Abstract
The primary sensory neurons that respond to noxious stimulation and project to the spinal cord are known to fall into two distinct groups: one sensitive to nerve growth factor and the other sensitive to glial cell-line-derived neurotrophic factor. There is currently considerable interest in the ways in which these factors may regulate nociceptor properties. Recently, however, it has emerged that another trophic factor—brain-derived neurotrophic factor (BDNF)—may play an important neuromodulatory role in the dorsal horn of the spinal cord. BDNF meets many of the criteria necessary to establish it as a neurotransmitter/neuromodulator in small-diameter nociceptive neurons. It is synthesized by these neurons and packaged in dense core vesicles in nociceptor terminals in the superficial dorsal horn. It is markedly up-regulated in inflammatory conditions in a nerve growth factor-dependent fashion. Postsynaptic cells in this region express receptors for BDNF. Spinal neurons show increased excitability to nociceptive inputs after treatment with exogenous BDNF. There are both electrophysiological and behavioral data showing that antagonism of BDNF at least partially prevents some aspects of central sensitization. Together, these findings suggest that BDNF may be released from primary sensory nociceptors with activity, particularly in some persistent pain states, and may then increase the excitability of rostrally projecting second-order systems. BDNF released from nociceptive terminals may thus contribute to the sensory abnormalities associated with some pathophysiological states, notably inflammatory conditions.
Studies on null-mutant mice have provided ample evidence for the neurotrophic hypothesis—that discrete groups of neurons receive from their targets specific trophic support that is essential for the survival of those neurons. For small-diameter primary sensory neurons of neural-crest origin that project to the spinal cord, a member of the neurotrophin family, nerve growth factor (NGF) seems to fulfil this role in development. However, as animals mature, these small-diameter neurons, which are virtually all nociceptive in nature, show several changes in their trophic requirements: first, the cells loose their dependence on NGF for survival, a process that emerges in the first 2 weeks of postnatal life in the rat; second, the same cells change their expression of trophic factor receptors. At birth, virtually all these small cells express the high-affinity NGF receptor trkA. Again, over the first 2 postnatal weeks, about one-half of the cells down-regulate trkA and simultaneously up-regulate receptor components for another factor, glial cell-line-derived neurotrophic factor (GDNF). Thus, in the mature state, the large group of small-diameter dorsal root ganglion (DRG) neurons forms two distinct groups, one sensitive to NGF and the other sensitive to GDNF (1). It is therefore not surprising that much effort has been directed at elucidating the biological effects and roles of these two factors on nociceptive systems (2).
A third member of the neurotrophin family, brain-derived neurotrophic factor (BDNF), seems to be a target-derived trophic factor for many placode-derived sensory neurons (e.g., those in the nodose ganglion). Given the modest sensory neuron loss in BDNF null mutant mice, BDNF does not seem to act in this fashion for the neural-crest-derived sensory neurons. However, BDNF does interact with this population of sensory neurons in maturity. BDNF is important in mature animals for regulating the mechanosensitivity of slowly adapting mechanoreceptors, myelinated fibers required for fine tactile discrimination (3). In addition, there is now evidence accruing that BDNF has a distinct role in nociceptive processing in the adult nervous system, a role distinct from that played by NGF or GDNF. The evidence to date suggests that BDNF may function as a central neuromodulator: not only is BDNF synthesized by some nociceptors, but it also may be released in the spinal cord in an activity-dependent fashion, thereby regulating the excitability of rostrally projecting second-order systems. This article will consider the evidence relating to the hypothesis that BDNF may function as a central modulator of pain.
BDNF Is Constitutively Expressed Within a Subpopulation of Primary Sensory Neurons.
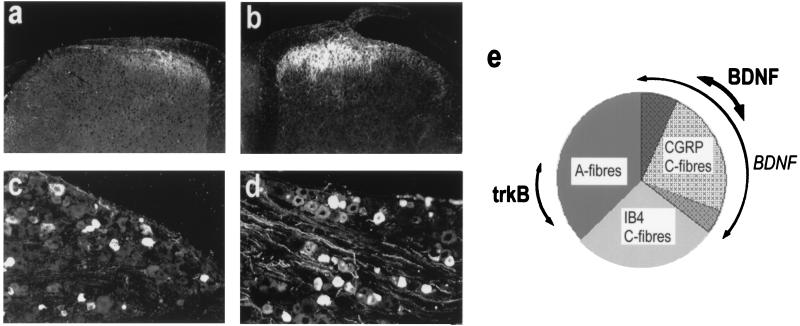
BDNF shares one of the defining characteristics of the neurotrophin family of molecules in that it is retrogradely transported by responsive sensory neurons to the DRG (4). In addition, BDNF shows an unusual property for this family of molecules in that it is constitutively expressed by adult sensory neurons (5–8). Fig. 1 illustrates that, in the lumbar DRG of the rat, the distribution of BDNF protein is not uniform but is localized to a restricted number of primary sensory neurons. Recently, the type of neurons that synthesize BDNF has been elucidated (8). Although, under normal circumstances, BDNF mRNA and protein seem to be present in several different subtypes, the overwhelming contribution is from those sensory neurons that express the neuropeptide CGRP and the high-affinity NGF receptor trkA. Perhaps surprisingly, cells that express trkB (the high-affinity receptor for BDNF) do not, for the most part, express either BDNF mRNA or protein (8). This pattern of localization has important functional implications, because those sensory neurons that express CGRP and trkA are considered to be predominantly nociceptive in function. This nociceptive function is supported further by observations on the spinal localization of BDNF. BDNF is anterogradely transported to both to the periphery and to the spinal cord where it accumulates in the central terminals in the superficial laminae (I and II) and colocalizes with CGRP-containing arborizations (refs. 8 and 9; see Fig. 1). Notably, BDNF immunoreactivity is not abundant in intrinsic spinal-cord neurons nor does it coexist within the primary afferent terminals or cell bodies marked by binding of the lectin IB4. This population of sensory neurons does not constitutively express neuropeptides but expresses GDNF receptor components and is responsive to this factor (10). BDNF immunoreactivity is present only sparingly within deeper laminae. Thus, most of the BDNF protein in spinal cord seems to be localized in the terminals of primary sensory nociceptors, specifically those that express trkA and are known to be sensitive to NGF (11). In these sensory neurons, BDNF is located in dense core vesicles (12), suggesting that it is likely to be released with activity in nociceptors. The superficial laminae of the spinal cord are of course, important sites for integration of nociceptive information. Full-length trkB receptors are present in the cells in this region of the spinal cord (13).
Figure 1.
BDNF expression within the DRG and spinal dorsal horn. (a and c) Under normal circumstances, modest BDNF immunoreactivity is observed within lumbar DRG and superficial dorsal horn. (e, bold arrow) Constitutively expressed BDNF colocalizes with the neuropeptide calcitonin-gene-related peptide (CGRP) and is present mainly within small-diameter sensory neurons. These neurons mostly do not express trkB. BDNF expression is regulated by NGF. (b and d) After systemic NGF treatment, BDNF immunoreactivity is enhanced within lumbar ganglia and dorsal horn. (e, light arrow) After NGF treatment, ≈80–90% of trkA-expressing sensory neurons contain BDNF immunoreactivity. These neurons coexpress CGRP.
The possible interaction of BDNF with nociceptive pathways is highlighted further by the fact that the levels of BDNF are regulated by another neurotrophin, NGF (Fig. 1). Several recent studies have shown that BDNF levels within sensory neurons may be increased by exogenous administration of NGF (6, 8): NGF increases the number of DRG cells showing BDNF immunoreactivity and mRNA. Although there is some debate as to which cells express BDNF after NGF treatment, it is apparent that considerable numbers of these neurons express trkA (and are thus directly responsive to NGF). In one study (8) for instance, 80–90% of those sensory neurons expressing BDNF immunoreactivity after intrathecal NGF belonged to the trkA group.
The peripheral synthesis of NGF is known to be increased in a variety of inflammatory conditions, particularly after injury or experimentally induced inflammation. Under such pathophysiological states BDNF may be up-regulated in nociceptors. Cho et al. (7) showed that intraplantar Freund’s adjuvant injection was associated with a significant increase in BDNF mRNA with a time course consistent with the known increase of NGF in this model. Moreover, the increase in BDNF mRNA was prevented by coinjection of NGF antibody, suggesting that BDNF expression depends on peripheral NGF production. Because there is good evidence that some of the sensory abnormalities associated with inflammation depend on NGF production, this finding in consistent with the notion that BDNF itself contributes to altered sensory processing.
The expression of BDNF is altered in an interesting manner in another pathophysiological state, that associated with peripheral nerve injury. After sciatic axotomy, BDNF is up-regulated in large-diameter DRG cells, and anterograde transport to the dorsal horn of the spinal cord increases (14). Thus, with peripheral nerve injury, BDNF expression decreases in nociceptors but increases in large-diameter sensory neurons (these large-diameter neurons are generally considered to subserve transmission of innocuous sensations from the periphery). The signal for the expression in large cells is not yet known, although reduced retrograde transport of neurotrophin-3 by large-diameter sensory neurons is one obvious possibility. The BDNF in damaged large neurons is also transported to the central terminals of these fibers. It is tempting to speculate that this BDNF also may have similar postsynaptic effects to those described below. Of course, if so, such effects are likely to be found in deeper dorsal horn laminae, the known normal termination sites of large afferents.
BDNF Has Postsynaptic Actions Within the Spinal Dorsal Horn.
Modulation of spinal reflex excitability. Although there is evidence for the activity-dependent secretion of neurotrophins from hippocampal slices (15), there is, as yet, no such evidence that BDNF is released within the spinal cord after afferent fiber activation. The localization of BDNF and its injury-associated regulation detailed above, however, are strongly consistent with a possible neuromodulatory role. Recently, we tested this hypothesis further by examining the effect of exogenous BDNF delivery on spinal reflex excitability.
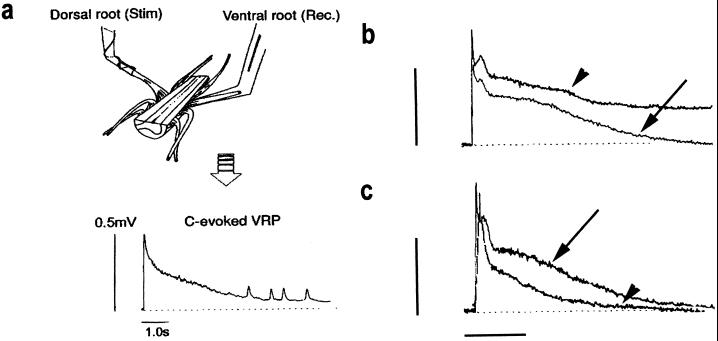
Because neurotrophins do not penetrate the spinal cord readily after systemic delivery, we used an in vitro spinal-cord preparation to study BDNF’s actions. The in vitro hemisected spinal cord is used routinely in our and other laboratories to monitor alterations in synaptic excitability. Reflex responses to C fiber inputs may be recorded from the ventral root, close to the motoneuron cell bodies, with close-fitting glass suction electrodes, and these reflex responses appear as prolonged ventral root potentials (VRPs). These extracellularly recorded potentials are a good measure of spinal excitability (16) and, under control conditions, will remain stable for several hours (Fig. 2a). These preparations were superfused the with BDNF at 2- 1,000 ng/ml for 30 min. The C fiber-evoked responses were recorded before BDNF exposure and for several hours after cessation of BDNF superfusion. A significant and sustained increase in the C fiber-evoked reflex response was observed repeatedly (Fig. 2b; refs. 17 and 18). This enhancement of C fiber-evoked excitability was sustained, outlasting the duration of BDNF administration, and was dose-dependent. The threshold dose to elicit an increased spinal excitability was in the range 100–200 ng/ml BDNF, which suggests a specific and receptor-mediated event. Coadministration of the BDNF-sequestering molecule trkB-IgG to spinal-cord preparations eliminates the facilitatory effect of BDNF and further indicates that the effect of BDNF is likely to be mediated by interaction at the trkB receptor. Superfusion of spinal-cord preparations with NGF (200 ng/ml) does not produce similar effects on C fiber-evoked reflex excitability.
Figure 2.
Exogenous BDNF enhances postsynaptic activity in the spinal cord and endogenous BDNF contributes to reflex excitability. (a) Spinal reflex excitability was assessed from the VRP evoked after electrical stimulation of C fibers in the dorsal root of an isolated hemisected rat spinal-cord preparation. Under normal circumstances, the VRP evoked by C fiber activation is characterized by a prolonged, slowly decaying potential, and this potential remains stable from trial to trial. (b) After a 30-min superfusion with BDNF (200 ng/ml), the amplitude and duration of the C fiber-evoked VRP increased significantly relative to pretreatment activity (arrow, control trace; arrowhead, trace after BDNF superfusion). (c) The contribution of endogenous BDNF to reflex excitability was assessed after a 30-min superfusion of spinal-cord preparations with trkB-IgG (500 ng/ml). Under these circumstances C fiber-evoked activity was reduced significantly compared with pretreatment control responses (arrow, control response; arrowhead, response after trkB-IgG superfusion). (b and c; bars = 0.5 mV and 5.0 s.)
The mechanism of action of BDNF within the spinal cord is, at present, unknown. There is evidence from primary cultures of hippocampal neurons that BDNF enhances spontaneous release of glutamate and acetylcholine, suggesting that BDNF functions there as a retrograde modulator of presynaptic transmitter release (19). It is unlikely that such a mechanism will operate within the spinal cord, because those sensory neurons that express BDNF do not in general display the trkB receptor and are therefore likely to be insensitive to the synaptically released neurotrophin. There is also good evidence, again from the hippocampus, that postsynaptic injection of protein kinase inhibitors will block the effect of BDNF on synaptic enhancement, suggesting that phosphorylation of postsynaptic receptors is a critical step in the BDNF effect. Recently, it has been shown that BDNF rapidly and specifically enhances phosphorylation of the postsynaptic N-methyl-d-aspartic acid (NMDA) receptor (20). Again in the hippocampus, BDNF will potentiate NMDA responses via a 3-fold increase in NMDA receptor open time (20). In cultured rat neurons in the central nervous system, BDNF will also induce changes in NR2A and NR2B NMDA receptor subunit expression (21). NR2 NMDA receptor subunits define the pharmacological and biophysical properties of this receptor (22). In the spinal cord, NMDA receptor activation plays a central role in the induction and maintenance of central sensitization. In this well studied phenomenon, the recruitment of the NMDA receptor seems to be the pivotal event in increasing the sensitivity of nociceptive spinal circuits to sensory inputs (23).
Central sensitization is believed to underlie some aspects of altered sensibility found in chronic pain states. Thus, again, the available data are consistent with the idea that BDNF may be a key mediator of central sensitization within the spinal cord via an interaction at the NMDA receptor site. Several other molecules have been implicated in this process of central sensitization, most notably the tachykinin substance P (23). It is possible that BDNF is simply one of a series of modulators capable of triggering postsynaptic change—perhaps via a common mechanism of NMDA receptor phosphorylation. It is interesting that substance P is itself regulated in a very similar fashion to that described above for BDNF. That is, it is up-regulated in trkA-expressing cells by exogenous NGF or by inflammatory stimuli in an NGF-dependent fashion. Moreover, its expression falls in these cells after peripheral axotomy, but the same stimulus apparently can induce expression in some large neurons (24). The clear effects of trkB-IgG coupled with the rather modest phenotype of substance P and NK1 null-mutant animals (25) may indicate that BDNF is a more important mediator of these central changes, and antagonism of this molecule may provide a novel therapeutic target.
Modulation of gene expression.
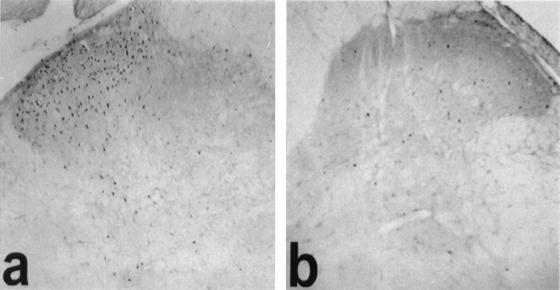
In addition to the effect of BDNF on short-term modulation of the NMDA receptor, there is a strong possibility that trk receptor activation and phosphorylation may underlie more prolonged changes in synaptic excitability. Kang and Schuman (26) showed that some effects of BDNF on synaptic plasticity in the hippocampus may occur in the presence of the NMDA receptor antagonist AP-5, suggesting that alternative mechanisms may exist. There is now evidence that BDNF will interact with signal transduction pathways that underlie longer-lasting forms of synaptic plasticity (27). We have examined the ability of BDNF to induce alterations in immediate early genes expressed in dorsal horn neurons in the spinal cord (18). Intrathecal administration of BDNF to adult rats induces a large increase in c-fos immunoreactivity in the superficial dorsal horn (Fig. 3), whereas NGF does not. In the spinal cord, it is likely therefore that BDNF interacts with signal transduction pathways to induce long-term changes in synaptic excitability that depend on gene transcription and protein synthesis. It is well recognized that activity in primary sensory neurons, and specifically in nociceptive sensory neurons, is capable of inducing c-fos in spinal neurons. However, it is not yet clear whether the c-fos induced by BDNF represents some nociceptive action or it relates to some other function.
Figure 3.
BDNF induces alterations in immediate early gene expression within the spinal cord. (a) A large increase in c-fos immunoreactivity is observed within the superficial dorsal horn 2 h after intrathecal administration of BDNF (5 μg). (b) This increase in immunoreactivity is not observed after intrathecal administration of NGF or saline.
Endogenous BDNF Contributes to Nociceptive Responses.
A key question for the hypothesis put forward here is whether endogenous BDNF contributes to behavioral responses after peripheral injury. To address this important question, we attempted to neutralize synaptically released BDNF with the sequestering antibody trkB-IgG. This molecule is a recombinantly produced fusion protein based on the extracellular domain of the trkB receptor. It is soluble and has the ability to bind with high affinity and thereby sequester endogenous BDNF (as well as the other trkB ligand, neurotrophin-4/5). It effectively serves as an antagonist of BDNF.
We have examined the effects of trkB-IgG on nociceptive afferent-evoked reflex responses in the spinal cord. We have again used the in vitro hemisected spinal-cord preparation and maximized levels of BDNF within the spinal cord by pretreating animals with NGF. Under such circumstances, the afferent-evoked response is reduced significantly after superfusion with trkB-IgG (Fig. 2c), strongly suggesting that BDNF is released from afferent fibers under these conditions.
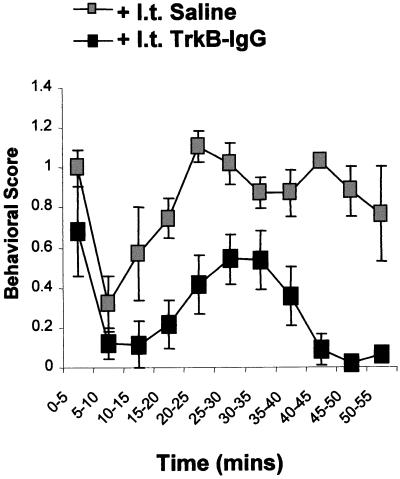
To date, most of the work that has examined the effects of BDNF on synaptic transmission has been undertaken on isolated preparations in vitro. There is evidence, however—again from work on the hippocampus—that BDNF will induce long-lasting enhancement of synaptic transmission in vivo (28). We have recently tested this idea more stringently by assessing the effect of trkB-IgG on nociceptive behavioral responses in adult rats. Behavioral nociceptive responses evoked after subcutaneous injection of dilute formalin into one hind paw were significantly reduced by prior intrathecal administration of trkB-IgG (Fig. 4). Both phases of the formalin response were affected, although the second phase, known to depend in part on the induction of central sensitization, was reduced to a greater degree.
Figure 4.
Endogenous BDNF release contributes to nociceptive behavioral responses in vivo. Animals were pretreated with NGF to maximize nociceptor BDNF levels. Intraplantar formalin [50 μl; 2% (vol/vol)] induces a characteristic two-phase nocifensor paw-withdrawal behavior. Intrathecal administration of trkB-IgG (but not saline) 30 min before formalin injection significantly reduces both phases of the formalin response.
Thus, these findings show that under some conditions, specifically those that model persistent pain states (e.g., inflammation), BDNF is released with activity from nociceptive afferent terminals and contributes to the postsynaptic responses in spinal-cord neurons.
CONCLUSIONS
As we review here, BDNF meets many of the criteria necessary to establish it as a neurotransmitter/neuromodulator in small-diameter nociceptive neurons. Thus, it is synthesized by these neurons and packaged in dense core vesicles. The BDNF-expressing nociceptive afferents terminate mostly in the superficial dorsal horn, and the postsynaptic cells in this region express full-length trkB receptors. However, the synaptic apposition of BDNF containing afferent profiles on neurons with trkB has not yet been established. Spinal neurons are responsive to exogenous BDNF, as evidenced both histochemically (by the induction of c-fos) and electrophysiologically (by an increased excitability to nociceptive inputs). BDNF is thus sufficient to elicit changes in postsynaptic excitability. Although the release of BDNF from nociceptive fibers with activity has not been shown, there are both electrophysiological and behavioral data indicating that antagonism of BDNF at least partially prevents some aspects of central sensitization. Thus, BDNF is also necessary for the full expression of this phenomenon.
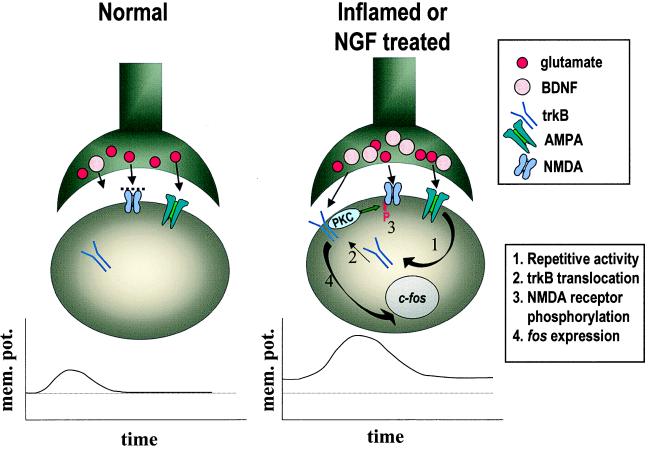
Currently, the mechanism of action of BDNF is not well understood. In Fig. 5, we illustrate several events that may contribute to the central modulatory role of BDNF. BDNF is constitutively expressed in some nociceptors. In normal animals, some BDNF is likely to released with noxious stimulation; however, such release may have limited postsynaptic effects, because much of the trkB receptor protein may not be present at the postsynaptic cell surface. Glutamate, also released with activity in nociceptors, may also have only restricted postsynaptic actions because of the limited responsiveness of NMDA receptors in these normal states. With peripheral inflammation, however, BDNF may play a much greater role. The expression is markedly up-regulated in trkA-expressing nociceptors in an NGF-dependent fashion. More BDNF is likely to be released with activity. The increased activity of postsynaptic neurons in these states may also lead to translocation of trkB receptors to the cell surface. The increased activation of trkB receptors in turn may lead to phosphorylation and increased responsiveness of NMDA receptors, perhaps independently of induction of immediate early genes. Together, these actions potentiate the efficacy of synaptic inputs from nociceptors and thus may contribute to the abnormal pain sensitivity found in these inflammatory conditions.
Figure 5.
Potential mechanisms by which BDNF may influence spinal nociceptive processing in normal and inflammatory conditions. See text for discussion. mem. pot., membrane potential; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
Acknowledgments
We would like to thank Matt Ramer for useful comments regarding the manuscript. Some of the work described in this article was supported by grants from the Medical Research Council of the United Kingdom, the Special Trustees of St. Thomas’ Hospital, and the Physiological Society (London).
ABBREVIATIONS
- NGF
nerve grown factor
- GDNF
glial cell-line-derived neurotrophic factor
- DRG
dorsal root ganglion
- BDNF
brain-derived neurotrophic factor
- VRP
ventral root potential
- NMDA
N-methyl-d-aspartic acid
- CGRP
calcitonin-gene-related peptide
References
- 1.Snider W D, McMahon S B. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 2.McMahon S B, Bennett D L H, Michael G J, Priestley J V. In: Neurotrophic Factors and Pain. Jensen T S, Turner J A, Wiesenfeld-Hallin Z, editors. Seattle: IASP; 1997. pp. 353–379. [Google Scholar]
- 3.Carroll P, Lewin G R, Koltzenburg M, Toyka K V, Thoenen H. Nat Neurosci. 1998;1:42–46. doi: 10.1038/242. [DOI] [PubMed] [Google Scholar]
- 4.DiStefano P S, Friedman B, Radziejewski C, Alexander C, Boland P, Schick C M, Lindsay R M, Wiegand S J. Neuron. 1992;8:983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 5.Ernfors P, Wetmore C, Olson L, Persson H. Neuron. 1990;5:511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- 6.Apfel S C, Wright D E, Wiideman A M, Dormia C, Snider W D, Kessler J A. Mol Cell Neurosci. 1996;7:134–142. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- 7.Cho H J, Kim J K, Zhou X F, Rush R A. Brain Res. 1997;764:269–272. doi: 10.1016/s0006-8993(97)00597-0. [DOI] [PubMed] [Google Scholar]
- 8.Michael G J, Averill S, Nitkunan A, Rattray M, Bennett D L, Yan Q, Priestley J V. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X F, Rush R A. Neuroscience. 1996;74:945–951. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]
- 10.Bennett D L H, Michael G J, Ramachandran N, Munson J B, Averill S, Yan Q, McMahon S B, Priestley J V. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMahon S B, Bennett D L H. In: Text Book of Pain. 4th Ed. Wall P D, Dubner R, editors. Edinburgh: Churchill Livingston; 1999. , in press. [Google Scholar]
- 12.Michael G J, Averill S, Nitkunan A, Rattray M, Bennett D L H, Yan Q, Priestley J V. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradbury E J, King V, Simmons L J, Priestley J V, McMahon S B. Eur J Neurosci. 1998;10:3058–3068. doi: 10.1046/j.1460-9568.1998.00307.x. [DOI] [PubMed] [Google Scholar]
- 14.Tonra J R, Curtis R, Wong V, Cliffer K D, Park J S, Timmes A, Nguyen T, Lindsay R M, Acheson A, DiStefano P S. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blochl A, Thoenen H. Eur J Neurosci. 1995;7:1220–1228. doi: 10.1111/j.1460-9568.1995.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 16.Thompson S W N, King A E, Woolf C J. Eur J Neurosci. 1990;2:638–649. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson S W N. Eur J Neurosci. 1998;10, Suppl. 10:48.03. (abstr.). [Google Scholar]
- 18.Kerr, B. J., Bradbury, E. J., Bennett, D. L. H., Trivedi, P. M., Dassan, P., French, J., Shelton, D. B., McMahon, S. B. & Thompson, S. W. N. (1999) J. Neurosci. 15, in press. [DOI] [PMC free article] [PubMed]
- 19.Lessmann V, Gottmann K, Heumann R. Neuroreport. 1994;6:21–25. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- 20.Levine E S, Crozier R A, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1998;95:10235–10238. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small D L, Murray C L, Mealing G E A, Poulter M O, Buchan A M, Morley P. Neurosci Lett. 1998;252:211–214. doi: 10.1016/s0304-3940(98)00587-4. [DOI] [PubMed] [Google Scholar]
- 22.Sucher N J, Awobuluyi M, Choi Y B, Lipton S A. Trends Pharmacol Sci. 1996;17:348–355. [PubMed] [Google Scholar]
- 23.Urban L, Thompson S W N, Dray A. Trends Neurosci. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi K, Dubner R, De Leon M, Senba E, Ruda M A. J Neurosci Res. 1994;37:596–603. doi: 10.1002/jnr.490370506. [DOI] [PubMed] [Google Scholar]
- 25.Woolf C J, Mannion R J, Neumann S. Neuron. 1998;20:1063–1066. doi: 10.1016/s0896-6273(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 26.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 27.Kang H, Welcher A A, Shelton D, Schuman E M. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 28.Messaoudi E, Bardsen K, Srebo B, Bramham C R. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]