Abstract
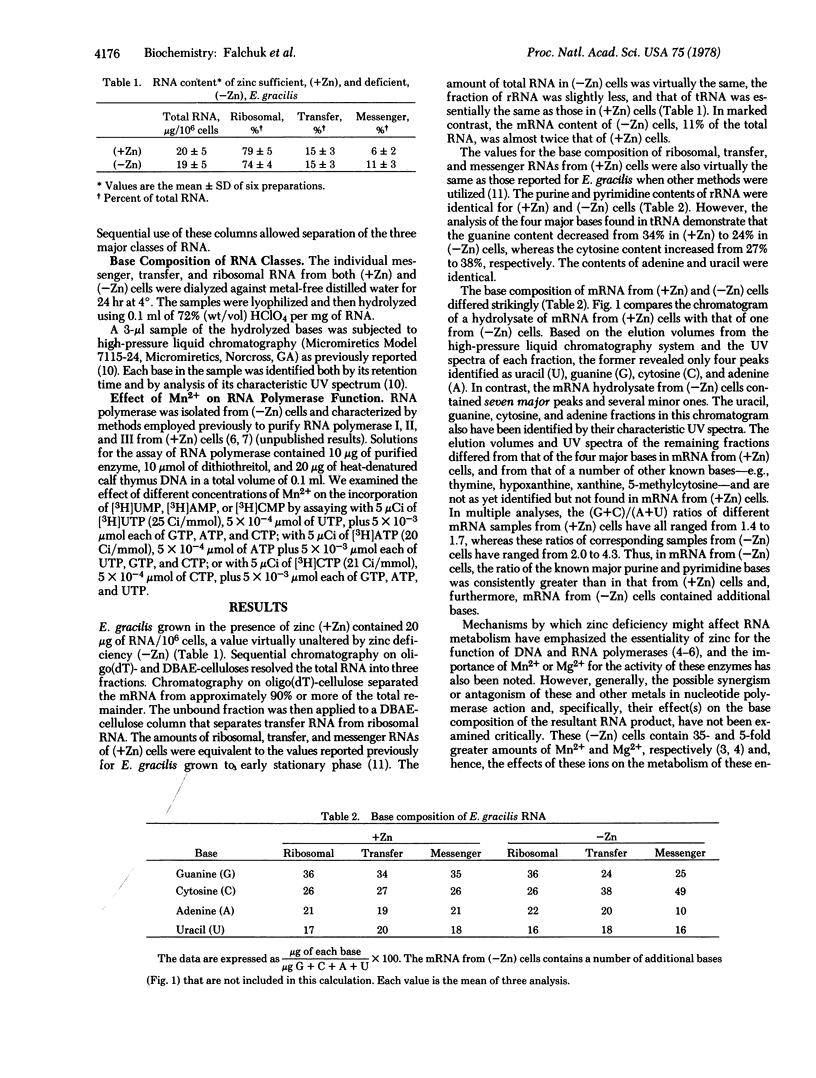
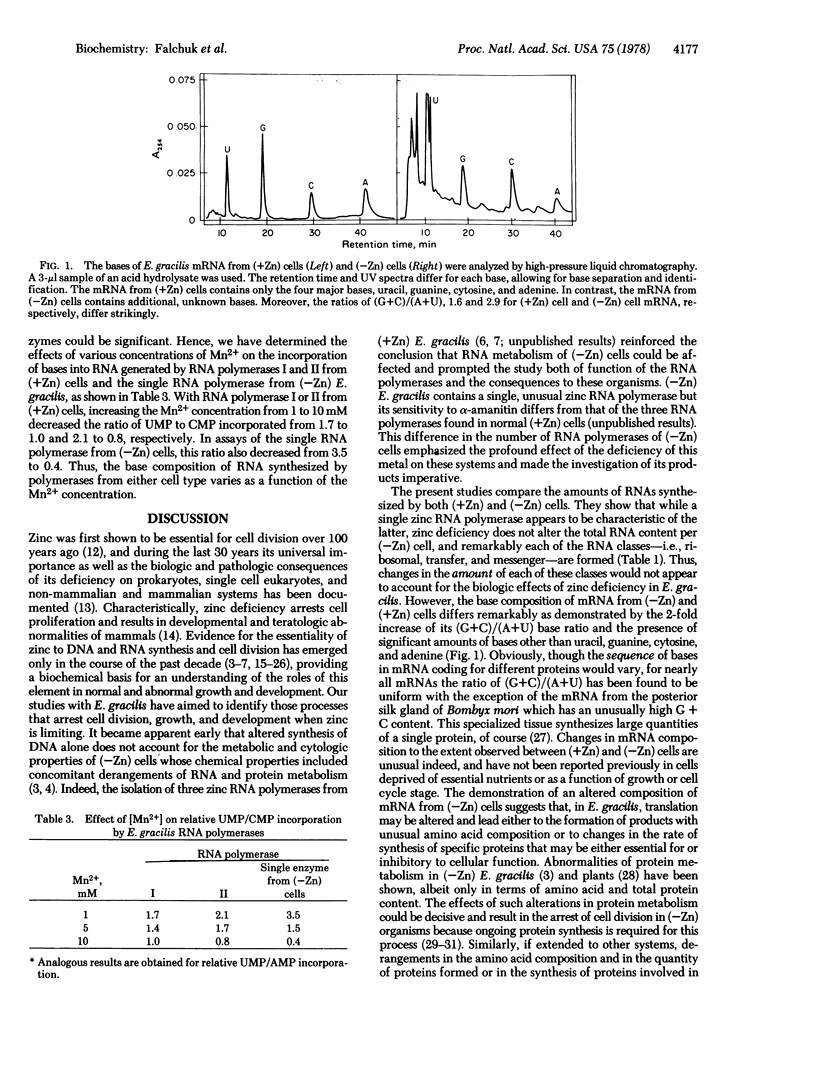
The three major RNA classes from zinc-sufficient [(+Zn)] and zinc-deficient [(=Zn)] Euglena gracilis have been separated by affinity chromatography on oligo(dT)- and N-[N'-[m-(dihydroxyboryl)phenyl]succinamoyl]aminoethyl (DBAE)-celluloses. The total RNA content and the ribosomal and transfer RNA fractions are the same in (+Zn) and (=Zn) cells. IN (-Zn) cells, the messenger RNA fraction increases, and its altered base composition reveals additional bases and a 2-fold increase in the (G+C)/(A+U) ratio. Since the intracellular content of manganese increases in (-Zn) cells, we have examined its role in determining these changes in RNA composition. An increase in the Mn2+ content from 1 to 10 mM in assays with RNA polymerases I and II from (+Zn) cells and those with the single RNA polymerase from (-Zn) cells decreases the ratio of UMP to CMP incorporated from 1.7 to 1.0, 2.1 to 0.8 and 3.5 to 0.4, respectively. Thus, Mn2+ concentration can significantly alter the products of the enzymatic action of RNA polymerases from both (+Zn) and (-Zn) E. gracilis cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Atsuya I. Yeast RNA polymerase I: a eukaryotic zinc metalloenzyme. Biochem Biophys Res Commun. 1976 Mar 22;69(2):548–554. doi: 10.1016/0006-291x(76)90555-6. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. Reverse transcriptase from avian myeloblastosis virus: a zinc metalloenzyme. Biochem Biophys Res Commun. 1974 Apr 23;57(4):967–972. doi: 10.1016/0006-291x(74)90790-6. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. Zinc reverse transcriptases from mammalian RNA type C viruses. Biochem Biophys Res Commun. 1975 Jan 20;62(2):296–302. doi: 10.1016/s0006-291x(75)80137-9. [DOI] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Brunori A., Avanzi S., D'Amato F. Chromatid and chromosome abberrations in irradiated dry seeds of Vicia faba. Mutat Res. 1966 Aug;3(4):305–313. doi: 10.1016/0027-5107(66)90037-6. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Drishan A., Vallee B. L. DNA distribution in the cell cycle of Euglena gracilis. Cytofluorometry of zinc deficient cells. Biochemistry. 1975 Jul 29;14(15):3439–3444. doi: 10.1021/bi00686a023. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Fawcett D. W., Vallee B. L. Role of zinc in cell division of Euglena gracilis. J Cell Sci. 1975 Jan;17(1):57–78. doi: 10.1242/jcs.17.1.57. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Hardy C., Ulpino L., Vallee B. L. RNA polymerase, manganese and RNA metabolism of zinc sufficient and deficient E. gracilis. Biochem Biophys Res Commun. 1977 Jul 11;77(1):314–319. doi: 10.1016/s0006-291x(77)80198-8. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Mazus B., Ulpino L., Vallee B. L. Euglena gracilis DNA dependent RNA polymerase II: a zinc metalloenzyme. Biochemistry. 1976 Oct 5;15(20):4468–4475. doi: 10.1021/bi00665a021. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H., Ulpino L., Mazus B., Vallee B. L. E. gracilis RNA polymerase I: a zinc metalloenzyme. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1206–1212. doi: 10.1016/0006-291x(77)91646-1. [DOI] [PubMed] [Google Scholar]
- Fuwa K., Wacker W. E., Druyan R., Bartholomay A. F., Vallee B. L. NUCLEIC ACIDS AND METALS, II: TRANSITION METALS AS DETERMINANTS OF THE CONFORMATION OF RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1960 Oct;46(10):1298–1307. doi: 10.1073/pnas.46.10.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas R. E., Roberts R. J. One predominant 5'-undecanucleotide in adenovirus 2 late messenger RNAs. Cell. 1977 Jul;11(3):533–544. doi: 10.1016/0092-8674(77)90071-x. [DOI] [PubMed] [Google Scholar]
- Hirsch M., Penman S. Post-transcriptional addition of polyadenylic acid to mitochondrial RNA by a cordycepin-insensitive process. J Mol Biol. 1974 Feb 25;83(2):131–142. doi: 10.1016/0022-2836(74)90384-2. [DOI] [PubMed] [Google Scholar]
- Holzer H. Regulation of enzymes by enzyme-catalyzed chemical modification. Adv Enzymol Relat Areas Mol Biol. 1969;32:297–326. doi: 10.1002/9780470122778.ch7. [DOI] [PubMed] [Google Scholar]
- Hurley L. S. Zinc deficiency in the developing rat. Am J Clin Nutr. 1969 Oct;22(10):1332–1339. doi: 10.1093/ajcn/22.10.1332. [DOI] [PubMed] [Google Scholar]
- Kotsiopoulos P. S., Mohr S. C. Protein synthesis elongation factor 1 from rat liver: a zinc metalloenzyme. Biochem Biophys Res Commun. 1975 Dec 1;67(3):979–987. doi: 10.1016/0006-291x(75)90771-8. [DOI] [PubMed] [Google Scholar]
- Litman R. M. The differential effect of magnesium and manganese ions on the synthesis of poly (dGd.C) and Micrococcus luteus DNA by Micrococcus luteus DNA polymerase. J Mol Biol. 1971 Oct 14;61(1):1–23. doi: 10.1016/0022-2836(71)90203-8. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo A. O., Littau V. C., Allfrey V. G., Mirsky A. E. Modification of ribonucleic Acid synthesis in nuclei isolated from normal and regenerating liver: some effects of salt and specific divalent cations. Proc Natl Acad Sci U S A. 1967 Mar;57(3):743–750. doi: 10.1073/pnas.57.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prask J. A., Plocke D. J. A Role for Zinc in the Structural Integrity of the Cytoplasmic Ribosomes of Euglena gacilis. Plant Physiol. 1971 Aug;48(2):150–155. doi: 10.1104/pp.48.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. A., Vallee B. L. Euglena gracilis, A Test Organism for Study of Zinc. Plant Physiol. 1962 May;37(3):428–433. doi: 10.1104/pp.37.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton M. C., Wu C. W., Goldthwait D. A. The presence and possible role of zinc in RNA polymerase obtained from Escherichia coli. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2497–2501. doi: 10.1073/pnas.68.10.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. A., Eichhorn G. L. Interactions of metal ions with polynucleotides and related compounds. XI. The reversible unwinding and rewinding of deoxyribonucleic acid by zinc (II) Ions through temperature manipulation. Biochemistry. 1968 Mar;7(3):1026–1032. doi: 10.1021/bi00843a022. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Metal activation of DNA synthesis. Biochem Biophys Res Commun. 1976 Jun 7;70(3):812–817. doi: 10.1016/0006-291x(76)90664-1. [DOI] [PubMed] [Google Scholar]
- Slater J. P., Mildvan A. S., Loeb L. A. Zinc in DNA polymerases. Biochem Biophys Res Commun. 1971 Jul 2;44(1):37–43. doi: 10.1016/s0006-291x(71)80155-9. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Shapiro B. M., Ginsburg A., Kingdon H. S., Denton M. D. Regulation of glutamine synthetase activity in Escherichia coli. Brookhaven Symp Biol. 1968 Jun;21(2):378–396. [PubMed] [Google Scholar]
- Suzuki Y., Brown D. D. Isolation and identification of the messenger RNA for silk fibroin from Bombyx mori. J Mol Biol. 1972 Feb 14;63(3):409–429. doi: 10.1016/0022-2836(72)90437-8. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L. Biochemistry, physiology and pathology of zinc. Physiol Rev. 1959 Jul;39(3):443–490. doi: 10.1152/physrev.1959.39.3.443. [DOI] [PubMed] [Google Scholar]
- WACKER W. E. Nucleic acids and metals. III. Changes in nucleic acid, protein, and metal content as a consequence of zinc deficiency in Euglena gracilis. Biochemistry. 1962 Sep;1:859–865. doi: 10.1021/bi00911a019. [DOI] [PubMed] [Google Scholar]
- Wandzilak T. M., Benson R. W. Yeast RNA polymerase III: a zinc metalloenzyme. Biochem Biophys Res Commun. 1976 May 23;76(2):247–252. doi: 10.1016/0006-291x(77)90718-5. [DOI] [PubMed] [Google Scholar]



