fig.3C, F–J) or at E18.5 (Fig.4). The dramatic decrease in testis size in GATA4SF mutants (Fig.1D) cannot be attributed to excessive apoptosis, as the TUNEL assay revealed no increase in cell death (Fig.3D and data not shown).
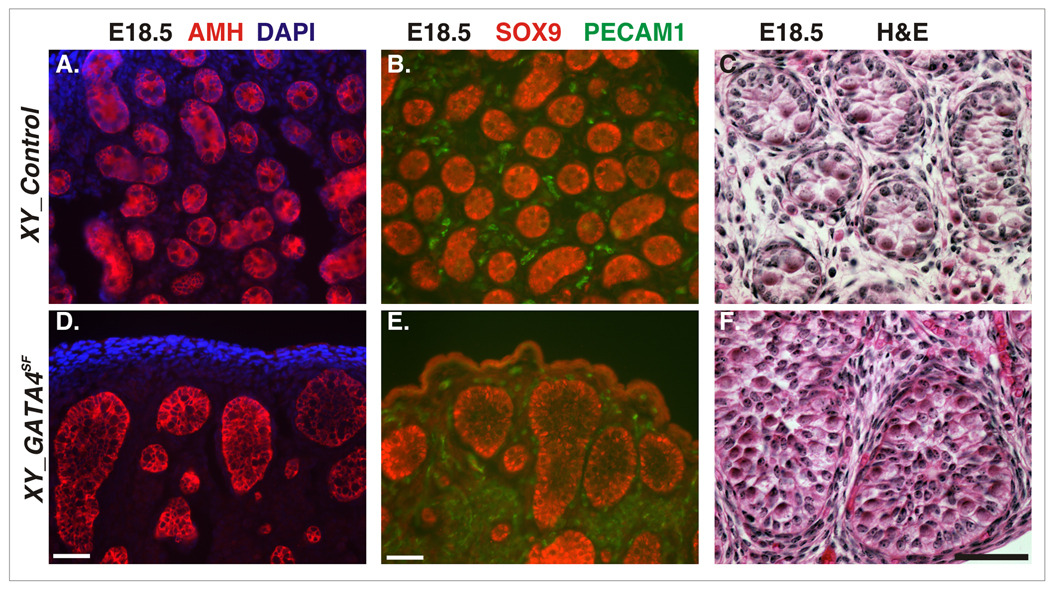
Fig.4.
Testis cord development in GATA4SF testis. Frozen testis sections from the control (A–C) and GATA4SF E18.5 testis (D–F) were stained with anti-AMH (red in A,D); co-stained with anti-SOX9 (red in B,E) and PECAM-1 (green in B,E) or histological stains (C,F). Note that, despite normal expression of the Sertoli cell markers (AMH and SOX9), testis cords in the mutant appeared highly irregular. Scale bars 100µ.
However, in contrast to the homogenous appearance of the testis cords in E18.5 control males (Fig.4A–C), cord structures in the GATA4SF mutants were clearly asymmetrical (Fig.4D–F). Upon closer examination, the irregularity in cord formation was already apparent at E14.5 (Fig.3C). An additional atypical feature of mutant E18.5 cords was the absence of an anuclear central space (pseudolumen); instead the mutant cords appeared to be packed with cell nuclei (Fig.4F). This last feature of the phenotype resembled that in Dmrt1 null testis as described in more detail below.
Dmrt1 Gene Expression in Sertoli Cells requires GATA4
DMRT1 (Doublesex and Mab-3 Related Transcription factor-1) is an evolutionarily conserved transcriptional regulator that harbors a DNA-binding domain (the DM domain) highly homologous to the DNA binding module in transcription factors that regulate sexual differentiation in flies (DOUBLESEX) and worms (MAB-3) (for a recent review, see (Graves, 2009; Koopman, 2009)). It has been proposed that DMRT1 performs a fundamental conserved function in testis development of all vertebrates (Raymond et al., 1998). In mice (as well as in other vertebrates) Dmrt1 expression is restricted to the developing indifferent gonads in both sexes (De Grandi et al., 2000; Raymond et al., 1999) and both somatic and germ cells express this protein shortly before sex determination at E10.5–E11.5 (Lei et al., 2007). A clear dimorphism for DMRT1 in males becomes apparent by E12.5, with robust expression of DMRT1 in both Sertoli and germ cells. In contrast, in females at E12.5 DMRT1 expression is markedly lower in somatic cells than in germ cells and somatic expression becomes extinct at E13.5 (Lei et al., 2007). Studies of Dmrt1 null males revealed this gene’s role in testis differentiation (Raymond et al., 2000). Sex determination in these animals initially proceeded as normal, but after birth (beginning ~at P14) the seminiferous tubules of Dmrt1−/− testis became crowded (similar to Fig.4F) with immature Sertoli cells that failed to exit the cell cycle and differentiate (Raymond et al., 2000).
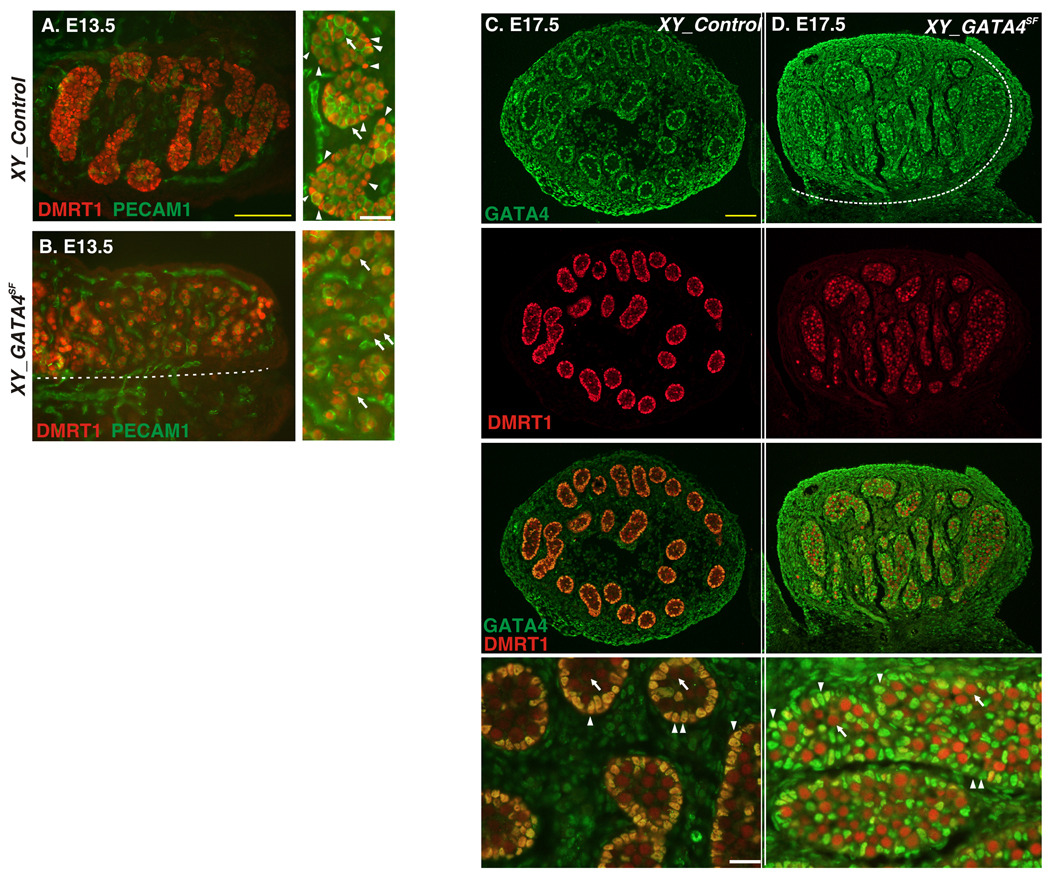
The perceived similarity between Dmrt1−/− and GATA4SF testis phenotypes led us to ask if GATA4 is required for DMRT1 expression. We found that unlike other proteins (e.g. SOX9 and AMH (MIS), Figs.3, 4) DMRT1 expression was dramatically downregulated in Sertoli cells of GATA4SF males as early as E13.5, while DMRT1 expression in germ cells appeared unabated (Fig.5B). DMRT1 staining remains depleted in GATA4SF mutants throughout embryogenesis (Fig.5D). Consistent with this result, Gata4 was previously described to regulate Dmrt1 expression in vitro (Lei and Heckert, 2004). Loss of DMRT1 expression in GATA4SF testis lends further support to the notion that GATA4 regulates Dmrt1.
Fig.5.
Loss of Dmrt1 Gene Expression in Sertoli Cells of GATA4SF mutants. A–D. Frozen sections of E13.5 (A and B) or E17.5 (C and D) XY gonads from the control (A and C) or GATA4SF mutants (B and D) were stained with anti-DMRT1 (red) and anti-PECAM1 (green) antibodies (A and B), or anti-DMRT1(red) and anti-GATA4S (green) antibody (C and D). PECAM-1 identifies germ cells in (A, B) and GATA4S labels somatic cells in (C and D). Dashed line (B,D) indicates a gonad-mesonephros border. Notice that DMRT1 staining is virtually absent in Sertoli (arrowheads) but not germ cells (arrows) in the GATA4SF mutants. In the GATA4SF mutant sample anti-GATA4S antibody (green) recognizes inactive partial GATA4 peptide. Scale bars: 100µ (yellow) and 25µ (white).
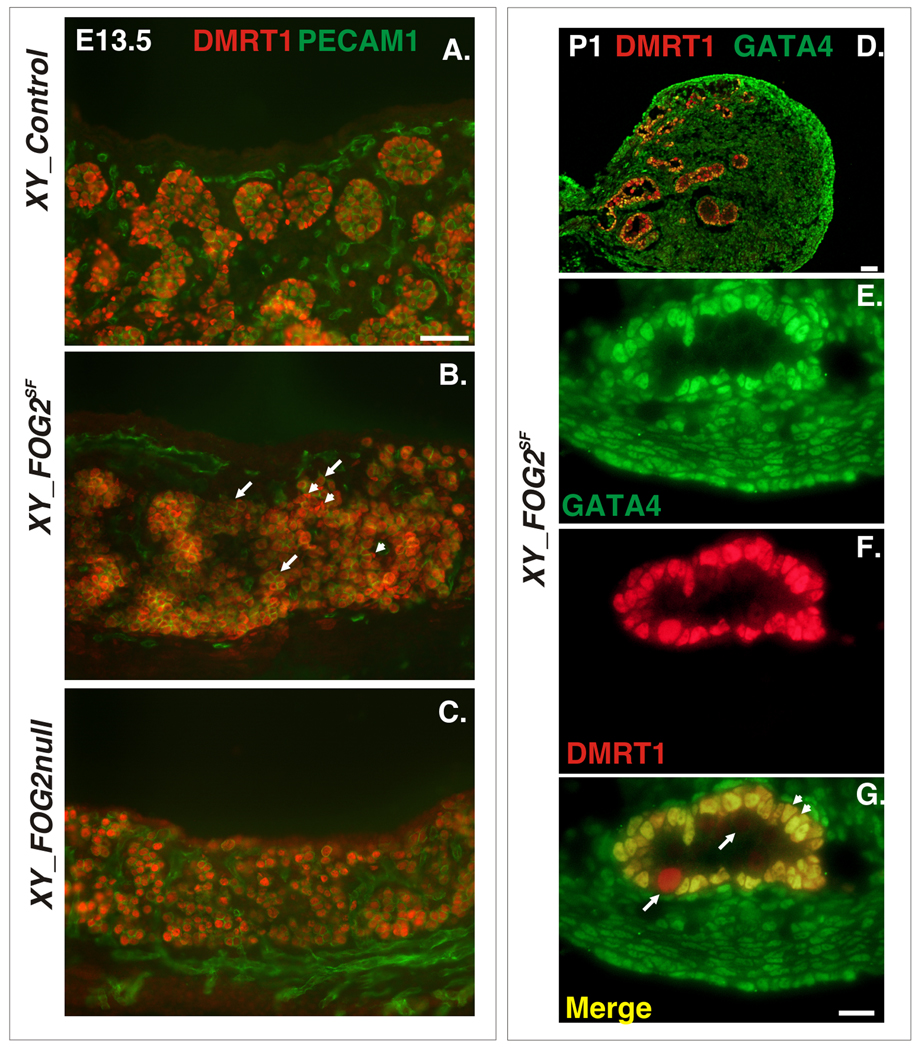
In contrast to GATA4SF males, XY FOG2SF mice continued expressing DMRT1 in the remaining Sertoli cells in early and late embryogenesis (Fig.6A,B and D–G). We concluded that in differentiated Sertoli cells FOG2 is not required to support DMRT1 expression. The previous study also proposed a role for FOG2 in Dmrt1 regulation based on the qRT-PCR analysis of XY Fog2 null gonadal samples (Lei and Heckert, 2004). Hence we also examined DMRT1 expression in XY Fog2 null gonads, which never expressed FOG2. Unlike FOG2SF testes, XY Fog2−/− gonads did not express somatic DMRT1 (Fig.6C). This was expected as XY Fog2 null gonads (and previously described GATA4ki gonads) had a complete early block in Sertoli cell development and did not express any Sertoli-specific genes (e.g. Sox9 or Amh/Mis) besides Sry (Tevosian et al., 2002). In summary, both proteins (i.e. GATA4-FOG2 interaction) are required for the differentiation of pre-Sertoli cells (Tevosian et al., 2002); however, once the cells are differentiated, GATA4 but not FOG2 is necessary to retain somatic Dmrt1 expression in the testis.
Fig.6.
The Residual Sertoli Cells in FOG2SF Mutants Retain DMRT1 Expression. A–G. Frozen sections of E13.5 (A–C) or P1 (D–G) XY gonads from the control (A), FOG2SF (B, D–G) or FOG2 null mutants (C) were co-stained with anti-DMRT1 (red) and anti-PECAM1 (green) antibodies (A–C), or anti-DMRT1 (red) and anti-GATA4 (green) antibody (D–G). Notice that DMRT1 staining is normal both in the Sertoli (arrowheads) and germ (arrows) cells in the FOG2SF mutants (B, G); notice also that DMRT1 expression is lost in the somatic cells of the XY Fog2 null gonadal sample (C). Scale bars: 100µ (A and D) and 25µ (G); E–G are higher magnification of a section adjacent to (D).
DMRT1 Expression in the Sertoli Cells does not Require Continuous GATA4 Presence
Our data suggests that SOX9 expression in Sertoli cells is maintained in the absence of GATA4 (e.g., Figs. 3 and 4). In contrast, GATA4 is required for somatic DMRT1 expression (Fig.5). It was therefore possible that, unlike that of Sox9 or Amh, maintenance of Dmrt1 expression requires a continuous presence of GATA4 throughout the life of the animal. To evaluate this possibility we took advantage of tamoxifen-inducible CreERT2 expressed from the Wt1 locus (Wt1CreERT2) (Zhou et al., 2008). Upon induction with tamoxifen, Wt1-driven CreERT2 was active in Sertoli cells and germ cells of the adult testis (Supp_Fig.4E,F).
To induce Gata4 loss in the adult testis we used Gata4flap animals (Ma et al., 2008). Gata4flap/flap;Wt1CreERT2 males were injected with tamoxifen at 4 weeks of age and their testes examined two weeks later. As was expected, in the control testis both GATA4 and DMRT1 were robustly expressed (Fig.7A, C and (Raymond et al., 2000; Viger et al., 1998)). Wt1-driven Cre efficiently abolished GATA4 expression in adult Sertoli cells. Despite GATA4 loss, DMRT1 expression remained unscathed (Fig.7B); similarly, adult-stage testicular inactivation of Gata4 had no observable consequences on testis appearance and spermatogenesis (Fig.7B, D). This contrasted with embryonic loss of GATA4 in GATA4SF mutants that led to the dramatic reduction of somatic DMRT1 staining (Fig.5). SOX9 expression was similarly unperturbed upon GATA4 loss (Fig.7D). This was not entirely surprising given that SOX9 expression was not diminished in GATA4SF mutants as well (Figs.3 and 4). We conclude that, once initiated, DMRT1 expression in Sertoli cells does not require a continuous presence of GATA4.
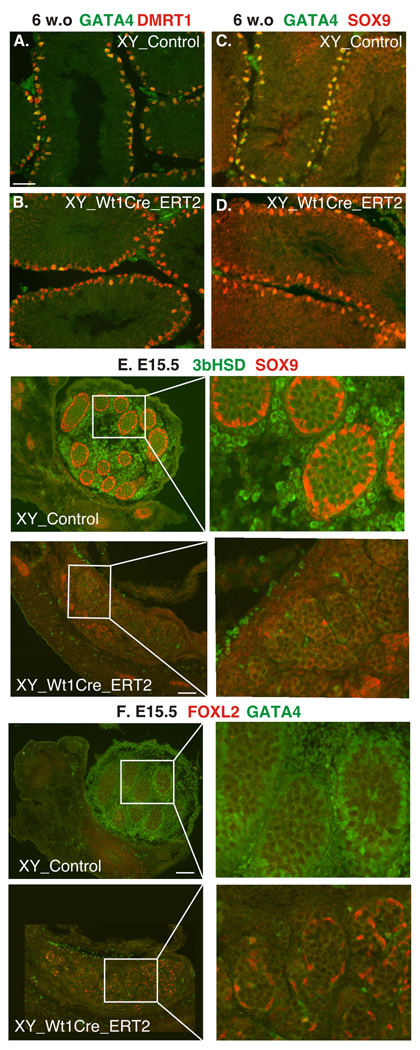
Fig.7.
The Specific Timing of GATA4 Loss Ascertains Divergent Phenotypes in XY gonads. A, B. Immunofluorescent staining of gonadal sections from the control (top) and Wt1CreERT2; Gata4flap/flap (bottom) 6 week-old male mice with anti-GATA4 (green) and anti-DMRT1 (red, A) or anti-SOX9 (red, B) antibodies. Cre function was induced at 4 weeks of age. Note that while GATA4 expression (green) was absent in the somatic cells in the mutant, DMRT1 and SOX9 expression were unscathed. C, D. Immunofluorescent staining of gonadal sections from the control (top) and Wt1CreERT2; Gata4flap/flap (bottom) E15.5 XY embryos either with anti-3bHSD (green) and anti-SOX9 (red) in (C) or anti-FOXL2 (red) and anti-GATA4 (green) antibodies in (D). Note the dramatic reduction of both Sertoli and Leydig cell marker expression upon Gata4 excision in the mutant. Cre function was induced by tamoxifen injection at E10.5. Scale bars: 50µ (A,B) and 200µ (C,D); right panels in C and D are magnifications of the areas delimited on the left.
The Early Loss of Somatic GATA4 Expression Leads to a Block in Male Development and Sex Reversal
Our previous work convincingly showed that constitutive loss of interaction between GATA4 and FOG2 is incompatible with normal sex determination and expression of most sexually dimorphic genes (Tevosian et al., 2002; Tevosian and Manuylov, 2008). In contrast, Sf1Cre-mediated deletion of the Gata4 gene at E12.5 resulted in definitive embryonic testes and markers of male development were expressed (Figs.1–4). The milder phenotype of conditional GATA4SF gonads compared to the constitutive disruption of GATA4-FOG2 interaction (in Fog2 null or Gata4ki/ki mutants (Tevosian et al., 2002)) led us to hypothesize that gonadal GATA4 activity prior to excision by Sf1Cre at E12.5 was sufficient for normal sex determination and expression of sexually dimorphic genes. To test this hypothesis we used inducible WT1CreERT2 described above to delete Gata4 early in gonadal development. Using a reporter strain, we determined that tamoxifen injection at E10.5 induced broad WT1CreERT2–mediated recombination in the developing genital ridge (Supp_Fig.4A–D). To delete Gata4, Gata4flap/flap females were crossed to Wt1CreERT2/+;Gata4flap/flap males and injected with tamoxifen at E10.5. Analysis of E15.5 XY embryos that underwent Wt1CreERT2-mediated deletion of Gata4 showed an early block of male sexual differentiation and sex reversal (Fig.7E, F). The gonads in these embryos had a dramatically diminished expression of Sertoli (e.g., SOX9) and Leydig (e.g., 3bHSD) cell markers (Fig.7E). Instead, expression of the early ovary-specific marker FOXL2 was observed (Fig.7F).
This result clearly differs from that in GATA4SF mutants where definitive testes developed and sex reversal was not observed (Fig.3), or from that in the adult where GATA4 loss appeared to have no deleterious effect in the testis (Fig.7A–D). This result is also in agreement with our previous assertion that GATA4-FOG2 complex is essential in testis differentiation during the initial sex determination stage (Tevosian et al., 2002). In summary, these observations imply that GATA4 performs different functions at different stages of gonadal development.
Timing of Gene Deletion is Critical for the Outcome of Gata4 Loss
In this work we aimed to determine the function of GATA4 protein during testis development. To examine embryonic gonads deficient in Gata4 we used two different Cre recombinase drivers that led to different phenotypes, with embryonically-induced Wt1Cre-mediated excision clearly being more severe (e.g., compare Figs. 3,4 and Fig.7E, F). We reasoned that the difference in outcomes could be due to an earlier activation of Wt1-driven Cre in the gonad leading to an earlier loss of Gata4. We also noted that while Wt1Cre is active in the coelomic epithelium (e.g., Supp_Fig.4D), Sf1Cre excises inefficiently in these cells. To determine whether a phenotype observed with Sf1Cre could be recapitulated using Wt1_CreERT2, we induced Wt1_CreERT2 one day (24hours) later, at E11.5. As expected, in contrast to GATA4SF testis (e.g., Fig.8C), GATA4 expression is lost from Wt1Cre-excised coelomic cells, regardless of later injection (Fig.8A–B). However, unlike an E10.5-induced excision (Fig.7E,F), E11.5-induced embryos expressed SOX9, AMH and 3bHSD (Fig.8D,E) and their irregular testis cord structure strongly resembled that in GATA4SF testis (compare to Figs.3–5). Most importantly, Wt1_CreERT2; Gata4flox/flox animals similarly cease DMRT1 expression in their Sertoli cells (compare Fig.8G to Fig.5). We conclude that the requirement for GATA4 in embryonic testis development is highly dynamic: early on GATA4 is required for Sertoli cell differentiation and later plays a more restricted but essential role in controlling particular genes (e.g. Dmrt1; see also Supp_Fig.6). We also reason that continuous GATA4 expression in coelomic cells (at least past E11.5) is dispensable for testis development.
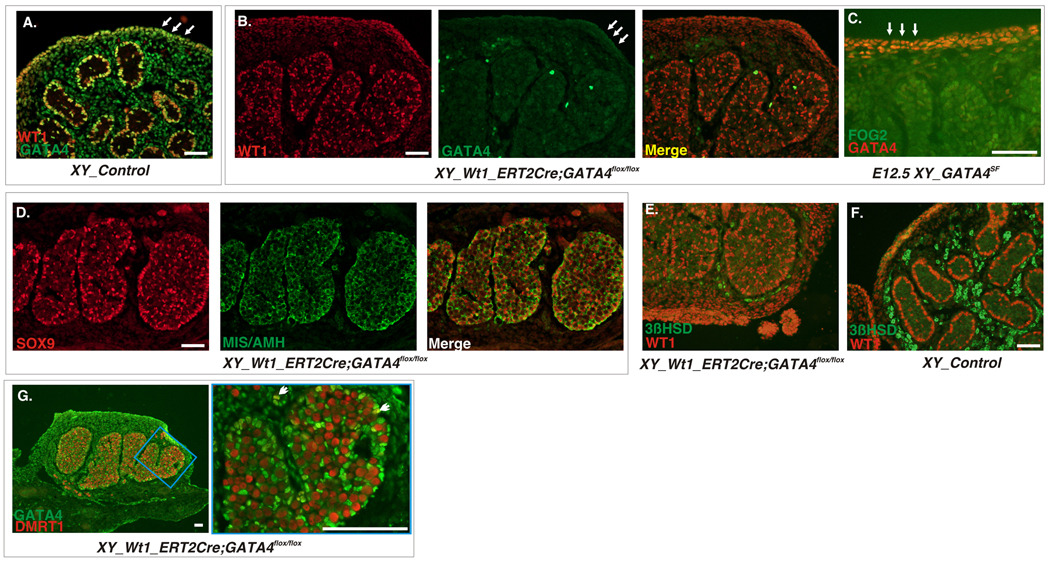
Fig. 8.
Timing of Gene Deletion is Critical for the Outcome of the Gata4 Gene Loss. A–B. Frozen sections of E15.5 XY gonads from the control (A) or Wt1CreERT2; Gata4flox/flox mutants (B) were stained with anti-GATA4 (green) and anti-WT1 (red) antibodies. Cre function was induced by tamoxifen injection at E11.5. Note virtual absence of GATA4 staining in the mutant sample (B), including cells of the coelomic domain (arrows in A–C). C. A frozen section of E12.5 XY gonads from the GATA4SF mutant stained with anti-GATA4 (red) and anti-FOG2 (green) antibodies is shown. Note that most cells in the coelomic domain retain GATA4 protein (arrows). D and E. Sections adjacent to those in (B) stained with anti-SOX9 (red) and anti-AMH (green) antibodies (D) or stained with anti-WT1 (red) and anti-3βHSD antibodies (green) (E) show ample presence of testis-associated proteins despite WT1-Cre mediated GATA4 loss. A section from the control E15.5 testis stained for 3βHSD and WT1 is shown for comparison in (F). G. Same section as in (B) stained with anti-DMRT1 (red) and anti-GATA4S (green) antibody. Notice the abnormal cord structure similar to that in GATA4SF samples (e.g., Fig.4 and Fig.5D). Notice also that, similar to the GATA4SF mutant (Fig.5), the majority of Sertoli cells are no longer positive for DMRT1 (arrows indicate two rare DMRT1- positive cells). In the GATA4SF sample anti-GATA4S antibody (G, green) recognizes inactive partial GATA4 peptide. Scale bars: 100µ.
XY FOG2SF Gonads Undergo an Early Block in Male Development and Sex Reversal
Sf1-driven Cre recombinase allows for efficient and early deletion of either Gata4 or Fog2 genes in the differentiating gonads (Fig.2). While the loss of GATA4 staining was already prominent at E11.5 and practically complete by E12.5 in GATA4SF testis, we did not observe a similar loss of FOG2 expression in FOG2SF mutants until E13.5 (Fig.2 and data not shown). Despite this lingering expression, the male specific program was severely perturbed in E13.5–E14.5 XY FOG2SF gonads (Fig.9). Specifically, we observed a dramatic down-regulation of the Sertoli-associated gene expression (Sox9, Amh, Dhh; Fig.9A–C). This was in sharp contrast with contemporaneous GATA4SF testis (Fig.3). Additionally the coelomic vessel appeared underdeveloped (data not shown). However, in contrast to Fog2 null embryos that exhibited a complete failure in testis differentiation (Tevosian et al., 2002), Sf1Cre-mediated Fog2 excision resulted in a limited expansion of the male program and was compatible with expression of genes associated with differentiated Sertoli cells (i.e. Sox9; Fig.9A). Despite gross abnormalities in the Sertoli cell differentiation and testis cord development, the steroidogenic program in XY FOG2SF gonads appeared grossly normal, suggesting that the residual function of Sertoli cells in XY FOG2SF embryos was adequately supporting Leydig cells and steroidogenesis (Fig.9G–I).
Fig.9.
Testis differentiation was compromised in XY FOG2SF fetuses. A. Immunofluorescent staining of gonadal sections from the control (A, C) and FOG2SF (B, D) XY E14.5 embryos (A, B) or neonatal animals (C, D) with anti-SOX9 (red) antibody and either anti-PECAM1 (green) antibody or DAPI nuclear stain. E–I. Whole-mount ISH was performed on XY control or FOG2SF gonads with indicated probes. Note that while Sertoli cell marker expression was dramatically reduced in the FOG2SF samples (A–F), Leydig cell expression proceeded unabated (G–I). Scale bars 100µ (A,B) and 200µ (C,D) and 1mm (E).
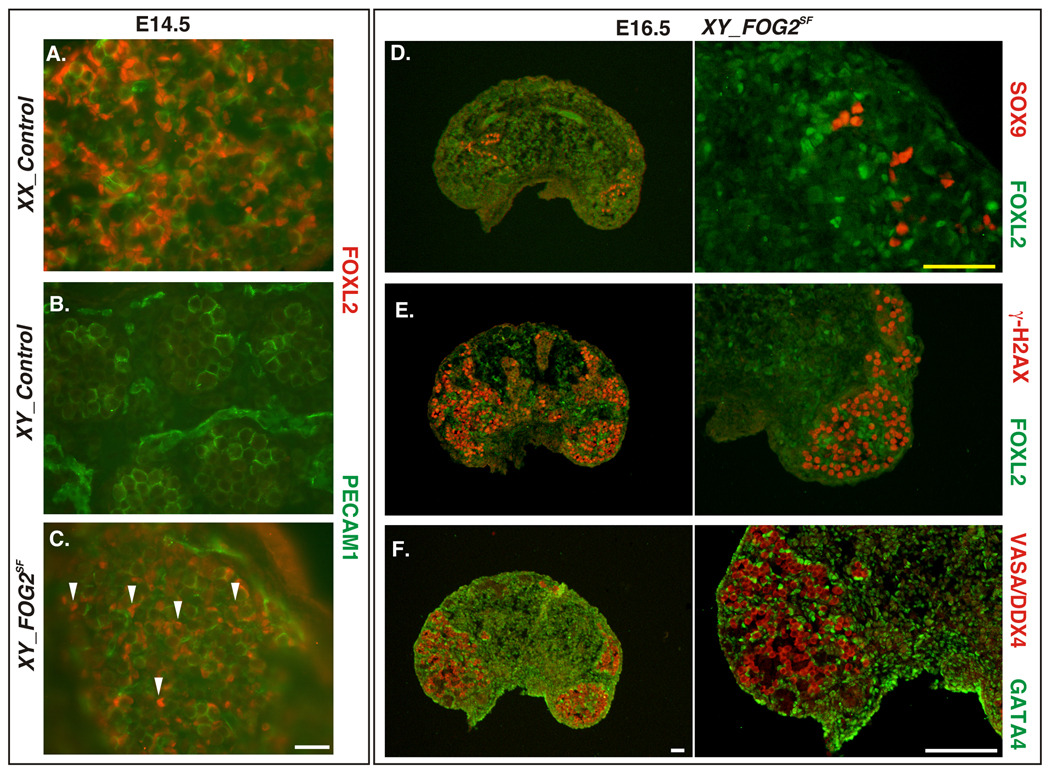
Our data demonstrate that the male differentiation program was severely compromised in XY gonads of FOG2SF mice. To examine whether this collapse of male differentiation leads to the resurgence of female pathway, we examined expression of female-specific genes in FOG2SF XY gonads. Consistent with the hermaphrodite phenotype of the FOG2SF males, we observed that Fog2 deletion was sufficient to cause partial sex reversal, with ectopic expression of the FOXL2 gene in the XY gonads (Fig.10A–C, Supp. Fig.5A–G). The gonads (but not individual cells) in XY FOG2SF animals co-express SOX9 and FOXL2 genes (Fig.10D; Supp_Fig.5D–F). Consistent with sex-reversal of the somatic cells, most gonocytes in these XY mutants express the marker of meiotic cells G-H2AX during embryogenesis (Fig.10E, F). These cells are lost prenatally and in the newborn XY FOG2SF animals the remaining germ cells are associated with the residual testis cords (Sup_Fig.5H,I; also Fig.6D). By 3 weeks of age the gonads in the XY FOG2SF mutants strongly resembled ovaries except for the presence of rare SOX9 positive cells (Supp_Fig.5J). While we observed robust FOXL2 expression in the somatic cells, no early stage (primordial or primary) follicles were detected in the cortex of the XY FOG2SF ovary (Sup_Fig.5J–L). In contrast to FOG2 mutants, ovarian-associated gene expression was not observed in GATA4SF testes (data not shown), consistent with largely preserved male gene expression in XY GATA4SF mice.
Fig.10.
Sex Reversal in XY FOG2SF gonads. A–C. Immunofluorescent staining of gonadal sections from control female (A), male (B) and FOG2SF XY (C) fetuses with anti-FOXL2 (red) antibody. Germ cells and vasculature are stained with anti-PECAM1 (green). Note that female-associated FOXL2 protein was expressed in the somatic cells in the ovary (A) and XY FOG2 mutant (C, arrowheads), but not in the control testis (B). D–F. Adjacent XY FOG2SF gonadal sections were stained with anti-SOX9 (red) and anti-FOXL2 antibodies (green, D); anti-γ-H2AX (red) and anti-FOXL2 antibodies (green, E), and anti-VASA/DDX4 (red) and anti-GATA4 antibodies (green, F). Right panels in D–F are higher magnifications of adjacent sections. Note that while SOX9 and FOXL2 expression is not segregated spatially, the proteins are not expressed in the same cell (D). Also note that the majority of the germ cells in the XY FOG2SF mutant also express the meiosis-associated marker, γ-H2AX (E,F). Scale bars: 100µ (white) and 50µ (yellow).
DISCUSSION
The Genetic Analysis in Mice Highlights the Complexity of GATA4:FOG2-dependent Transcriptional Regulation
The promoter studies in cultured cells provided important inroads into understanding GATA4 function in the testis. It had been suggested that GATA4 participates in the regulation of many testicular genes (e.g., Mis/Amh (Viger et al., 1998; Watanabe et al., 2000), Sf1 and aromatase (Cyp19a1) (Tremblay and Viger, 1999), Inha (Ketola et al., 1999), StAR (Nishida et al., 2008; Silverman et al., 2006) and Rhox5 (Bhardwaj et al., 2008); see (Lavoie and King, 2009; Viger et al., 2008) for review). Nevertheless, direct analysis of gene regulation in the absence of GATA4 previously has not been possible due to early embryonic lethality of Gata4−/− mice (Kuo et al., 1997; Molkentin et al., 1997). Now our results reveal the complexity of the GATA4- and FOG2-dependent regulation of gene expression and gonadal development, which could have only been exposed by genetically manipulating gene function in mice (Table 1).
Table 2.
Summary of the Gata4/Fog2 Loss-of-function Models and the Resulting Phenotypes.
| Genetic Sex |
Targeted Gene |
Modification | Time of Gene Loss |
Phenotype | References |
|---|---|---|---|---|---|
| XY | Fog2 | Deletion Fog2−/− |
Constitutive | Embryonic lethal ~ E13.0–E14.0 Complete block in testis development No sex reversal |
Tevosian et al., 2002 |
|
Excision with Sf1Cre |
Conditional; E13.5 |
Hermaphrodites Incomplete early block in testis differentiation Sex reversal |
This study | ||
| Gata4 | Point mutation, Gata4ki/ki |
Constitutive | Embryonic lethal ~ E13.0–E14.0 Complete block in testis development No sex reversal |
Tevosian et al., 2002 | |
|
Excision with Sf1Cre |
Conditional; E12.5 |
Undervirilized males Definitive testis development Testis cords defect Loss of Dmrt1 gene expression in Sertoli cells |
This study | ||
|
Excision with Wt1CreERT2 |
Conditional; Induced at E10.5 |
Early block in testis differentiation Sex reversal |
|||
|
Excision with Wt1CreERT2 |
Conditional; Induced at E11.5 |
Definitive testis development Testis cords defect Loss of Dmrt1 gene expression in Sertoli cells |
|||
|
Excision with Wt1CreERT2 |
Conditional; Induced at 4 w.o. |
Normal gene expression |
GATA4 is required for Normal Gonadal Development of Both Sexes in Mice
We have shown previously that loss of GATA4-FOG2 interaction (e.g. GATA4ki mutation as well as Fog2 gene deletion) leads to a comprehensive block in gonadal development in mice. The phenotype of GATA4ki animals firmly implicated GATA4 as an indispensable component in both sex differentiation programs. Importantly, Gata4ki mutation is not equivalent to Gata4 loss of function: the Gata4ki mutation results in the production of GATA4 protein that is impaired solely in its ability to interact with FOG co-factors (Crispino et al., 1999). To establish the specific requirements for GATA4 protein in sexual development we have now generated its loss of function mutation in gonads using two different Cre recombinase carrier strains.
Despite the loss of GATA4 expression by E12.5, GATA4SF mice did not recapitulate the early acute arrest of gonadogenesis that is characteristic of the GATA4ki phenotype. While gonads in the GATA4SF males eventually succumbed to a prominent block in their development (Fig.1D, F and Supp_Fig.1), testis differentiation in these animals appeared to initiate normally. As one expects a more dramatic defect in a complete rather than partial loss-of-function, the most likely explanation for the milder phenotype is the delayed timing of Gata4 gene loss in GATA4SF animals. Specifically, while the excision is practically complete in the gonad proper at E12.5 (Fig.2B and Fig.3A), at E11.5 (during the critical window for sex determination) many GATA4-positive cells were still present among the gonadal somatic cells (Fig.2A).
Inducible Wt1CreERT2 allowed us to incorporate a time-dependent deletion series that was informative. It showed that when induced at E10.5 Wt1CreERT2 excision of Gata4 leads to an early and broad failure of Sertoli cell differentiation and male development with concurrent sex reversal (Fig.7E, F). In contrast, by using Sf1Cre we have derived an insight for dedicated GATA4 function in the formation of testis cords and defined genes that critically depend on GATA4 presence as early as E12.5. With the majority of Sertoli-specific genes expressed as normal (e.g., Sox9, Amh/Mis; Figs.3, 4) our results unequivocally show that GATA4 is required for Dmrt1 expression in the context of otherwise normally developing Sertoli cells (Figs.5 and 8). The requirement for GATA4 in regulating Dmrt1 expression is not constant. Specifically, though GATA4 is obligatory during embryogenesis for retaining Dmrt1 expression in Sertoli cells, persistent Dmrt1 expression in the adult does not require GATA4 (likely due to GATA1 compensation).
Sf1Cre recombines inefficiently in coelomic epithelium (Bingham et al., 2006; Kim et al., 2007b; Manuylov et al., 2008) and GATA4 expression in these cells remained largely intact even at E12.5 (Fig.2B and 3A). Coelomic epithelium harbors the progenitor population for the somatic gonadal cells (Karl and Capel, 1998) and although these GATA4-positive cells do not express Sertoli cell markers (e.g. SOX9, Fig.3A), their continuous impact on gonadal development remained possible. In this respect, equivalent results were obtained with Wt1CreERT2 induced at E11.5 (with efficient deletion of Gata4 in coelomic cells) further strengthening the conclusion that the phenotype is determined by the timing rather than residual expression of GATA4 in coelomic cells (compare Fig.8 to Figs.3–5)
Dmrt1 is an Integral Transcriptional Target of GATA4, but not FOG2 in Embryonic Sertoli Cells
Among several gene targets of GATA4 in the developing testis (Supp_Fig.6 and Manuylov et al., unpublished), Dmrt1 is the most informative. DMRT1 expression is limited to the developing gonads (where both Sertoli and germ cells express the protein) and postnatal testis. In humans DMRT1 is implicated in embryonic testis development and sex determination, while in mice it appears to be required only after the time of birth. Specifically, murine DMRT1 is required autonomously for Sertoli cell postnatal differentiation, while germ cell DMRT1 promotes their radial migration to the tubule periphery and supports mitotic reactivation and viability (Kim et al., 2007a).
The regulatory cis-elements required for Dmrt1 expression were characterized in primary cultures of rat Sertoli cells (Lei and Heckert, 2004; Lei et al., 2009). The control region contributing to Sertoli cell-specific transcriptional activity was located between −3.2kb and −2.8kb and contained several critical regulatory elements capable of binding GATA4. Furthermore, reduced Dmrt1 mRNA levels were reported for the gonads from E13.5 Fog2−/− XY embryos, but not Fog2−/− XX embryos, providing additional support for the role of the GATA4-FOG2 complex in sex-specific Dmrt1 regulation (Lei and Heckert, 2004).
We have now shown that Dmrt1 expression is lost from the Sertoli cells of GATA4SF (Fig.5) and Wt1_CreERT2; Gata4fl/fl (Fig.8) embryonic testis, and that the expansion of Sertoli cells in GATA4 mutants partially resembles that in postnatal Dmrt1−/− mutants (e.g. compare Fig.4F in this work to Fig.5F in (Raymond et al., 2000)). Importantly, the testis cord defect in the GATA4 mutants is manifested considerably earlier; hence it cannot be attributed solely to the loss of Dmrt1 expression. While it is possible that a more severe phenotype of GATA4SF mutants results from down-regulation of other Dmrt family genes, these are not expressed markedly in the developing testis. We favor a hypothesis that deregulated gene expression in addition to Dmrt1 contributes to an earlier phenotype in GATA4SF mutants. In this respect, the WISH assay confirmed that, in addition to Dmrt1, at least two other genes, Cst9 (cystatin 9) and Clu/ApoJ (clusterin), are dramatically down regulated in GATA4SF testis as early as E14.5 (Supp_Fig.6). Analysis of clusterin (Bailey et al., 2002) and Cts9 (testatin) (Hasegawa et al., 2006; Tohonen et al., 2005) mutants did not reveal these genes’ definitive role in testis development. However, a combined loss of expression for these (and possibly other, yet unidentified) genes in addition to Dmrt1 could be responsible for the observed phenotype of the GATA4SF mutants.
While the complete roster of genes under GATA4 control remains to be elucidated, this data highlights a previously unappreciated role for GATA4 in Sertoli-specific Dmrt1 expression and testis cord development. In contrast to GATA4SF testis, DMRT1 staining remains strong in the Sertoli cells of the XY FOG2SF mutants (Fig.6) thus underscoring a specific role for GATA4 but not FOG2 in regulating somatic Dmrt1 expression in the testis.
Divergent Outcomes of Sf1Cre-induced GATA4 versus FOG2 Loss
Our previous work has demonstrated that FOG2 is strictly required for testis development (Tevosian et al., 2002). Here we specifically ablated Fog2 in gonadal somatic cells. In contrast to Fog2 null gonads where early sexual development is blocked completely (Tevosian et al., 2002), FOG2SF animals initiate Sertoli cell differentiation and the male pathway. Interestingly, the Leydig cell markers in the XY FOG2SF gonads are expressed at the levels comparable to that in the control testis indicating that residual Sertoli cell function is sufficient to adequately support the male steroidogenic program (Fig.9). Nevertheless, testis cord development in FOG2SF mutants was disrupted and other markers of Sertoli cell differentiation besides SOX9 were either not expressed or dramatically diminished (Fig.9). Moreover, XY FOG2SF gonads underwent sex reversal and expressed markers of early ovarian development (Fig.10; Supp_Figs.2, 5). This contrasts with GATA4SF testis where initial testis development proceeded with relatively little disruption (Figs.3, 4) and progressively deteriorated after birth. The parsimonious explanation for a different phenotype of FOG2SF gonads is a loss of GATA4-independent function upon Fog2 deletion; a GATA-independent function for FOG2 was recently suggested by others (Hyun et al., 2009).
We conclude here that our results support the notion that both proteins (GATA4-FOG2 complex) are required for somatic cell sex specification. In the developing testis, supporting cell progenitors (SCPs) rely on GATA4-FOG2 complex to successfully transition out of their undifferentiated state into Sertoli cells (Tevosian et al., 2002). In this respect, Gata4ki/ki or Fog2 null gonads express neither male (Sox9, Dhh, Mis, Dmrt1somatic) (Tevosian et al., 2002) nor female (Foxl2, Fst, Sprr2d) (Manuylov et al., 2008) markers. In contrast, in the conditional setting described here, gene deletions occur past the point of SCP specification and reveal separate functions for the two proteins. Shortly after differentiation, the bulk of Sertoli-specific genes becomes GATA4-independent (Fig.3). Sertoli cells still rely on GATA4 for the expression of essential genes (Dmrt1 being one important example, Fig.5); not surprisingly, GATA4-deficient Sertoli cells fail to mature and do not develop functional testis or support spermatogenesis. In contrast, in differentiated Sertoli cells a global requirement for Fog2 lingers on (Fig.9), but is likely becoming dispensable shortly after.
While compensation by another GATA protein able to associate with FOG2 in the Sertoli cells of GATA4SF mutants cannot be completely excluded, this possibility is remote since prior to sexual differentiation the phenotype of the GATA4ki mutation is identical to FOG2 null both in XY and XX animals (Manuylov et al., 2008; Tevosian et al., 2002). Moreover, thorough examination failed to reveal expression of other GATA family members in the somatic cells of developing testis at E13.5 (data not shown).
Research Highlights.
We have established that GATA4 is required for:
testis differentiation
for the expression of Dmrt1 gene,
and for testis cord morphogenesis.
Gonadal loss of Fog2 resulted in
an early partial block in male pathway and sex reversal.
We have determined that
testis sexual differentiation is sensitive to the timing of GATA4 loss
Our results now demonstrate that
Gata4 and Fog2 have non-overlapping essential functions in testis development.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Keith Parker’s kind gift of the Sf1Cre mouse strain. We also want to thank Marc Fellous, Reiner Veitia and David Zarkower for antibodies and Kenn Albrecht for his thorough read of the manuscript and for his many thoughtful suggestions. The Troma-I antibody contributed by Philippe Brulet and Rolf Kemler was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. We are grateful to members of the Tevosian laboratory for their comments on how to improve this work.
FUNDING
This work was supported by NIH grants to SGT (HD042751) and WTP (HL094683).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement
The authors declare no competing financial interests.
REFERENCES
- Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. FOG-2 and GATA-4 Are Coexpressed in the Mouse Ovary and Can Modulate Mullerian-Inhibiting Substance Expression. Biol Reprod. 2003;68:1333–1340. doi: 10.1095/biolreprod.102.008599. [DOI] [PubMed] [Google Scholar]
- Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous Inactivation of Sox9 Causes Complete XY Sex Reversal in Mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Rao MK, Kaur R, Buttigieg MR, Wilkinson MF. GATA factors and androgen receptor collaborate to transcriptionally activate the Rhox5 homeobox gene in Sertoli cells. Mol Cell Biol. 2008;28:2138–2153. doi: 10.1128/MCB.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- Bosse T, Piaseckyj CM, Burghard E, Fialkovich JJ, Rajagopal S, Pu WT, Krasinski SD. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006;26:9060–9070. doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Cantor AB, Orkin SH. Coregulation of GATA factors by the Friend of GATA (FOG) family of multitype zinc finger proteins. Semin Cell Dev Biol. 2005;16:117–128. doi: 10.1016/j.semcdb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Combes AN, Lesieur E, Harley VR, Sinclair AH, Little MH, Wilhelm D, Koopman P. Three-dimensional visualization of testis cord morphogenesis, a novel tubulogenic mechanism in development. Dev Dyn. 2009;238:1033–1041. doi: 10.1002/dvdy.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool J, Capel B. Mixed signals: development of the testis. Semin Reprod Med. 2009;27:5–13. doi: 10.1055/s-0028-1108005. [DOI] [PubMed] [Google Scholar]
- Coveney D, Cool J, Oliver T, Capel B. Four-dimensional analysis of vascularization during primary development of an organ, the gonad. Proc Natl Acad Sci U S A. 2008;105:7212–7217. doi: 10.1073/pnas.0707674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino JD, Lodish MB, MacKay JP, Orkin SH. Use of altered specificity mutants to probe a specific protein-protein interaction in differentiation: the GATA-1:FOG complex. Mol Cell. 1999;3:219–228. doi: 10.1016/s1097-2765(00)80312-3. [DOI] [PubMed] [Google Scholar]
- Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 2001;15:839–844. doi: 10.1101/gad.875201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grandi A, Calvari V, Bertini V, Bulfone A, Peverali G, Camerino G, Borsani G, Guioli S. The expression pattern of a mouse doublesex-related gene is consistent with a role in gonadal differentiation. Mech Dev. 2000;90:323–326. doi: 10.1016/s0925-4773(99)00282-8. [DOI] [PubMed] [Google Scholar]
- Fossett N, Tevosian SG, Gajewski K, Zhang Q, Orkin SH, Schulz RA. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci U S A. 2001;98:7342–7347. doi: 10.1073/pnas.131215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves JA. Sex determination: Birds do it with a Z gene. Nature. 2009;461:177–178. doi: 10.1038/461177a. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Chuma S, Tada T, Sakurai T, Tamura M, Suemori H, Nakatsuji N. Testatin transgenic and knockout mice exhibit normal sex-differentiation. Biochem Biophys Res Commun. 2006;341:369–375. doi: 10.1016/j.bbrc.2005.12.183. [DOI] [PubMed] [Google Scholar]
- Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS, Wilson DB. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138:3505–3514. doi: 10.1210/endo.138.8.5350. [DOI] [PubMed] [Google Scholar]
- Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139:1096–1108. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Ketola I, Pentikainen V, Vaskivuo T, Ilvesmaki V, Herva R, Dunkel L, Tapanainen JS, Toppari J, Heikinheimo M. Expression of transcription factor GATA-4 during human testicular development and disease. J Clin Endocrinol Metab. 2000;85:3925–3931. doi: 10.1210/jcem.85.10.6900. [DOI] [PubMed] [Google Scholar]
- Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, Huhtaniemi IT, Wilson DB, Heikinheimo M. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999;140:1470–1480. doi: 10.1210/endo.140.3.6587. [DOI] [PubMed] [Google Scholar]
- Kim S, Bardwell VJ, Zarkower D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev Biol. 2007a;307:314–327. doi: 10.1016/j.ydbio.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bingham N, Sekido R, Parker KL, Lovell-Badge R, Cape B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A. 2007b;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P. Sex determination: the power of DMRT1. Trends Genet. 2009;25:479–481. doi: 10.1016/j.tig.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- LaVoie HA. The role of GATA in mammalian reproduction. Exp Biol Med (Maywood) 2003;228:1282–1290. doi: 10.1177/153537020322801107. [DOI] [PubMed] [Google Scholar]
- Lavoie HA, King SR. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp Biol Med (Maywood) 2009;234:880–907. doi: 10.3181/0903-MR-97. [DOI] [PubMed] [Google Scholar]
- Lavoie HA, McCoy GL, Blake CA. Expression of the GATA-4 and GATA-6 transcription factors in the fetal rat gonad and in the ovary during postnatal development and pregnancy. Mol Cell Endocrinol. 2004;227:31–40. doi: 10.1016/j.mce.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Lei N, Heckert LL. Gata4 regulates testis expression of Dmrt1. Mol Cell Biol. 2004;24:377–388. doi: 10.1128/MCB.24.1.377-388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N, Hornbaker KI, Rice DA, Karpova T, Agbor VA, Heckert LL. Sex-specific differences in mouse DMRT1 expression are both cell type- and stage-dependent during gonad development. Biol Reprod. 2007;77:466–475. doi: 10.1095/biolreprod.106.058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei N, Karpova T, Hornbaker KI, Rice DA, Heckert LL. Distinct transcriptional mechanisms direct expression of the rat Dmrt1 promoter in sertoli cells and germ cells of transgenic mice. Biol Reprod. 2009;81:118–125. doi: 10.1095/biolreprod.108.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JR, McKinsey TA, Xu H, Wang DZ, Richardson JA, Olson EN. FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol Cell Biol. 1999;19:4495–4502. doi: 10.1128/mcb.19.6.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Zhou B, Pu WT. Reassessment of Isl1 and Nkx2–5 cardiac fate maps using a Gata4-based reporter of Cre activity. Dev Biol. 2008;323:98–104. doi: 10.1016/j.ydbio.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Fujiwara Y, Adameyko II, Poulat F, Tevosian SG. The regulation of Sox9 gene expression by the GATA4/FOG2 transcriptional complex in dominant XX sex reversal mouse models. Dev Biol. 2007a;307:356–367. doi: 10.1016/j.ydbio.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Leach L, Tevosian SG. Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development. 2008;135:3731–3743. doi: 10.1242/dev.024653. [DOI] [PubMed] [Google Scholar]
- Manuylov NL, Smagulova FO, Tevosian SG. Fog2 excision in mice leads to premature mammary gland involution and reduced Esr1 gene expression. Oncogene. 2007b;26:5204–5213. doi: 10.1038/sj.onc.1210333. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirment of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- Nel-Themaat L, Vadakkan TJ, Wang Y, Dickinson ME, Akiyama H, Behringer RR. Morphometric analysis of testis cord formation in Sox9-EGFP mice. Dev Dyn. 2009;238:1100–1110. doi: 10.1002/dvdy.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Miyagawa S, Vieux-Rochas M, Morini M, Ogino Y, Suzuki K, Nakagata N, Choi HS, Levi G, Yamada G. Positive regulation of steroidogenic acute regulatory protein gene expression through the interaction between Dlx and GATA-4 for testicular steroidogenesis. Endocrinology. 2008;149:2090–2097. doi: 10.1210/en.2007-1265. [DOI] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Qin Y, Bishop CE. Sox9 is sufficient for functional testis development producing fertile male mice in the absence of Sry. Hum Mol Genet. 2005;14:1221–1229. doi: 10.1093/hmg/ddi133. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215:208–220. doi: 10.1006/dbio.1999.9461. [DOI] [PubMed] [Google Scholar]
- Raymond CS, Murphy MW, O'Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14:2587–2595. doi: 10.1101/gad.834100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, Zarkower D. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391:691–695. doi: 10.1038/35618. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, Izumo S, Tabin CJ, Pu WT. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133:3607–3618. doi: 10.1242/dev.02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Silverman E, Yivgi-Ohana N, Sher N, Bell M, Eimerl S, Orly J. Transcriptional activation of the steroidogenic acute regulatory protein (StAR) gene: GATA-4 and CCAAT/enhancer-binding protein beta confer synergistic responsiveness in hormone-treated rat granulosa and HEK293 cell models. Mol Cell Endocrinol. 2006;252:92–101. doi: 10.1016/j.mce.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Svensson EC, Tufts RL, Polk CE, Leiden JM. Molecular cloning of FOG-2: a modulator of transcription factor GATA-4 in cardiomyocytes. Proc Natl Acad Sci U S A. 1999;96:956–961. doi: 10.1073/pnas.96.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A, Lovell-Badge R. Mammalian sex determination: a molecular drama. Genes Dev. 1999;13:755–767. doi: 10.1101/gad.13.7.755. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–4634. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Cantor AB, Rieff HI, Fujiwara Y, Corfas G, Orkin SH. FOG-2: A novel GATA-family cofactor related to multitype zinc-finger proteins Friend of GATA-1 and U-shaped. Proc Natl Acad Sci U S A. 1999;96:950–955. doi: 10.1073/pnas.96.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Manuylov NL. To β or not to β: canonical β-catenin signaling pathway and ovarian development. Dev Dyn. 2008;237:3672–3680. doi: 10.1002/dvdy.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohonen V, Frygelius J, Mohammadieh M, Kvist U, Pelliniemi LJ, O'Brien K, Nordqvist K, Wedell A. Normal sexual development and fertility in testatin knockout mice. Mol Cell Biol. 2005;25:4892–4902. doi: 10.1128/MCB.25.12.4892-4902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol. 1999;13:1388–1401. doi: 10.1210/mend.13.8.0330. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de RD, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22:781–798. doi: 10.1210/me.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development. 1998;125:2665–2675. doi: 10.1242/dev.125.14.2665. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK. Endogenous expression of Mullerian inhibiting substance in early postnatal rat sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc Natl Acad Sci U S A. 2000;97:1624–1629. doi: 10.1073/pnas.97.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Kong SW, Hu Y, Campbell PH, McGowan FX, Ackerman KG, Wu B, Zhou B, Tevosian SG, Pu WT. Fog2 is critical for cardiac function and maintenance of coronary vasculature in the adult mouse heart. J Clin Invest. 2009;119:1462–1476. doi: 10.1172/JCI38723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.