Abstract
Magnetotactic bacteria (MTB) are capable of swimming along magnetic field lines. This unique feature renders them suitable in the development of magnetic-guided, auto-propelled microrobots to serve in target molecule separation and detection, drug delivery, or target cell screening in a microfluidic chip. The biotechnology to couple these bacteria with functional loads to form microrobots is the critical point in its application. Although an immunoreaction approach to attach functional loads to intact MTB was suggested, details on its realization were hardly mentioned. In the current paper, MTB-microrobots were constructed by attaching 2 μm diameter microbeads to marine magnetotactic ovoid MO-1 cells through immunoreactions. These microrobots were controlled using a special control and tracking system. Experimental results prove that the attachment efficiency can be improved to ∼30% via an immunoreaction. The motility of the bacteria attached with different number of loads was also assessed. The results show that MTB can transport one load at a velocity of ∼21 μm/s and still move and survive for over 30 min. The control and tracking system is fully capable of directing and monitoring the movement of the MTB-microrobots. The rotating magnetic fields can stop the microrobots by trapping them as they swim within a circular field with a controllable size. The system has potential use in chemical analyses and medical diagnoses using biochips as well as in nano/microscale transport.
INTRODUCTION
Microrobotics has become the focus of attention worldwide with the development of the microelectromechanical system (MEMS) and robotics.1, 2, 3, 4, 5 Microrobots have many potential applications, such as in medical diagnosis, targeted delivery, invivo sensing, cell manipulation, carrying or assembling tasks in microfluidic chips, and material removal in the human body.6, 7, 8, 9 However, because of the size limitation, the energy supply is always one of the bottlenecks in microrobotic development.5, 10, 11, 12 Inspired by flagellar bacteria, microrobots using bacteria for energy have recently been rapidly developed and become a research focus.12, 13, 14, 15 The bacterial flagellum contains a rotary motor embedded in the cell wall, and a filament extending to the outer matrix.16, 17, 18 Driven by proton-ion or sodium-ion motors, flagella can rotate at frequencies of hundreds of hertz to promote forward motion.19, 20, 21 Therefore, bacteria are highly suitable in microrobot designs because they can overcome the drag force of fluid at a low Reynolds number.10, 15, 22 Tung et al.14 were the first to use bacteria as functional components. They fixed Escherichia coli to the inner surface of a microfluidic channel, and the liquid in the channel was pumped by the force generated by bacterial rotation. To pump fluid in a microfluidic channel, Darnton et al.13 attached Serratia marcescens to a polydimethylsiloxane (PDMS)-coated slide, forming bacterial carpets.
The control of a microrobotic system is a very important technology. Bacteria possess the property of taxies such as phototaxis, aerotaxis, chemotaxis, and magnetotaxis,23, 24 all of which could be used to control and track bacterial microrobots. Chlamydomonas reinhardtii phototaxis was used to control their transport of microscale loads.25 The negative phototaxis of S. marcescens was used to start/stop bacterial movement through ultraviolet light.26 The braking/start-up motion of S. marcescens microrobots was realized by adding chemical reagents to stop or resume the bacterial flagellar motors.12 The present paper used the magnetotactic bacteria (MTB) MO-1 to devise bacterial robots called MTB-microrobots. Magnetotactic bacteria are unique prokaryotes with flagella and intracellular membrane-surrounded magnetosomes. With these features, the microorganisms navigate along geomagnetic field lines to search for and remain in an optimal environment.27, 28, 29, 30 MO-1 cells are a kind of MTB with two lateral bundles of seven individual flagella enclosed in a special sheath.29, 31, 32 In particular, MO-1 cells are polar MTBs that can orient themselves in a magnetic field and swim along a fixed magnetic direction.31 Thus, MO-1 cells can be considered as both an actuator and a navigator of a microrobot. The navigation of the cells only requires a weak uniform magnetic field (0.05 mT to 0.2 mT). The magnetic field serves an orientation function, whereas the actuator function is provided by bacterial flagellum rotation.
An MO-1 cell itself is a simple microrobot, however, to implement a desired mission, bacterial microrobots should carry an artificially functional load composed of polystyrene (PS), PDMS, glass, or photoresist SU8. Of these loads, PS microbeads are the most commonly used.33 Thus, the technology for attaching functional loads to cells is a critical point in the construction of a bacterial microrobot. Two methods for the formation of microrobots have been described in the literature. One is by the direct adsorption between bacteria and loads. S.marcescens can be directly adsorbed onto PS, PDMS,13 or photoresist SU8 (Ref. 26) for linkage, but E. coli cannot.13 This finding indirectly suggests that attachment depends more on the bacterial surface properties than on those of artificially functional loads. Even if S.marcescens successfully adsorbs onto glass microbeads, the coupling is less robust.13 Thus, the method of direct adsorption is hardly used despite its simplicity. The other method of microrobot formation relies on some special molecules or functional groups in the bacterial wall and loads that can bind to one other via immunological or chemical reactions. Fernandes et al.34 used antibody-antigen binding and neutravidin-biotin binding to obtain an effective connection between E. coli and a nanostructure.
Martel et al.35, 36 first proposed the idea of an MTB-microrobot and used a microelectromagnetic array device to control an MTB-microrobot of the magnetotactic marine coccus strain MC-1. The technology for the attachment of MTB to functional carriers mainly relying on intermolecular forces is key to creating an MTB-microrobot. The same group also point that attachment using an antibody is a much more promising method for fabricating microrobots; however, they have not provided details of the method further.36 In the current paper, the properties and behaviors of MTB MO-1 microrobots constructed by antigen-antibody immune responses were analyzed. A microscopic system to control and trace the MO-1 microrobots was then developed. A microfluidic channel system was used to assess the motility and controllability of the MO-1 microrobots. The present work provides an efficient procedure for loading beads on MTB and monitoring the navigation of the MTB-microrobots. A foundation for future designs and applications of MTB-microrobots is also laid.
DESIGN AND FABRICATION OF THE EXPERIMENTAL SETUP
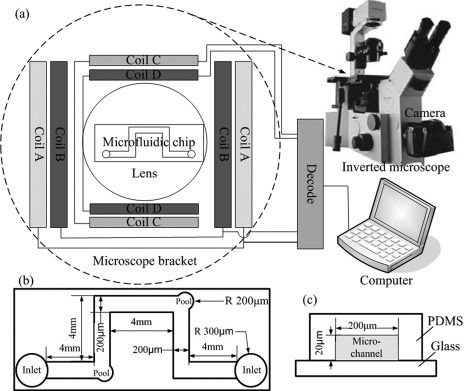
To control the movement of MTB or the MTB-microrobot under an optical microscope, a special control and tracking system (Fig. 1) was designed and constructed at the Institute of Electrical Engineering, Chinese Academy of Sciences. The system consists of an inverted microscope tracking device, a set of electromagnetic coil device embedded in the stage of the inverted microscope brackets, and a microfluidic chip at the center of the set of electromagnetic coil device.
Figure 1.
Schematic diagram of the control and tracking system: (a) The electromagnetic coil device, (b) top view of the microfluidic channels, (c) side view of the microfluidic channels. The geometry of the microfluidic channel is as in (b). For clarity, only one channel is shown.
Microscope tracking device
The microscope tracking device consists of an inverted microscope (OLYMPUS IX70) and a CCD video camera (Canon EOS 500D, Japan) installed through the camera interface and microscope [Fig. 1a]. The 20×, 40×, and 100× objectives were used with bright-field and phase-contrast modes.
Electromagnetic coil device
The electromagnetic coil device used to generate the magnetic fields consists of mutually perpendicular couples of rectangular coils [Fig. 1a]. Four groups of coils surrounded the 35 mm × 40 mm observation area at the center. The coils were constructed using an epoxy resin framework wound by a copper wire. Within the tolerances of the microscope, the coils were constructed to be nonidentical. They were connected to direct current (DC) sources, which were controlled by a computer. A software control system was designed according to the characteristics of the coils and the power sources to realize control by a computer and display the magnetic field state. The uniform magnetic fields were generated in the horizontal plane at adjustable intensities (0 mT to 1 mT), whereas the rotating magnetic field was generated at adjustable intensities (0 mT to 1 mT) and rotation frequencies (0 Hz to 50 Hz). The magnetic field was measured by a Gauss/Tesla meter (F. W. Bell Model 7010, Bell Technologies, Inc., USA). In the current study, the magnetic field intensity of both the static and rotating magnetic fields used to control MTB or the MTB-microrobots were 0.2 mT, and the frequency of the rotating magnetic field was 1 Hz. By using the information feedback of the real-time recording of the camera, the electromagnetic coil device is fully capable of controlling the movement of MTB or the MTB-microrobots.
Microfluidic chip
The microfluidic chip was made of PDMS because of its properties, such as transparency, high gas permeability, and biological compatibility, which are useful for microscope observation and cell survival.37, 38, 39, 40 A PDMS microfluidic chip was placed in the middle of the magnetic field. The plane geometry of the microfluidic channel is shown in Fig. 1b. The two corners of the runners are each designed for two micropools (40 μm diameter). The side view of the microfluidic channel (200 μm wide and 20 μm tall) is shown in Fig. 1c. The chips were manufactured at the National Center for Nanoscience and Technology of China, and the production process is described as follows: rapid photolithography41, 42 was used to fabricate the features in the photoresist SU-8 on silicon wafers. Masters were silanized using a vapor of trichlorosilane. PDMS was poured onto the master and thermally cured (60 °C). The PDMS was then carefully peeled from the silicon wafers, and the resulting PDMS layer contained the microfluidic channels. Outlets were punched on the PDMS. The glass slides and PDMS layers containing channels were placed under oxygen plasma and sealed by pressing the surfaces into contact.
MATERIALS AND METHODS
Bacterial culture
Magneto-ovoid strain MO-1 is our laboratory stock strain that was isolated from the Mediterranean Sea as described by Lefèvre et al.31 MO-1 cells were cultured in the EMS2 medium at room temperature (between 23 and 26 °C), as previously reported.31, 43 Cells were harvested and used for direct observation, preparation of polyclonal antibodies, or connection with beads.
Preparation and characterization of rabbit anti-MO-1 antibodies
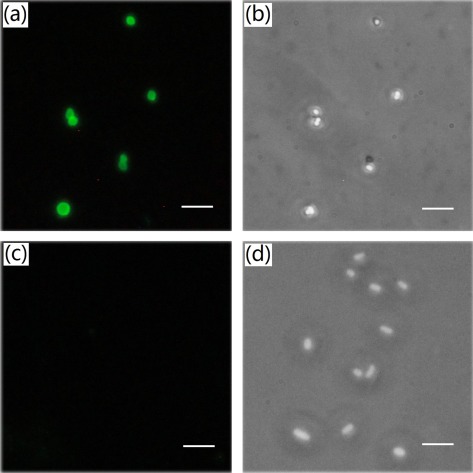
MO-1 cells were first inactivated using 1% formaldehyde. After that, cells suspension was washed with phosphate buffered saline (PBS, Na2HPO4 6.7 mM, NaH2PO4 3.3 mM, NaCl 145.4 mM, pH 7.0) for three times to remove formaldehyde and then resuspended with the same buffer to 1 × 109cells/ml. New Zealand big-ear rabbits were first immunized subcutaneously with 1.0 ml MO-1 cells in an equal volume of Freund’s complete adjuvant (Sigma, Beijing, China). For the next four times immunization, 0.5 ml MO-1 cells with the same volume of Freund’s incomplete adjuvant (Sigma, Beijing, China) were used by intravenous injection. The intervals for each immunization is 2 weeks.44 Two weeks after the last immunization, the antiserum was collected. The sensitivity of antibodies was tested by slide agglutination method and the results showed that the polyclonal antibody could agglutinate with MO-1 at the minimum concentration of 1:640. Additionally, we employed the indirect immune-fluorescent assay to assay the specificity of the antibodies. First, MO-1 cells were washed and adjusted to a concentration of 5 × 106 cells/ml. 100 μl cells suspension was incubated with the antibody at 4 °C for 40 min. After washed, MO-1 cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (ZhongShan, Beijing, China) at 4 °C for 30 min. The immunostained MO-1 cells were washed for three times and then coated them at a slide for the visualization using a fluorescent microscope. Similarly, E. coli cells were used as a negative control. As a result, the antibodies recognized only the antigens on the MO-1 surface and not those on the surface of E. coli [Fig. 2], revealing their high specificity.
Figure 2.
Indirect immune-fluorescent assay of rabbit anti-MO-1 polyclonal antibody. MO-1 cells (a) and (b) and E. coli cells (c) and (d) were immunostained by FITC-conjugated goat anti-rabbit immunoglobulin G. (a) and (c) were visualized under fluorescence microscope; (b) and (d) under phase contrast microscope. Scale bar: 5 μm.
Reagents
The PS beads (2 μm in diameter) modified by carboxyl groups on the surface were used and purchased from baseline (Tianjin, China) because their density (ρ = 1.05 g·cm−3) is very close to that of bacterial cells (ρ = 1.05 g·cm−3 to 1.07 g·cm−3) and their biocompatibility is very good.45 Recombinant protein A (Protein A, Bioss, Inc., USA) was dissolved to a concentration of 5 g/l in PBS (pH 7.4). 2-(N-morpholino)ethanesulfonic acid (MES), N-hydroxysuccinimide (NHS, MW 191.7) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC, MW 217.13) were purchased from Sigma (USA) and dissolved in MES at 10 mg/ml. The coupling buffer PBS (pH 7.4), the storage buffer [PBS(pH 7.4) with 0.5 g/L Tween-20, 0.1% BSA and 0.002 g/L NaN3], and the quenching solution (PBS with 0.1% BSA, pH 7.4) were prepared in our laboratory. All buffers were prepared in sterilized and deionized water under a clean environment.
Construction of MTB-microrobots
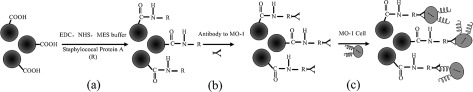
The MTB cells and PS microbeads were attached together to constitute the MTB-microrobots. An immunoreaction was used to improve the adsorption efficiency and robust connection between the cell and the microbead. The PS microbeads were functionalized with protein A through its carboxyl group using NHS and EDC.46, 47, 48, 49, 50 The PS beads coupled with protein A were then linked to the antibodies that reacted with the MO-1 cell wall antigens. The entire process is shown in Fig. 3.
Figure 3.
Scheme of MTB cells attached to the microbeads. The reaction has three steps: (a) carboxyl-modified PS microbeads 2 μm in diameter were reacted with staphylococcal protein A using NHS and EDC; (b) the resulting microbeads were used to capture the antibody against MO-1; and (c) the antibody-modified microbeads were attached to the MO-1 cells.
Step 1. Preparation of protein A-decorated beads
Covalent coupling51 was used to crosslink the beads and protein A [Fig. 3a]. The PS microbeads were first sonicated, and the NHS and EDC solutions were then added to the mixture of PS microbeads and MES to activate the carboxyl groups. The new mixture was stirred for 1 h and rinsed with a PBS solution. The resulting activated PS microbeads were mixed with staphylococcal protein A and allowed to react at room temperature for 2 h to 4 h. The reaction mixture was rinsed with a PBS-TBN solution three times and stored at 4 °C.
Step 2. Preparation of antibody-modified beads
Protein A-decorated beads were also supersonically treated and then mixed with anti-MO-1 antibodies for their attachment. The reaction was allowed to proceed at 4 °C for 1 h [Fig. 3b]. After reaction completion, the mixture was rinsed with a PBS solution to remove free antibodies and stored at 4 °C for subsequent use.
Step 3. Attachment of the MTB MO-1 cells to the antibody-modified microbeads
The attachment of the MTB MO-1 cells to the antibody-modified microbeads was performed via an antigen-antibody reaction [Fig. 3c]. After centrifugation, the modified microbeads were resuspended using the MO-1 culture EMS2 at a final concentration of 1 × 106 cells/ml. Approximately 50 μl antibody-modified microbeads were incubated with 50 μl MO-1 cells at the exponential phase growth for 20 min at room temperature in an Eppendorf tube containing 100 μl EMS2 buffer. Finally, the reaction product was observed under an optical microscope to determine the coupling efficiency.
Observation of the MTB-microrobot movement
A drop of MO-1 cells or MO-1 cells and the microbead reaction product suspension (MO-1 MTB-microrobots) was pushed into a microfluidic channel. The movement of the MO-1 cell swarm or the MO-1 MTB-microrobots tracked by the control and tracking system in the microfluidic channel was observed under 20× or 40× magnification in the dark-field mode. The swimming behaviors were recorded using a Canon 500D camera that recorded 640 pixel × 480 pixel images at 30 frames per second.
Statistics of attachment efficiency
The size and shape of the PS microbeads (2 μm in diameter) have no significant difference with those of the MO-1 cells (1.85 μm in diameter) when observed under 40× magnification. Interestingly, lipid storage globules are rich in MO-1 cells and are observed as bright spots,31 but PS microbeads appear as larger spheres under the phase-contrast mode using a 100× oil immersion objective. Consequently, the 100× oil immersion objective was used to differentiate among MO-1 cells, microbeads, and microrobots. The microbeads from three independent experiments were randomly analyzed, and the efficiency of microbead attachment to MO-1 cells was calculated.
RESULTS AND DISCUSSION
Navigation of the MO-1 cell swarm in the microfluidic chip
The experimental results show that both the PDMS channel and the glass substrate are not adsorbed onto the MO-1 cells. Thus, the cells can smoothly swim in the microfluidic channel, which is good for their controllability. We can track the MTB swarm in the microfluidic channel as well as control their forward, backward, turning, and rotary motion.
The swarm velocity of the MO-1 cells in the microfluidic channel is approximately 131 ± 29.7 μm/s. The MO-1 cells can survive in the microfluidic channel for about 3 h to 4 h. Fig. 4 shows that the MO-1 cells can swim, turn, and rotate in the microfluidic chip. A rotating magnetic field can also control the MTB within a small region. Hence, this approach is a possible braking/start-up motion control system for MTB-microrobots. This method inflicts no damage to cells and can more accurately control the MTB-microrobots than other methods, such as optical or chemical control. The rotational motion of the MO-1 swarm may also be sufficient to mix the solution in the microfluidic chip. Therefore, this approach also allows for a micromixer function in the microfluidic chip.
Figure 4.
(a) A swarm of MTBs is executing turning and rotation movements in the microfluidic channels under the navigational control of the system. The black ones are bacteria; (b) the movement route outlined by the MO-1 swarm in the microfluidic channel (enhanced online).
MO-1 cells attachment to microbeads to form MTB-microrobots
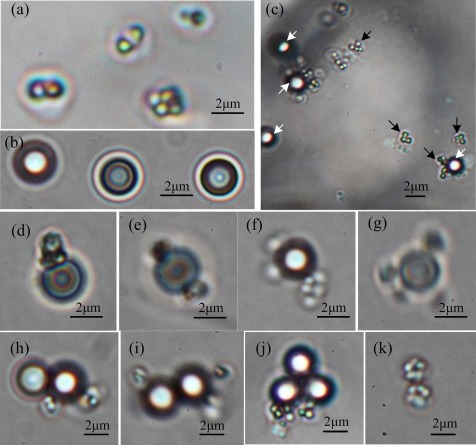
We developed a method of attaching PS microbeads to MO-1 cells. Adjusting the relative concentrations of the microbeads and cells ensured that the cell-to-microbead ratio was higher than 2:1. After a suspension of MO-1 cells was mixed with antibody-coated microbeads, they connect with each other via an immunoreaction. We used different cell:microbead ratios and finally chose the optimal one at 3:1. The attachment efficiency of the microbeads to the MO-1 cells was analyzed under a 100× oil phase-contrast immersion objective; typical photos are shown in Fig. 5. The forms of attachment of the MO-1 cells to the microbeads vary, as shown in Figs. 5d, 5e, 5f, 5g, 5h, 5i, 5j, demonstrating that the course of attachment is random. To ensure that the solution contained no free antibodies, the microbeads were cleaned at least three times after they were modified. Thus, few cell aggregations are observed (∼2%), as shown in Fig. 5k. This phenomenon may also be due to cell division. We then randomly selected and analyzed 100 to 150 microbeads (353 microbeads in total) from each independent experiment and calculated their attachment efficiency. The ratio of the microspheres attached to MO-1 cells to the total microbeads is 31.01% ± 2.27% (n = 3). The proportion of all kinds of attachments was also calculated, and the results are shown in Table TABLE I.. The bead-to-cell ratio that makes up the largest proportion is 1:1 followed by 1:2. Previously we used the antiserum directly to bind microbeads without protein A on the surface and found that the attachment ratio of microbeads to MO-1 cells was very low (<1%). This finding proves that the attachment efficiency can be improved using protein A. Compared with other known techniques,13, 36 the proposed attachment method is an effective one.
Figure 5.
Attachment of PS microbeads to the MO-1 cells under a phase-contrast microscope. (a) MO-1 cell, (b) PS microbeads, (c) MO-1 cells attached with antibody-coated microbeads, the black arrows indicate cells and the white arrows indicate PS microbeads, (d) one microbead and one cell, (e) one microbead and two cells, (f) one microbead and three cells, (g) one microbead and four cells, (h) two microbeads and two cells, (i) two microbeads and three cells, (j) three microbeads and two cells, and (k) two cells.
TABLE I.
Component ratios of the different attachment modes.
| Attachment mode (bead:cell) | 1:1 | 1:2 | 2:1 | 2:2 | 3:2 | 1:4 |
|---|---|---|---|---|---|---|
| Component ratio (%) | 52.70 ± 15.83 | 28.59 ± 6.87 | 5.60 ± 1.16 | 5.43 ± 4.45 | 5.75 ± 4.96 | 1.93 ± 1.76 |
Speed of the MTB-microrobots in the microfluidic chip
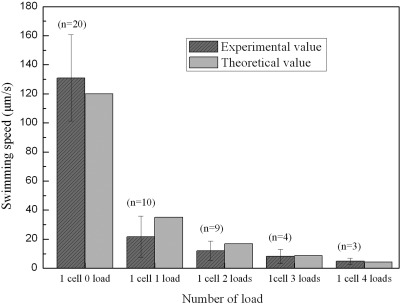
We placed the microbeads and MO-1 cell reaction product into the microfluidic channels to observe their motion in a magnetic field. The majority of motile cells are found to carry one or two loads, and occasionally, a cell transports larger number of loads. We then analyzed the speed of the MTB-microrobots carrying different number of loads; the results are shown in Fig. 6. The velocity of the MO-1 cells modified with beads is generally reduced. The speed of the MO-1 cell decreases exponentially as the number of loads it carries increased. In addition, the average speed of the MO-1 cells is significantly reduced in the microchannel (from 150 ± 34 μm/s in the unbound fluid to 131 ± 29.7 μm/s in the microchannel).
Figure 6.
Experimental and theoretical speeds of MTB-microrobots with different number of loads.
We also performed a theoretical analysis on the speed. Given the small sizes of the bacteria, the Reynolds number (Re) is very low (Re ∼ 2.5 × 10−4 in our experiment). Thus, the Stokes equations can be used to estimate the drag force exerted on the cell.10, 52, 53 On the other hand, because of the viscous drag force, the MO-1 cells swim in an equilibrium state, indicating that their thrust force equals the viscous drag force exerted on them. The thrust force of the MTB is given by
| (1) |
where V and db are the velocity and length scale of an MO-1 cell, respectively (V ≈ 150 μm/s, db ≈ 1.85 μm), and η is the dynamic viscosity of the fluid (for marine water, η = 1.08 × 10−3 Pa/s). A thrust force of 2.82 pN is obtained using Eq. 1 and the 150 μm/s speed for MTB.
When a cell is attached to one or more loads, the viscous drag force exerted on the MTB-microrobot is approximately expressed as
| (2) |
where Vr is the velocity of the MTB-microrobot, dm is the length of the load (dm = 2 μm in our experiment), and n is the number of loads. If n is not very large (that is, Re ≪ 1), the MTB-microrobot also swims in the equilibrium state () because of the viscous drag force. Assuming that the MTB MO-1 cell maintains the thrust force as calculated above (2.82 pN), the speed of the cell significantly decreases [∼150/(n + 1), assuming that the beads have the same size as the bacteria] with increasing n.
When the MTB-microrobots move in the microchannel, the speed decreases because of the wall effect.15, 54 This effect becomes more significant as the diameter of the loads approaches the diameter of the microchannel. By considering the wall effect, the swim speed is calculated as follows:
| (3) |
where Vw is the velocity of the MTB-microrobot with the wall effect and D is the depth of the microchannel (D ≈ 20 μm in our experiment). Using Eq. 3, the theoretical speed of an MO-1 cell attached with different number of loads can be calculated (Fig. 6). The speed of an MTB without a load (n = 0) in the microchannel is calculated as 120 μm/s. Thus, we established a method of estimating the swim speed of MTB-microrobots. Fig. 6 shows that the theoretical result agrees well with the experimental one.
A large amount of coupling MO-1 cells show low activities and some are even immotile. The coupling position on the surface of the MO-1 cells possibly affects their velocity. For instance, if the coupling position is near the flagellum, the rotation of the flagellum is affected.
MTB-microrobot navigation in the microfluidic chip
The control and tracking system can smoothly manipulate the forward, backward, and turning movements of MTB-microrobots along the microfluidic channels. Fig. 7 shows an MTB-microrobot pushing a microbead to traverse the microfluidic channel, and then making a turn along the channel of the navigation device. Compared with a microelectromagnetic coil array prepared via MEMS technology,55 our electromagnetic coil device is capable of controlling the movement of MTB within a large area in the microfluidic channels but is unable to independently control a single robot in different positions. In addition, we also observed that a cell with six loads can still be motile. However, in this case, when the magnetic field direction changes, the robot with six loads cannot immediately change its direction to that of the magnetic field, but will move forward in a curve to turn to the magnetic field direction. This action makes controlling inaccurate because the Re value of the MTB-microrobot increases as the load is increased. In this case, the inertial force increases and cannot be ignored, and the motion of the MTB-microrobot cannot be described by the Stokes equations. Hence, we speculate that the motion of a robot can be accurately controlled when the load is less than four.
Figure 7.
Image of an MTB-microrobot, composed of a PS bead (2 μm in diameter) and an MO-1 cell, within the microfluidic channels executing a turn under the control of the control and tracking system. The dashed circles are used to mark and trace the trajectory of the bead, and the directions of the cut heads are that of the applied magnetic field (enhanced online).
To test the endurance and reliability of the constructed MO-1 microrobots, we measured the distance that the MTB cells covered along the microfluidic channel under the tracking navigation system. Attached with one microbead, a cell swam a distance of approximately 0 cm to 38 cm for about 0 min to 30 min, at an average velocity of 21 μm/s before being blocked by obstacles in the channels. We also measured the endurance and reliability of a cell with two loads. The cells are found to maintain the same endurance and high reliability as that with one load. These results show that MO-1 cell performance is very reliable when the load volume is twice its own volume. Of course, other microstructures or microparticles apart from microbeads can be used to attach to MO-1 cells. The resulting microrobots can similarly execute carrying or transporting tasks in microfluidic channels through the precise control of the magnetic field. If the microbeads are coated with antigens or some other interesting molecules, the MTB-microrobot can act as a biosensor to execute detection or screening tasks.
CONCLUSION
An efficient method of attaching microbeads to MTB and the corresponding device were developed and described in the current paper. The purpose was to facilitate the efficient control of MTB-microrobots in a microfluidic chip and monitor their swimming behavior under optical microscopy. First, a special electromagnetic coil device embedded in a microscope bracket and a PDMS microfluidic chip were fabricated. Second, a method for attaching PS microbeads to MO-1 cells to form MTB-microrobots via an immunoreaction was realized. The results demonstrate that the method not only improves the attachment efficiency but also results in MTB-microrobots with a very good reliability and endurance. The control and tracking device can fully manipulate the movement of the MTB-microrobots or MTB within the microfluidic channels and trap the swimming cells within a circular magnetic field. Based on these results, this method of trapping MTB in a circular field can be realized the stop controlling. The system can be used as a mixer in some environments that need stirring in a microfluidic chip. It can also be applied in microfluidic systems for chemical analyses and medical diagnoses as well as in nano/microscale transport. Our future work will include pathogen detection and capture using the MTB-microrobots.
ACKNOWLEDGMENTS
This work was supported by the State Key Program of National Natural Science of China (51037006). The authors wish to thank Professor Xingyu Jiang and Dr. Bo Yuan from National Center for Nanoscience and Technology of China, for advice on microfluidic chip fabrication. We also thank Dr. Weidong Pan and Dr. Leng Nie from Institute of Electrical Engineering Chinese Academy of Sciences, and Dr. Dingbin Liu from National Center for Nanoscience and Technology of China for valuable discussions about covalent coupling method.
References
- Pawashe C., Floyd S., and Sitti M., Int. J. Robot. Res. 28, 1077 (2009). 10.1177/0278364909341413 [DOI] [Google Scholar]
- Yesin K. B., Vollmers K., and Nelson B. J., Int. J. Robot. Res. 25, 527 (2006). 10.1177/0278364906065389 [DOI] [Google Scholar]
- Donald B. R., Levey C. G., McGray C. D., Paprotny I., and Rus D., J. Microelectromech. Syst. 5, 1 (2006). 10.1109/JMEMS.2005.863697 [DOI] [Google Scholar]
- Frutiger D. R., Vollmers K., Kratochvil B. E., and Nelson B. J., Int. J. Robot. Res. 29, 613 (2010). 10.1177/0278364909353351 [DOI] [Google Scholar]
- Zhang L., Abbott J. J., Dong L., Kratochvil B. E., Bell D., and Nelson B. J., Appl. Phys. Lett. 94, 064107 (2009). 10.1063/1.3079655 [DOI] [Google Scholar]
- Jager E. W. H., Inganäs O., and Lundström I., Science 288, 2335 (2000). 10.1126/science.288.5475.2335 [DOI] [PubMed] [Google Scholar]
- Fischer T., Agarwal A., and Hess H., Nat. Nanotechnol. 4, 162 (2009). 10.1038/nnano.2008.393 [DOI] [PubMed] [Google Scholar]
- Clemmens J., Hess H., Doot R., Matzke C. M., Bachand G. D., and Vogel V., Lab Chip 4, 83 (2004). 10.1039/b317059d [DOI] [PubMed] [Google Scholar]
- Abbott J. J., Peyer K. E., Lagomarsino M. C., Zhang L., Dong L., Kaliakatsos I. K., and Nelson B. J., Int. J. Robot. Res. 28, 1434 (2009). 10.1177/0278364909341658 [DOI] [Google Scholar]
- Abbott J. J., Nagy Z., Beyeler F., and Nelson B. J., IEEE Rob. Autom. Mag. 14, 92 (2007). 10.1109/MRA.2007.380641 [DOI] [Google Scholar]
- Martel S., Felfoul O., Mathieu J. B., Chanu A., Tamaz S., Mohammadi M., Mankiewicz M., and Tabatabaei N., Int. J. Robot. Res. 28, 1169 (2009). 10.1177/0278364908104855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behkam B. and Sitti M., Appl. Phys. Lett. 90, 023902 (2007). 10.1063/1.2431454 [DOI] [Google Scholar]
- Darnton N., Turner L., Breuer K., and Berg H. C., Biophys. J. 86, 1863 (2004). 10.1016/S0006-3495(04)74253-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung S., Kim J. W., Malshe A., Lee C., and Pooran R., in Proceedings of the 12th International Conference on Solid State Sensors, Actuators and Microsystems (Transducers ‘03), Boston, MA, 8–12 June 2003 (IEEE, 2003), p. 678.
- Lu Z. and Martel S., The Nanotechnology Conference and Trade Show (NSTI) Nanotech, Boston, MA, May 7–11 (2006).
- Kubori T., Shimamoto N., Yamaguchi S., Namba K., and Aizawa S. I., J. Mol. Biol. 226, 433 (1992). 10.1016/0022-2836(92)90958-M [DOI] [PubMed] [Google Scholar]
- Berg H. C., Annu. Rev. Biochem. 72, 19 (2003). 10.1146/annurev.biochem.72.121801.161737 [DOI] [PubMed] [Google Scholar]
- Samatey F. A., Matsunami H., Imada K., Nagashima S., Shaikh T. R., Thomas D. R., Chen J. Z., DeRosier D. J., Kitao A., and Namba K., Nature (London) 431, 1062 (2004). 10.1038/nature02997 [DOI] [PubMed] [Google Scholar]
- Berry R. M., Encyclopedia of Life Sciences (Wiley, 2001) (online). [Google Scholar]
- Berry R. M., Turner L., and Berg H. C., Biophys. J. 69, 280 (1995). 10.1016/S0006-3495(95)79900-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Biochemistry 72, 19 (2003). 10.1146/annurev.biochem.72.121801.161737 [DOI] [Google Scholar]
- Purcell E. M., Am. J. Phys. 45, 3 (1977). 10.1119/1.10903 [DOI] [Google Scholar]
- Taylor B. L., Zhulin I. B., and Johnson M. S., Annu. Rev. Microbiol. 53, 103–128 (1999). 10.1146/annurev.micro.53.1.103 [DOI] [PubMed] [Google Scholar]
- Armitage J. P., Bacterial Taxis (Wiley, 2001) (online). [Google Scholar]
- Weibel D. B., Garstecki P., Ryan D., DiLuzio W. R., Mayer M., Seto J. E., and Whitesides G. M., Proc. Natl. Acad. Sci. U.S.A. 102, 11963 (2005). 10.1073/pnas.0505481102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steager E., Kim C. B., Patel J., Bith S., Naik C., Reber L., and Kim M. J., Appl. Phys. Lett. 90, 263901 (2007). 10.1063/1.2752721 [DOI] [Google Scholar]
- Blakemore R., Science 190, 377 (1975). 10.1126/science.170679 [DOI] [PubMed] [Google Scholar]
- Schüler D. and Frankel R. B., Appl. Microbiol. Biotechnol. 52, 464 (1999). 10.1007/s002530051547 [DOI] [PubMed] [Google Scholar]
- Lefevre C. T., Song T., Yonnet J. P., and Wu L. F., Appl. Microbiol. Biotechnol. 75, 3835 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazylinski D. A. and Frankel R. B., Nat. Rev. Microbiol. 2, 217 (2004). 10.1038/nrmicro842 [DOI] [PubMed] [Google Scholar]
- Lefèvre C. T., Bernadac A., Yu. Zhang K., Pradel N., and Wu L. F., Environ. Microbiol. 11, 1646 (2009). 10.1111/j.1462-2920.2009.01887.x [DOI] [PubMed] [Google Scholar]
- Lefèvre C. T., Santini C. L., Bernadac A., Zhang W. J., Li Y., and Wu L. F., Mol. Microbiol. 78, 1304 (2010). 10.1111/j.1365-2958.2010.07404.x [DOI] [PubMed] [Google Scholar]
- Verpoorte E., Lab Chip 3, 60 (2003). 10.1039/b313217j [DOI] [PubMed] [Google Scholar]
- Fernandes R., James T., Zuniga M. C., Li N., Ngan S., and Gracias D. H., 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, Washington, 2–6 October 2011.
- Martel S., Mohammadi M., Felfoul O., Lu Z., and Pouponneau P., Int. J. Robot. Res. 28, 571 (2009). 10.1177/0278364908100924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel S., Tremblay C. C., Ngakeng S., and Langlois G., Appl. Phys. Lett. 89, 233903 (2006). 10.1063/1.2402221 [DOI] [Google Scholar]
- Ma D., Chen H., Li Z., and He Q., Biomicrofluidics 4, 044107 (2010). 10.1063/1.3516038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B., Kurkuri M., Shi J. Y., Lwin L. E. M. P., and Palms D., Biomicrofluidics 4, 032205 (2010). 10.1063/1.3480573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepke M. W. and Beebe D. J., Lab Chip 6, 1484 (2006). 10.1039/b612140c [DOI] [PubMed] [Google Scholar]
- Lee C. C., Sui G., Elizarov A., Shu C. J., Shin Y. S., Dooley A. N., Huang J., Daridon A., Wyatt P., and Stout D., Science 310, 1793 (2005). 10.1126/science.1118919 [DOI] [PubMed] [Google Scholar]
- Anderson J. R., Chiu D. T., Jackman R. J., Cherniavskaya O., McDonald J. C., Wu H., Whitesides S. H., and Whitesides G. M., Anal. Chem. 72, 3158 (2000). 10.1021/ac9912294 [DOI] [PubMed] [Google Scholar]
- Duffy D. C., McDonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70, 4974 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- Zhang W. J., Chen C., Li Y., Song T., and Wu L. F., Environ. Microbiol. Rep. 2, 646 (2010) 10.1111/j.1758-2229.2010.00150.x [DOI] [PubMed] [Google Scholar]
- Darwish I. A., Al-Obaid A. R. M., and Al-Malaq H. A., J. Immunoassay Immunochem. 32, 57 (2011). 10.1080/15321819.2010.538109 [DOI] [PubMed] [Google Scholar]
- Cao Y. C., Wang Z., Jin X., Hua X. F., Liu M. X., and Zhao Y. D., Colloids Surf., A 334, 53 (2009). 10.1016/j.colsurfa.2008.10.002 [DOI] [Google Scholar]
- Staros J. V., Wright R. W., and Swingle D. M., Anal. Biochem. 156, 220 (1986). 10.1016/0003-2697(86)90176-4 [DOI] [PubMed] [Google Scholar]
- Grabarek Z. and Gergely J., Anal. Biochem. 185, 131 (1990). 10.1016/0003-2697(90)90267-D [DOI] [PubMed] [Google Scholar]
- Wong S. S., Chemistry of Protein Conjugation and Cross-Linking (CRC, 1991). [Google Scholar]
- Lee J., Edwards H., Pereira C., and Samii S., J. Mater. Sci. Mater. Med. 7, 531 (1996). 10.1007/BF00122176 [DOI] [Google Scholar]
- Anderson G. W., Zimmerman J. E., and Callahan F. M., J. Am. Chem. Soc. 86, 1839 (1964). 10.1021/ja01063a037 [DOI] [Google Scholar]
- Bangs L. and No T. N., 205: Covalent Coupling (Bangs Laboratories, Fishers, IN, 2008). [Google Scholar]
- Happel J. and Brenner H., Low Reynolds Number Hydrodynamics: With Special Applications to Particulate Media (Springer, 1983). [Google Scholar]
- Namdeo S., Khaderi S. N., den Toonder J. M. J., and Onck P. R., Biomicrofluidics 5, 034108 (2011). 10.1063/1.3608240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A. W., Physics 4, 403 (1933). 10.1063/1.1745151 [DOI] [Google Scholar]
- Lee H., Purdon A. M., and Westervelt R. M., App. Phys. Lett. 85, 1063 (2004). 10.1063/1.1776339 [DOI] [Google Scholar]