Abstract
We have detected nucleotide sequences related to the transforming gene of avian sarcoma vius (ASV) in the DNA of uninfected vertebrates. Purified radioactive DNA (cDNAsarc) complementary to most of all of the gene (src) required for transformation of fibroblasts by ASV was annealed with DNA from a variety of normal species. Under conditions that facilitate pairing of partially matched nucleotide sequences (1.5 M NaCl, 59 degrees), cDNAsarc formed duplexes with chicken, human, calf, mouse, and salmon DNA but not with DNA from sea urchin, Drosophila, or Escherichia coli. The kinetics of duplex formation indicated that cDNAsarc was reacting with nucleotide sequences present in a single copy or at most a few copies per cell. In contrast to the preceding findings, nucleotide sequences complementary to the remainder of the ASV genome were observed only in chicken DNA. Thermal denaturation studies of the duplexes formed with cDNAsarc indicated a high degree of conservation of the nucleotide sequences related to src in vertebrate DNAs; the reductions in melting temperature suggested about 3--4% mismatching of cDNAsarc with chicken DNA and 8--10% mismatching of cDNAsarc with the other vertebrate DNAs.
Full text
PDF


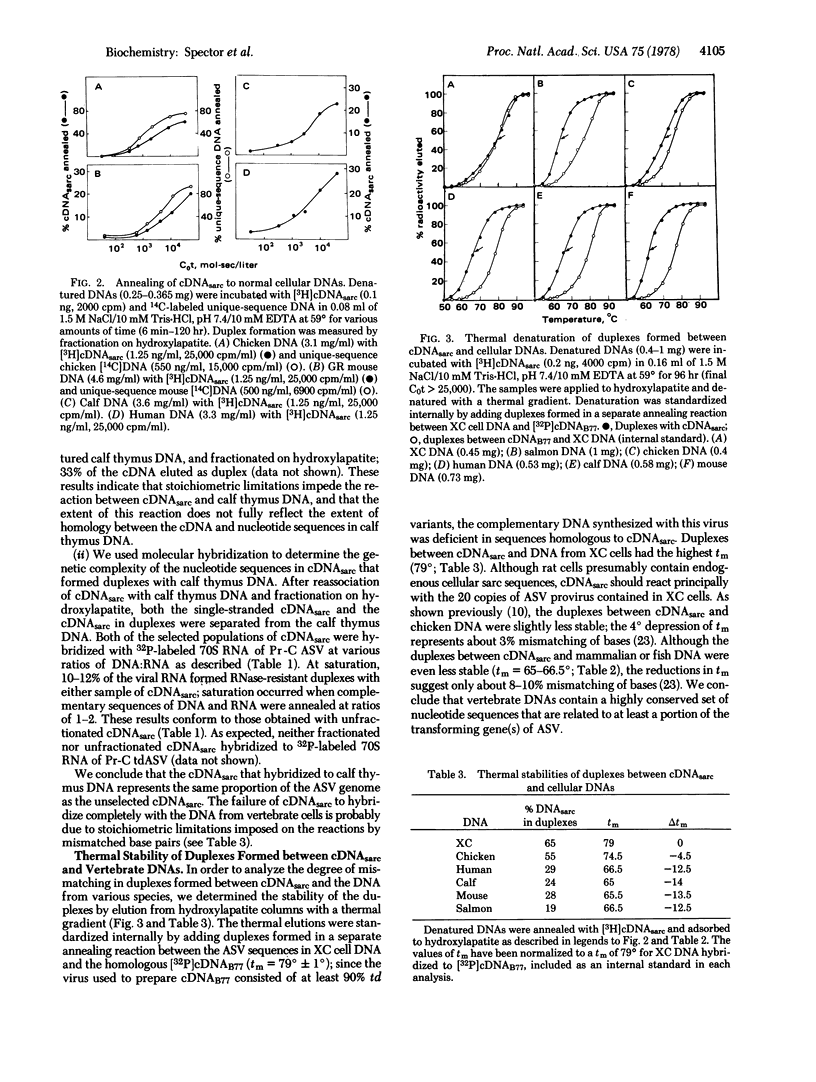

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: I. Nucleic acid from baboon type C virus as a measure of divergence among primate species. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4513–4518. doi: 10.1073/pnas.71.11.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein A., MacCormick R., Martin G. S. Transformation-defective mutants of avian sarcoma viruses: the genetic relationship between conditional and nonconditional mutants. Virology. 1976 Mar;70(1):206–209. doi: 10.1016/0042-6822(76)90254-3. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich R., Morris V. L., Goodman H. M., Bishop J. M., Varmus H. E. Differences between genomes of two strains of mouse mammary tumor virus as shown by partial RNA sequence analysis. Virology. 1976 Jul 15;72(2):330–340. doi: 10.1016/0042-6822(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H., Horst J., Vogt P. K. Avian tumor virus RNA: a comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2266–2270. doi: 10.1073/pnas.70.8.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B. J., Farquhar M. N. The rate of change of DNA in evolution. Brookhaven Symp Biol. 1972;23:1–43. [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., McMillin C., MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. doi: 10.1128/jvi.13.4.837-846.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVOBODA J., CHYLE P., SIMKOVIC D., HILGERT I. Demonstration of the absence of infectious Rous virus in rat tumour XC, whose structurally intact cells produce Rous sarcoma when transferred to chicks. Folia Biol (Praha) 1963 Apr;9:77–81. [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Homology between avian oncornavirus RNAs and DNA from several avian species. J Virol. 1975 Dec;16(6):1492–1502. doi: 10.1128/jvi.16.6.1492-1502.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Smith K., Padgett T., McCombe P., Roulland-Dussoix D., Moscovici C., Varmus H. E., Bishop J. M. Uninfected avian cells contain RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):371–379. doi: 10.1016/0092-8674(78)90205-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Tereba A., Skoog L., Vogt P. K. RNA tumor virus specific sequences in nuclear DNA of several avian species. Virology. 1975 Jun;65(2):524–534. doi: 10.1016/0042-6822(75)90057-4. [DOI] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. II. Effects of deamination of cytosine. Biochim Biophys Acta. 1973 Feb 4;294(1):416–424. doi: 10.1016/0005-2787(73)90096-8. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Bishop J. M. Use of DNA-DNA annealing to detect new virus-specific DNA sequences in chicken embryo fibroblasts after infection by avian sarcoma virus. J Virol. 1974 Oct;14(4):895–903. doi: 10.1128/jvi.14.4.895-903.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]