Abstract
Doxorubicin (DOX) is one of the most powerful and widely prescribed chemotherapeutic agents to treat divergent human cancers. However, the clinical use of DOX is restricted due to its severe cardiotoxic side-effects. There has been ongoing search for cardioprotectants against DOX toxicity. Inorganic nitrate has emerged as a bioactive compound that can be reduced into nitrite and nitric oxide in vivo and in turn plays a therapeutic role in diseases associated with nitric oxide insufficiency or dysregulation. In this review, we describe a novel concept of using dietary supplementation of inorganic nitrate to reduce DOX-induced cardiac cellular damage and dysfunction, based on our recent promising studies in a mouse model of DOX cardiotoxicity. Our data show that chronic oral ingestion of sodium nitrate, at a dose equivalent to ~400% of the Acceptable Daily Intake of the World Health Organization, alleviated DOX-induced left ventricular dysfunction and mitochondrial respiratory chain damage. Such cardioprotective effects were associated with reduction of cardiomyocyte necrosis/apoptosis, tissue lipid peroxidation, and mitochondrial H2O2 generation following DOX treatment. Furthermore, proteomic studies revealed enhanced cardiac expression of mitochondrial antioxidant enzyme – peroxiredoxin 5 in the nitrate-treated animals. These studies suggest that inorganic nitrate could be an inexpensive therapeutic agent for long-term oral administration in preventing DOX-induced cardiac toxicity and myopathy during the prolonged pathological process. Future clinical trials in the cancer patients undergoing DOX chemotherapy are warranted to translate these experimental findings into an effective new therapy in preventing the DOX-induced cardiomyopathy.
Keywords: anthracycline, cardioprotection, cardiotoxicity, mitochondria, nitrate, ventricular function
1. Introduction
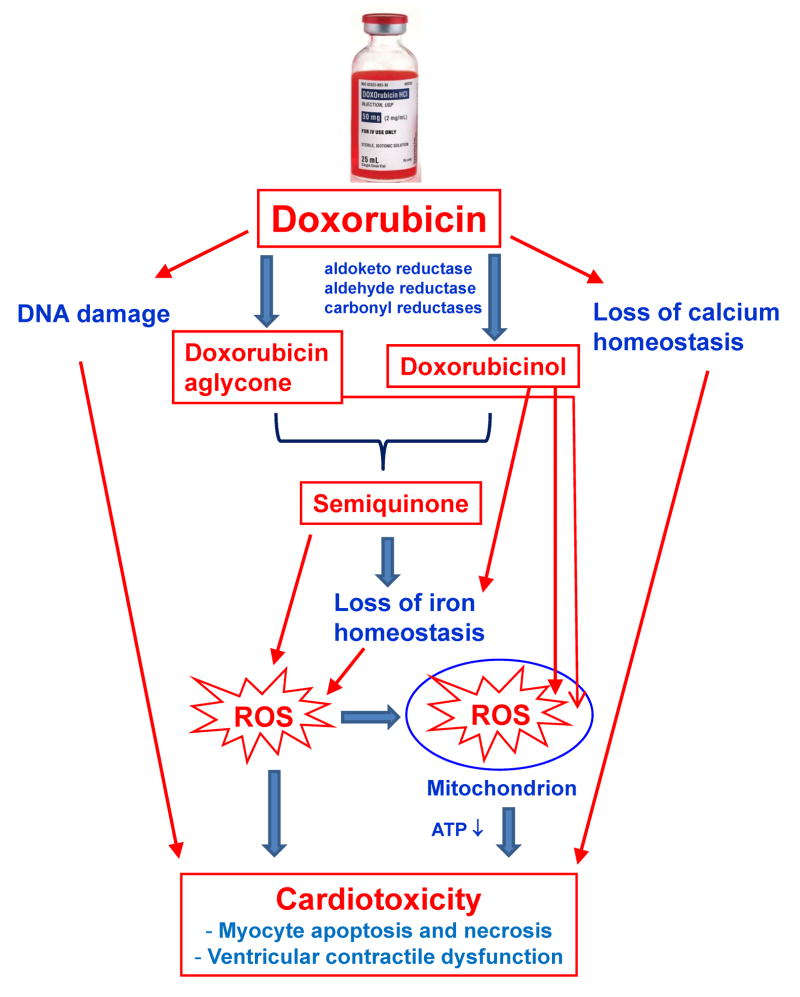
Doxorubicin (DOX) is a broad-spectrum and potent anthracycline antibiotic that has been widely used as a chemotherapeutic agent (trade names: Adriamycin®, Rubex®) to treat a variety of human cancers (1). Despite its anti-cancer efficacy, the clinical use of DOX is often limited by dose-dependent cardiotoxicity, which may lead to irreversible dilated cardiomyopathy and congestive heart failure (CHF) (2; 3). As illustrated in Fig. 1, the current popular theories for explaining DOX cardiotoxicity include the DOX-induced increase of oxidative stress in cardiomyocytes (4), alteration of mitochondrial energetics (5; 6), and direct effect on DNA. Anthracyclines may promote the formation of reactive oxygen species (ROS) through redox cycling of their aglycones as well as their anthracycline-iron complexes (7). The glycosidic DOX bond can be cleaved to yield 7-deoxydoxorubicinone, again producing ROS (4). Other proposed explanations of cardiotoxicity involve platelet-activating factor, prostaglandins, histamine, calcium, and C-13 hydroxy anthracycline metabolites, etc. (8; 9). DOX is metabolized to doxorubicinol, which has been implicated for its cardiotoxicity, possibly by causing perturbation of the iron homeostasis (4). The DOX-to-doxorubicinol metabolism is mediated by aldo-keto reductase, aldehyde reductase, and carbonyl reductases - CBR1 and CBR3 (9). For example, a recent study suggested that the functional CBR3 V244M polymorphism may have an impact on the risk of anthracycline-related CHF among childhood cancer survivors by modulating the intracardiac formation of cardiotoxic anthracycline alcohol metabolites (10). The potential role of genetic risk factors in anthracycline-related CHF remains to be further defined.
Figure 1.
Major pathways of cardiotoxicity of with doxorubicin and its metabolites. ROS – reactive oxygen species.
Due to the incomplete understanding of the multi-factorial cellular and molecular drivers underlying DOX cardiotoxicity, the optimal therapeutic approaches for protection against DOX cardiotoxicity have not yet been identified. This is because most of the tested agents (such as anti-oxidants and β-adrenergic receptor blockers) have pronounced clinical disadvantages including decline in high-density lipoprotein levels, inability to prevent mortality, weight loss, and potentiation of myelosuppression (a compromised ability of bone marrow to produce blood cells) (11). In addition, dexrazoxane, the only drug currently approved by the U.S. Federal Drug Administration (FDA) for treating DOX cardiotoxicity, acts by displacing iron from anthracycline-iron complexes or by chelating free cellular iron and in turn preventing the site-specific iron-catalyzed ROS overproduction (7). However, there has been a critical reassessment of the so-called “ROS and iron” hypothesis. Most notably, numerous exogenous antioxidants have failed to alleviate DOX cardiotoxicity in clinical trials (7). Several chelators that are stronger and more selective for iron did not protect against DOX cardiotoxicity (12; 13). There is also concern about dexrazoxane for its adverse effects of worsening myelosuppression and interfering with the anti-cancer efficacy of DOX. Therefore, there is an ongoing and urgent need to search for a better and safer cardioprotectant against DOX toxicity.
In this review, we provide an overview of our recent works on the use of inorganic nitrate in alleviating DOX cardiotoxicity at the systemic, cellular, organelle, and molecular levels (14; 15).
2. Role of nitric oxide (NO) and NO synthase (NOS) in DOX-induced cardiac injury
In addition to ROS, reactive nitrogen species are also implicated in DOX cardiotoxicity via disruption of NO regulation (16). DOX-induced cardiac dysfunction results from formation of peroxynitrite from the rapid reaction of NO and superoxide (17; 18). Previous studies have suggested that DOX-enhanced levels of peroxynitrite may negatively affect ventricular contractile function, which could result from the peroxynitrite-caused inhibition of myofibrillar creatine kinase (19), impairment in myocyte Ca2+ cycling (20), as well as the peroxynitrite-enhanced protein phosphatase activity that promotes the interaction of phospholamban with protein phosphatase 2a leading to cardiac dysfunction (21). In addition, previous studies also demonstrated the detrimental role of inducible and endothelial NOS (iNOS and eNOS, respectively) in rodent models of DOX cardiotoxicity (17; 18; 22; 23).
Conversely, NO is essential for the integrity of cardiovascular system and the decreased production and/or bioavailability of NO leads to the development of cardiovascular disorders (24) and heart failure (25). Numerous studies since 1999 have shown that iNOS/eNOS-derived NO protects against ischemia/reperfusion (I/R) injury induced by ischemic, hypoxic, and pharmacological preconditioning (26–32). Furthermore, our group first reported a beneficial role of NO in sildenafil-induced protection against DOX cardiotoxicity (33). There is also a possible link between the increased NO generation and diminished DOX cardiotoxicity under treatment of β-adrenergic blockers (34) or statin (35), because these drugs are known to enhance iNOS/eNOS mRNA and/or protein levels in myocardium (36) and cardiomyocytes (37). Hence, the opposing views regarding NO and NOS in DOX cardiotoxicity remain, similar to the overall image of NO as a double-edged sword.
3. NOS-independent nitrate/nitrite/NO pathway for cardiovascular protection
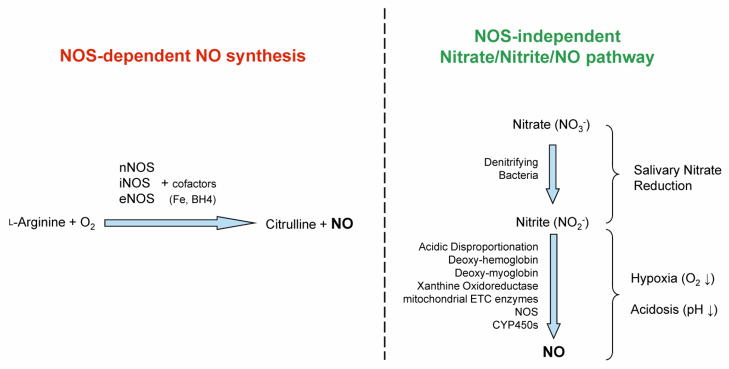
Nitrate and nitrite have been traditionally considered as the inert end-products of NO oxidation metabolism with limited intrinsic biological activity (38). This concept of nitrate/nitrite has recently been revised and inorganic nitrate/nitrite have emerged at the forefront of NO biology because they represent a major storage form of NO in blood and tissues (39). As summarized in Fig. 2, in addition to the L-arginine-NOS-dependent NO synthesis, the NOS-independent mechanism of NO generation also converts nitrate to nitrite to NO in vivo via both enzymatic reduction [e.g. xanthine oxidoreductase (40)] and non-enzymatic acidic disproportionation (41). There are numerous colonies of bacteria in the mammalian oral cavity and intestine which can reduce nitrate to nitrite in vivo (42). During the past decade, there has been an increasing recognition of the nitrate-nitrite-NO pathway as a complementary or alternative system to the NOS-dependent pathway for ensuring sufficient NO production, especially under pathophysiological situations including hypoxia and acidosis when the oxygen-dependent NOS activity is compromised (43; 44). Since a substantial portion of nitrate/nitrite in the blood and tissue is derived from dietary sources, the dietary supplementation of nitrate or nitrite protects against myocardial I/R injury (45; 46), cardiac arrest-resuscitation, reduces hypertension (47), and improves endothelial and platelet function as well as exercise performance (48). Table 1 provides an updated list of 14 key published studies in humans and rodents since 2002, which have consistently demonstrated the beneficial effects of nitrate supplementation against various cardiovascular disorders and risk factors (14; 15; 40; 45; 49–58).
Figure 2.
Distinctive pathways for the nitric oxide synthase (NOS) dependent and independent generation of nitric oxide (NO) in living organisms.
Table 1.
List of the key published studies demonstrating cardiovascular benefits of dietary inorganic nitrate.
| Authors | Species (disease) | Targeted pathologies, nitrate dose, and main end-points | Beneficial effects | Key cellular and molecular mechanisms |
|---|---|---|---|---|
| Richardson et al. [54] | Human | Blood-borne risk factors; KNO3 2 mmol once; blood samples | Platelet aggregation ↓ | RSNO ↑ |
| Larsen et al. [57] | Human | Hypertension; NaNO3 (0.1 mmol/kg/day); blood pressure | DBP ↓ | NOx ↑ |
| Bryan et al. [45] | Mouse | Ischemia–reperfusion; NaNO3 (1 g/L); myocardial infarct size | Infarct size ↓ | NOx ↑ |
| Webb et al. [49] | Human | Hypertension; beetroot juice; blood pressure, tissue perfusion, blood samples | DBP ↓ SBP ↓ platelet aggregation ↓ endothelial dysfunction ↓ | NOx ↑ |
| Jansson et al. [40] | Rat | Ischemia–reperfusion; NaNO3 (1 g/L); post-ischemic blood flow | Post-ischemic blood flow ↑ | |
| Petersson et al. [50] | Rat | Hypertension; KNO3 (0.06–0.35 mmol/kg/day); blood pressure | DBP ↓ SBP ↓ | Oral bacteria to convert nitrate to nitrite |
| Kapil et al. [56] | Human | Hypertension; KNO3 or beetroot juice (0.06–0.35 mmol/kg/day); blood pressure | DBP ↓ SBP ↓ | NOx ↑ |
| Carlström et al. [55] | Mouse (eNOS- KO); rat | Metabolic syndrome; NaNO3 (0.1 mmol/kg/day); glucose and lipid profiles; visceral fat; blood pressure | Body weight/fat ↓ triglycerides ↓ glucose tolerance ↑ MAP ↓ | Acute NOx ↑ independent of eNOS |
| Sobko et al. [52] | Human | Hypertension; Japanese traditional diet (nitrate 19 mg/kg/day); blood pressure | DBP ↓ | |
| Presley et al. [51] | Human (aged) | Aging-related decrease in brain circulation; arterial spin labeling MRI; tissue perfusion | Regional cerebral perfusion ↑ | NOx ↑ |
| Carlström et al. [53] | Rat (SHBP) | High salt-induced hypertension; NaNO3 (1 mmol/kg/day); myocardial infarct size | MAP ↓ cardiac hypertrophy ↓ cardiac fibrosis ↓ | Blood lipid peroxidation ↓ |
| Zhu et al. [14] | Mouse | DOX cardiotoxicity; NaNO3 (1 g/L) for 13 days; plasma nitrate 60 lM; echocardiography; Millar catheter; heart tissues; cultured cardiomyocytes | LV function ↑ necrosis/apoptosis ↓ | Mito-complex I activity ↑ mito-H2O2 ↓ cardiac lipid peroxidation ↓ NOx ↑ |
| Xi et al. [15] | Mouse | DOX cardiotoxicity; NaNO3 (1 g/L) for 13 days; heart tissues; proteomics with 2D-DIGE and mass spectroscopy | See Zhu et al. [14] | Prx5 ↑ |
| Kenjale et al. [58] | Human (PAD) | PAD; beetroot juice; walking distance before onset of claudication pain; blood pressure | Pain-free walking distance ↑ DBP ↓ | NOx ↑ |
Abbreviations: NOx, total sum of nitrate and nitrite; RSNO, S-nitrosothiols; DOX, doxorubicin; Prx5, peroxiredoxin 5; 2D-DIGE, two-dimensional differential in-gel electrophoresis; LV, left ventricle; MRI, magnetic resonance imaging; eNOS-KO, endothelial nitric oxide synthase knockout; SHBP, salt-induced high blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure; MAP, mean arterial pressure; ED, endothelial dysfunction; PAD, peripheral arterial disease.
The main focus of this review is to summarize and discuss our recently published data that demonstrate protective effects of nitrate against DOX cardiotoxicity (14; 15) and to emphasize the potential clinical implications of these novel findings as well as some necessary precautions for dietary ingestion of high dose of inorganic nitrate in conjunction with DOX chemotherapy.
4. Nitrate supplementation ameliorates DOX-induced LV contractile dysfunction
We employed a widely used protocol of DOX-induced acute cardiotoxicity. Adult male CF-1 outbred mice were given a single dose of DOX intraperitoneally (15 mg/kg, dissolved in saline) or volume-matched saline (0.2 mL). In the nitrate-treated groups, the mice received sodium nitrate (NaNO3) added into their drinking water at a concentration of 1 g/L (~12 mmol/L) for 7 days before the DOX injection on Day 8. The nitrate regimen was continued throughout the 5-day post-DOX period (14; 15). This oral dose of NaNO3 was chosen based on its reported cardioprotective property against myocardial ischemia-reperfusion injury (45).
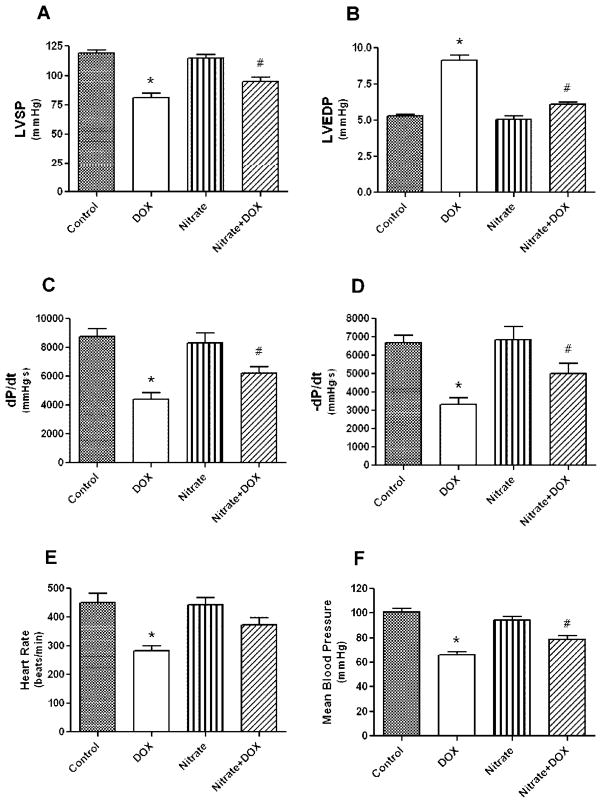
Left ventricular (LV) function was assessed using echocardiography (with Vevo770™ imaging system) under light anesthesia and subsequently with a Millar micro-tip pressure-volume catheter inserted into the LV cavity via right carotid artery under surgical anesthesia (14). LV ejection fraction and fractional shortening decreased significantly in the DOX group as compared with the control group after 5 days of DOX treatment and the LV function was improved with nitrate supplementation. Similarly, the DOX-induced LV systolic (Figs. 3A, 3C) and diastolic (Figs. 3B, 3D) dysfunction was significantly improved by nitrate. The decreases in heart rate (Fig. 3E) and mean aortic blood pressure (Fig. 3F) caused by DOX were partially attenuated by nitrate (P<0.05 Nitrate+DOX versus DOX)
Figure 3.
Effect of nitrate supplementation on doxorubicin (DOX)-induced left ventricular dysfunction. Nitrate intake restored the DOX-induced impairment in systolic pressure (A), end-diastolic pressure (B), positive, negative dP/dtmax (C, D), heart rate (E), and mean aortic blood pressure (F). Data are Mean±SEM (n=8 per group). * indicates P<0.05 versus all other groups; # indicates P<0.05 versus Control or Nitrate groups. Adopted from (14).
5. Nitrate supplementation reduces DOX-induced cardiomyocyte death in vitro
In a parallel series of experiments, we isolated cardiomyocytes from LV of the mice pretreated with 1 g/L nitrate for 7 days and exposed the cells to 1 μmol/L DOX added in the culture medium. Myocyte necrosis and apoptosis were quantified 1 hour and 18 hours later with trypan blue exclusion and TUNEL assays, respectively. The number of necrotic and apoptotic cardiomyocytes increased following exposure to DOX in vitro, whereas the cardiomyocytes from nitrate-treated mice were more resistant to DOX cytotoxicity and had less cell death (14).
6. Nitrate supplementation preserves mitochondrial function
Mitochondria are abundant in cardiomyocytes and may take up to 35% of the cell volume. Cardiac cells rely upon ATP to sustain contractile function and any interference with structural or functional integrity of mitochondria is likely to cause selective toxicity to the heart (5). Hence, mitochondria have been considered as the primary targets for the cardiotoxicity of DOX (5; 6). DOX aglycones have been shown to accumulate in the inner mitochondrial membrane where they interfere with electron carriers of the respiratory chain and cause release of cytochrome c (59). Furthermore, DOX has been shown to inhibit the net accumulation of calcium by isolated cardiac mitochondria (60).
It is widely accepted that DOX interferes with mitochondrial respiration at several levels of electron transport chain (ETC). It diverts electrons from complex I of the respiratory chain to generate the semiquinone free radical, initiating a futile redox cycle leading to a stimulation of ROS generation (61–63). The cardiotoxicity of DOX is apparently dose-dependent, i.e. a low concentration of DOX selectively damages NADH dehydrogenase by increasing oxidative stress, whereas high concentration of DOX induces non-oxidative inactivation of the complexes at ETC through the formation of a DOX-cardiolipin complex (64; 65). To demonstrate the effect of nitrate supplementation on oxidative phosphorylation following DOX treatment, we also studied oxidative phosphorylation in the cardiac mitochondria isolated from various treated groups using glutamate+malate (complex I) and succinate (complex II) as substrates to localize defects within the ETC. Enzyme activities of complex I (NADH:duroquinone oxidoreductase) and NADH dehydrogenase (proximal segment of complex I) were determined. DOX treatment markedly decreased the complex I-controlled oxidative phosphorylation, whereas the rate of succinate oxidation was not altered in DOX treated group. Nitrate supplementation restored oxidative phosphorylation, showing protection of complex I against DOX-induced damage. We further localized the site of defect in complex I by measuring the enzyme activities of complex I and NADH dehydrogenase (proximal part of complex I). We found that DOX decreased the activities of complex I and its NADH dehydrogenase (P<0.05 versus Saline). Nitrate supplementation protected both complex I and its NADH dehydrogenase against DOX-induced damage (14). These data suggest that the attenuated damage at mitochondrial complex I level may at least partially explain the nitrate-induced protection against cardiotoxicity.
7. Nitrate supplementation enhances plasma levels of nitrate and nitrite
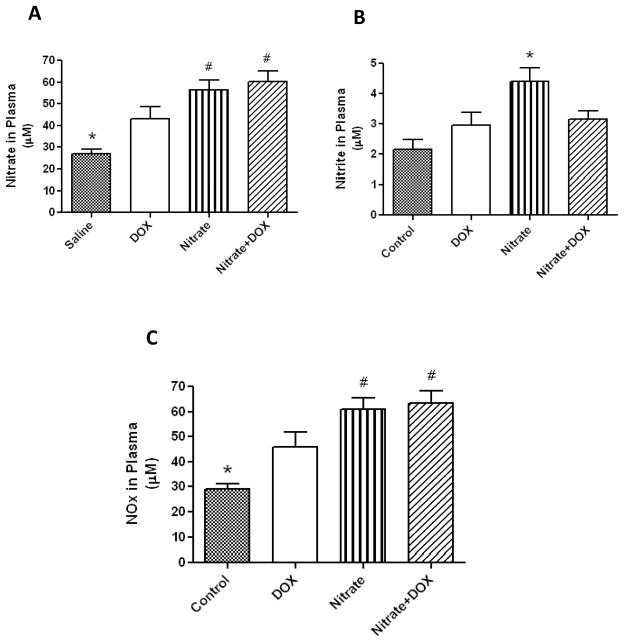
We measured the plasma levels of nitrate and nitrite in the tested animals with a SIEVERS chemiluminescence nitric oxide analyzer. We found that oral nitrate supplementation enhanced plasma nitrate (Fig. 4A) and nitrite (Fig. 4B) as compared with Control group (P<0.05). DOX treatment also elevated plasma nitrate level (likely through the enhanced NOS-dependent NO synthesis triggered by DOX). In contrast, the nitrite levels did not increase in DOX-treated groups with or without nitrate supplementation (Figs. 4A, 4B), suggesting an impeded nitrate-to-nitrite conversion. However, the overall NO oxidation products (NOx) were increased in Nitrate or Nitrate+DOX group compared with Control or DOX group (Fig. 4C, P<0.05) (14).
Figure 4.
Assessment of plasma levels of nitrate (A), nitrite (B), and sum of nitrate and nitrite, i.e. NOx (C). Data are Mean±SEM (n=6 per group). * indicates P<0.05 versus all other groups; # indicates P<0.05 versus DOX group. Adopted from (14).
Apparently the cardioprotective effects of oral nitrate supplementation were associated with a concomitant increase in plasma levels of nitrate+nitrite (i.e. NOx, Fig. 4C) – an indicator of enhanced NO production. However it is notable that DOX elevated plasma nitrate levels but not the nitrite levels (with or without nitrate ingestion). The reason is not entirely clear. From the current literature, we found absolutely no evidence for a direct reaction between DOX and nitrite, although indirect interaction between DOX and nitrite through the ROS-controlled oxidation of nitrite cannot be ruled out (66). Another interpretation for these results is that DOX enhances NOS-dependent endogenous NO synthesis that leads to the augmented plasma levels of nitrate and NOx, while the NOS-independent, oral bacterial-driven conversion of nitrate to nitrite could be impeded by the potent antibiotic property of DOX, indicated by the blunted nitrite response under DOX treatment (Fig. 4B). It is interesting that even with the small change in plasma nitrite levels, high dietary nitrate resulted in a larger increase in NOx level (Fig. 4C). Despite the possible inhibition of the nitrate-nitrite-NO pathway by DOX, a substantial amount of NO could still be generated via NOS-dependent pathway (Fig. 2), since both cardiac iNOS and eNOS tended to be further upregulated by nitrate supplementation (our unpublished observations). Nevertheless, future studies are needed to examine the role of NOS isoforms in nitrate-induced cardioprotection against DOX cardiotoxicity.
8. Antioxidant effects of inorganic nitrate
As mentioned above, DOX cardiotoxicity is often associated with ROS generation in mitochondria (65; 67), a separate mechanism from its anti-neoplastic activity (68). Complex I and complex III of the mitochondria are the key sites for ROS generation (69) and DOX enhances ROS generation through its bioreductive activation that converts DOX to a semiquinone radical via univalent reduction (65). Complex I, especially the NADH dehydrogenase (the initial segment of complex I), is the key site for DOX bioreductive activation in cardiac cells (61; 62). The increased generation of ROS from the ETC in turn damages mitochondria and induces cell injury (69; 70). Therefore, the novel observations described below on the antioxidant property of dietary nitrate may represent a key mechanistic explanation for the reduction of DOX cardiotoxicity by nitrate supplementation.
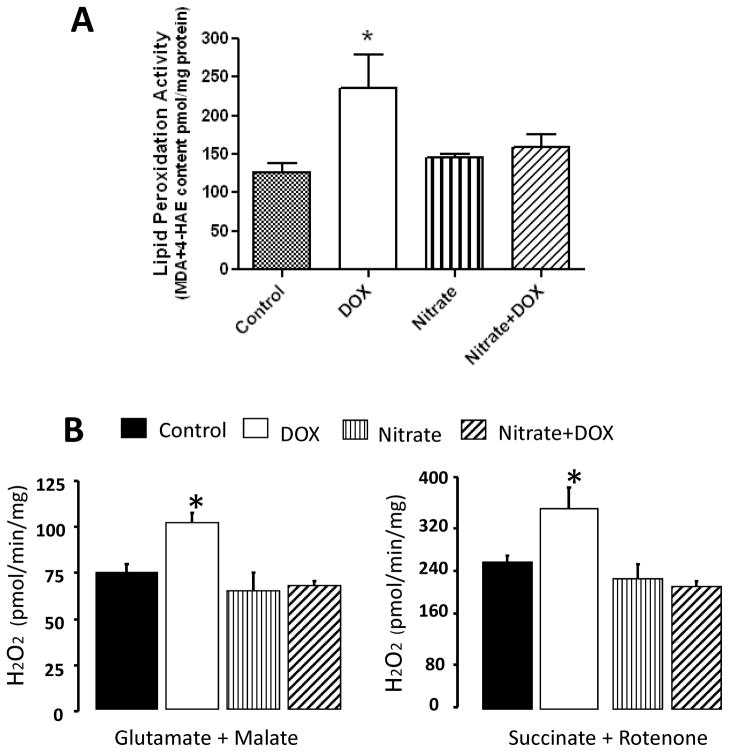
In our studies (14), we determined tissue lipid peroxidation by measuring the levels of malondialdehyde and 4-hydroxyalkenals in heart samples using a colorimetric assay kit. Lipid peroxidation in the DOX group was increased by 38% compared to control group (P<0.05, Fig. 5A), which was completely suppressed by nitrate supplementation. We also measured the rate of H2O2 generation in mitochondria by monitoring oxidation of fluorogenic indicator amplex red in the presence of horseradish peroxidase. H2O2 was increased in mitochondria isolated from the DOX-treated mice as compared with the control group when glutamate+malate were used as complex I substrate (P<0.05; Fig. 5B). Nitrate supplementation significantly decreased the H2O2 generation triggered by DOX. Similarly, nitrate supplementation attenuated DOX-induced H2O2 generation when succinate+rotenone were used as complex II substrate (Fig. 5B).
Figure 5.
Effect of nitrate supplementation on DOX-induced tissue lipid peroxidation (A) and H2O2 generation from isolated mitochondria (B). Complex I substrate (glutamate + malate) and complex II substrate (succinate + rotenone) were utilized to identify the specific site(s) of H2O2 generation in the mitochondria. Data are Mean±SEM; n=6 per group for (A); n=5–8 per group for (B). * indicates P<0.05 versus all other groups; # indicates P<0.05 versus DOX group. Adopted from (14).
Furthermore, concerning the increased mitochondrial ROS emission observed in the DOX-treated mice (Fig. 5B), there is a possibility that DOX, similar to other major anticancer alkylating agents and platinum-containing compounds, may target thioredoxin reductase (TrxR) and in turn promote oxidative stress. For example, Stanley and coworkers recently demonstrated that inhibiting thioredoxin 2 (Trx2) by auronofin dramatically increased H2O2 emission in cardiac mitochondria (71). Thus, a possible inhibitory effect of DOX on TrxR2 may be considered as a contributing factor for DOX triggered ROS production and cytotoxicity. However, this assumption appears to contradict with the published results, which demonstrated that, in contrast to the alkylating agents, anthracyclines (DOX and daunorubicin) are not inhibitors but poor substrates of TrxR (72).
9. Inorganic nitrate induces mitochondrial antioxidant enzyme - peroxiredoxin 5
To further explore the molecular mechanisms underlying nitrate-induced protection against DOX cardiotoxicity, we utilized a globally non-biased proteomic approach with two-dimensional differential in-gel electrophoresis (2D-DIGE) and MALDI TOF/TOF tandem mass spectrometry (15). The advantages of this method over conventional 2-D gels in detecting global protein expression and post-translational modifications have been increasingly appreciated in recent years (73; 74). In particular, the conventional 2-D gel methods are subject to inherent gel-to-gel variability and errors, which can be eliminated by multiplex, high-resolution 2D-DIGE technique. This technique uses both size- and charge-matched, spectrally resolvable fluorophores (CyDye) to simultaneously separate up to three samples on a single 2-D gel. As a result, every protein spot on the gel has its own internal standard and direct comparisons between the samples can be made with high sensitivity without any gel-to-gel variability (73; 74).
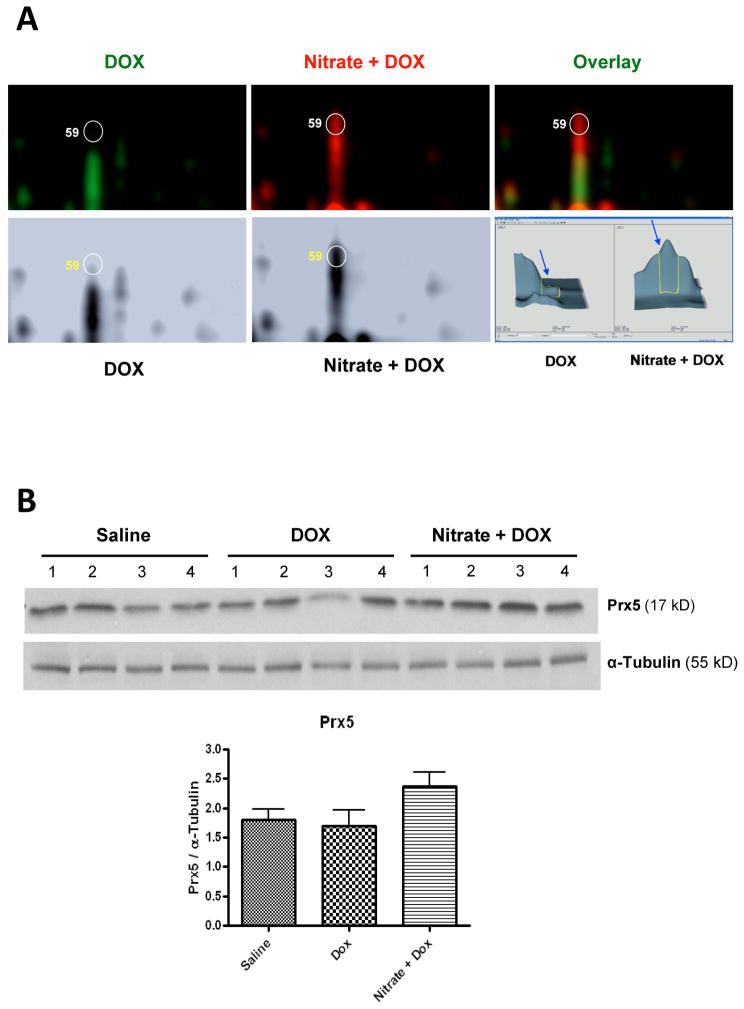
As highlighted in Fig. 6, our proteomic and Western blot data show that peroxiredoxin 5 (Prx5) was the only thiol-based antioxidant enzyme that was up-regulated by the cardioprotective regimen of nitrate (15). Peroxiredoxin represents a new type of peroxidase distinct from catalase and glutathione peroxidase, in terms of abundance in expression, broad intracellular distribution, and high affinity for hydrogen peroxide (75; 76). Among the total of 6 identified Prx isoforms, only Prx3 and Prx5 localize in mitochondria. Currently the role of Prx5 in any forms of cardioprotection is virtually unknown, although its importance in cytoprotection against injuries caused by ROS (particularly hydrogen peroxide and peroxynitrite) has been suggested by several studies in non-cardiac cells (77–80). Further physiological studies are required to confirm a cause-and-effect relationship between induction of cardiac Prx5 and cardioprotection against DOX cardiotoxicity by nitrate supplementation.
Figure 6.
Graph A shows the representative magnified 2D-DIGE gel images focusing on protein spot #59, which was identified as peroxiredoxin 5 (Prx5) by MALDI TOF/TOF tandem mass spectrometry. Note the remarkable difference between DOX alone and Nitrate+DOX groups in the labeled fluorescent dye colors (top panel), spot density of the converted black-and-white images (bottom left), as well as the integrated 3 dimensional DeCyder software analysis output (bottom right) for this protein. Graph B [partially adopted from (15)] presents Western blots for Prx5 and α-Tubulin (loading control) in heart tissue and densitometric quantification of the α-Tubulin-normalized expression of Prx5 (Mean±SEM; n=4/group).
It is noteworthy that although our proteomic study did not include the nitrate per se treatment group (15), we observed later with Western blots an upregulated level of Prx5 in cardiac mitochondria from the mice treated with nitrate alone (our unpublished data). On the other hand, our preliminary results as well as a recent proteomic study by Perlman et al. (81) indicated that nitrite per se seemed to have no significant effect on cardiac Prx5 expression. The underlying mechanisms for such a different response of Prx5 to nitrate versus nitrite need to be further elucidated. In addition, DOX and/or nitrate treatment may not only affect the overall abundance of Prx5 in the heart, but also its redox status, i.e. reduced form versus oxidized form (71). Therefore it is important to find out if nitrate ingestion also preserves the ratio of reduced/oxidized Prx5.
10. Elevated nitrate level does not hamper cancer-killing efficacy of DOX
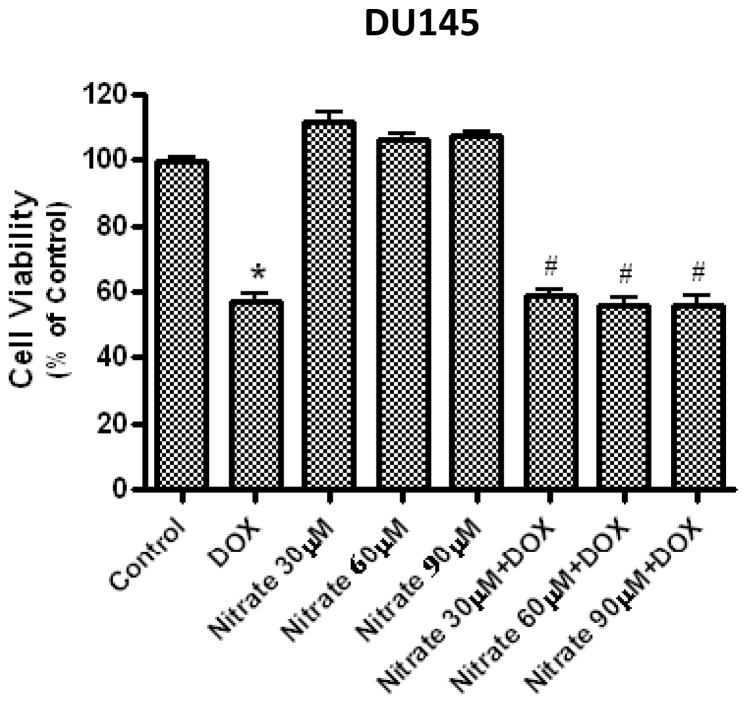
Importantly, our preliminary study with cell viability assay also showed that the cardioprotective plasma concentration of nitrate (~60 μmol/L) (14) did not interfere with anti-cancer efficacy of DOX in the cultured human prostate cancer cell line, DU145. As shown in Fig. 7, proliferation of the DU145 cells was significantly reduced by DOX (0.2 μmol/L for 48 hours) in vitro, whereas co-treatment with nitrate (30, 60 or 90 μmol/L) had no effect on DOX-induced toxicity in the cancer cells. Nevertheless, these in vitro findings need further confirmation in other types of cancer cells as well as in xenograft tumor models in vivo.
Figure 7.
Effect of nitrate on the cancer-killing efficacy of DOX in vitro. The human prostate cancer cell line (DU145) were incubated with DOX (0.2 μmol/L) for 48 hours with or without co-incubation with 30, 60, or 90 μmol/L of nitrate in the cell culture medium. Cell proliferation was measured with a CellTiter 96 AQueous One Solution Cell Proliferation assay kit (Promega Corp.). Data are Mean±SEM (n=3 per group). * P<0.05 vs. Control; # P<0.05 vs. the corresponding dose of Nitrate group.
11. Therapeutic implications and applicability
DOX-induced cardiomyopathy remains a clinical dilemma in oncology and cardiology practices and has severely limited the therapeutic potential of this potent anti-cancer drug (2). The above discussed results from our recent studies (14; 15) clearly suggest that nitrate supplementation could be a novel treatment modality in alleviating DOX-induced cardiotoxicity in cancer patients. The current upper limit of the World Health Organization Acceptable Daily Intake (WHO-ADI) for nitrate is 222 mg per day for an adult with 60 kg body weight (i.e. 3.4 mg/kg) (82). The oral nitrate dose used in our recent mouse studies is about 180 mg/kg/day, which converts to 14.6 mg/kg/day of equivalent human dose (according to the FDA conversion formula, i.e. mouse dose divided by 12.3). Therefore this nitrate dose is less than 400% of the WHO-ADI and highly physiological, considering only a cup of raw spinach contains 926 mg of nitrate (82). In fact, comparable oral doses of nitrate supplementation have been safely used in human subjects and afforded impressive beneficial effects under several cardiovascular disorders (see Table 1 for details). In addition, nitrate supplementation also alleviates some chronic degenerative diseases such as type-2 diabetes (55) and it could decrease whole body oxygen cost during exercise and improving maximal performance and mitochondrial efficiency of skeletal muscles (83–86). Our recent studies in the mouse model of DOX cardiotoxicity (14; 15) further underscore the potential utility of inorganic nitrate therapy as an effective and affordable therapy for protecting cancer patients from the devastating cardiotoxicity of DOX (87).
12. Concerns and precautions on potential adverse effects
Over the past 50 years, there has been a substantial disagreement among scientists over the interpretation of evidence on the issue of harmful effects of nitrate on human health (88). The evidence for nitrate as a cause of two serious diseases - infant methemoglobinemia and cancers of the digestive tract remains controversial (89–91). Despite the proven cardiovascular benefits of nitrate supplementation that would likely outweigh its potential side-effects, we should be cautious about the possibility that the sub-population of cancer patients receiving DOX may be more susceptible than healthy individuals to the adverse health effects of nitrate. Nevertheless, our recent data showed that nitrate-supplemented mice had better survival rate (93%) than the controls (80%) 5 days after DOX treatment (P<0.05), whereas a 13-day course of high nitrate intake alone did not cause any mortality or other apparent adverse effects in the mice (15).
12.1 Carcinogenesis
Nitrate has also received considerable bad press over the years. Nitrate salts have been widely used as preservative in processed meats and some epidemiological studies revealed a weak link between processed meats and gastric cancer (89–91). It was suggested that nitrate promotes digestive cancers partially due to its ability to form carcinogenic N-nitroso compounds in the digestive tract. This concept resulted in the imposed strict limits on nitrate as a food additive in many countries. To the contrary, a number of studies in animals and humans did not support the hypothesis that nitrate is carcinogenic (88; 92; 93). No difference was found in the incidence of gastric cancer and all causes of mortality between the nitrate fertilizer plant workers and the general population (94). In particular, the greatest dietary source of nitrate in the present time is vegetables, particularly leafy greens and red beet (90; 91). In addition, there should be less concern on the questionable carcinogenesis during the course of nitrate supplementation, since the targeted cancer patients are already receiving anti-cancer therapy with DOX.
12.2 Methemoglobinemia
Infant methemoglobinemia is a potentially lethal disease related to the high nitrate levels in well water in certain geographic areas (95). The nitrate-stimulated nitric oxide production in the gut would lead to its reaction with oxyhemoglobin in blood and converting it into methemoglobin. However, re-evaluation of the original studies in 1940s indicate that cases of methemoglobinemia always occurred when wells were contaminated with human or animal excrement and that the well water contained appreciable numbers of bacteria and high concentrations of nitrate, which strongly suggests that methemoglobinemia resulted from the presence of bacteria in the well water rather than nitrate per se (88). Therefore we cautiously believe that without bacterial contamination, nitrate per se should not cause methemoglobinemia in the case of nitrate use for protecting against DOX cardiotoxicity. In addition, methemoglobinemia rarely occurs in children or adults due to the physiological induction of methemoglobin reductase during the post-weaning developmental period (91).
12.3 Hypotension
One of the most common adverse effects of DOX is hypotension with a reported prevalence of 20% in high dose DOX-treated cancer patients (96). Hypotension is also a top perioperative cardiovascular complication in the patients with a history of anthracycline drug therapy, which was associated with or without depressed LV function (97). DOX enhances secretion of vasoactive substances such as histamine although the release of vasoactive substances may not play a prevalent role in the development of DOX cardiotoxicity (98). Because nitrate is a known vasodilator, its use may theoretically worsen DOX-induced hypotension. On the other hand, the nitrate-induced improvement in cardiac contractility and output may at least partially compensate for the vasodilatory effect and actually lead to a net increase of aortic blood pressure that was observed in our recent study (Fig. 3F) (14). Nevertheless, a great caution or contraindication should be applied when administering nitrate to the subgroup of patients with preexisting severe hypotension and a careful monitoring of peripheral blood pressure is always recommended under the nitrate therapy.
13. Concluding remarks
The above review summarized the cardioprotective effects of chronic dietary nitrate supplementation against cardiac injuries caused by DOX, which include ventricular dysfunction, cell death, oxidative stress, and mitochondrial respiratory chain damage. Importantly, the anticancer efficacy of DOX is not hindered by the cardioprotective concentration of nitrate. Further in-depth research is needed to identify the yet unknown molecular components and links involved in the cellular signaling cascade leading to the nitrate-enhanced resistance to DOX cardiotoxicity. In terms of translational value, the nitrate dose used in our studies is equivalent to less than 400% of WHO-ADI for human and therefore such a dose is highly physiological and can be easily attained through dietary ingestion of inorganic nitrate salts or the vegetables/vegetable juices containing high levels of nitrate. Based on the preclinical evidence from our laboratory, we believe that the use of oral nitrate supplementation could turn out to be a novel preventive and therapeutic approach for effectively reducing cardiotoxicity and financial burden of the thousands of cancer patients receiving DOX chemotherapy. Future clinical trials are clearly warranted to achieve this goal.
Highlights.
Doxorubicin is a potent cancer chemotherapy drug, but also causes cardiotoxicity.
Dietary nitrate limits cardiac dysfunction, cell death, and mitochondrial damage.
Nitrate reduces oxidative stress and induces antioxidant enzyme – peroxiredoxin 5.
Elevated levels of nitrate do not hamper anti-cancer efficacy of doxorubicin in vitro.
Inorganic nitrate may be an affordable therapy for alleviating drug cardiotoxicity.
Acknowledgments
Our original studies discussed in this review were supported by the grants from the National Institutes of Health (HL51045, HL79424, HL93685 to RCK; AG15885 to EJL), the American Heart Association (National Scientist Development Grant #0530157N to LX; Mid-Atlantic Affiliate Beginning Grant-in-Aid #0765273U to AD), and Medical Research Service, Department of Veterans Affairs (Merit Review Award to EJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bristow MR, Mason JW, Billingham ME, Daniels JR. Doxorubicin cardiomyopathy: evaluation by phonocardiography, endomyocardial biopsy, and cardiac catheterization. Ann Intern Med. 1978;88:168–175. doi: 10.7326/0003-4819-88-2-168. [DOI] [PubMed] [Google Scholar]
- 2.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin - A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 4.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 5.Wallace KB. Adriamycin-induced interference with cardiac mitochondrial calcium homeostasis. Cardiovasc Toxicol. 2007;7:101–107. doi: 10.1007/s12012-007-0008-2. [DOI] [PubMed] [Google Scholar]
- 6.Lebrecht D, Kokkori A, Ketelsen UP, Setzer B, Walker UA. Tissue-specific mtDNA lesions and radical-associated mitochondrial dysfunction in human hearts exposed to doxorubicin. J Pathol. 2005;207:436–444. doi: 10.1002/path.1863. [DOI] [PubMed] [Google Scholar]
- 7.Simunek T, Sterba M, Popelova O, Adamcova M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 8.Olson RD, Mushlin PS. Doxorubicin cardiotoxicity - analysis of prevailing hypotheses. FASEB J. 1990;4:3076–3086. [PubMed] [Google Scholar]
- 9.Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, Kawashima TI, Davies SM, Relling MV, Robison LL, Sklar CA, Stovall M, Bhatia S. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H : Quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–2795. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Chen Z, Chua CC, Ma YS, Youngberg GA, Hamdy R, Chua BH. Melatonin as an effective protector against doxorubicin-induced cardiotoxicity. Am J Physiol Heart Circ Physiol. 2002;283:H254–H263. doi: 10.1152/ajpheart.01023.2001. [DOI] [PubMed] [Google Scholar]
- 12.Hasinoff BB, Patel D, Wu X. The oral iron chelator ICL670A (deferasirox) does not protect myocytes against doxorubicin. Free Radic Biol Med. 2003;35:1469–1479. doi: 10.1016/j.freeradbiomed.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Hasinoff BB, Patel D. The iron chelator Dp44mT does not protect myocytes against doxorubicin. J Inorg Biochem. 2009;103:1093–1101. doi: 10.1016/j.jinorgbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi L, Zhu SG, Hobbs DC, Kukreja RC. Identification of protein targets underlying dietary nitrate-induced protection against doxorubicin cardiotoxicity. J Cell Mol Med. 2011;15:2512–2524. doi: 10.1111/j.1582-4934.2011.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fogli S, Nieri P, Breschi MC. The role of nitric oxide in anthracycline toxicity and prospects for pharmacologic prevention of cardiac damage. FASEB J. 2004;18:664–675. doi: 10.1096/fj.03-0724rev. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Ther. 2000;294:396–401. [PubMed] [Google Scholar]
- 18.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 19.Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA. Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Br J Pharmacol. 2002;135:581–588. doi: 10.1038/sj.bjp.0704495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katori T, Donzelli S, Tocchetti CG, Miranda KM, Cormaci G, Thomas DD, Ketner EA, Lee MJ, Mancardi D, Wink DA, Kass DA, Paolocci N. Peroxynitrite and myocardial contractility: in vivo versus in vitro effects. Free Radic Biol Med. 2006;41:1606–1618. doi: 10.1016/j.freeradbiomed.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Kohr MJ, Davis JP, Ziolo MT. Peroxynitrite Increases Protein Phosphatase Activity and Promotes the Interaction of Phospholamban with Protein Phosphatase 2a in the Myocardium. Nitric Oxide. 2009;20:217–221. doi: 10.1016/j.niox.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA, Jr, Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36:11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 23.Deng S, Kruger A, Schmidt A, Metzger A, Yan T, Godtel-Armbrust U, Hasenfuss G, Brunner F, Wojnowski L. Differential roles of nitric oxide synthase isozymes in cardiotoxicity and mortality following chronic doxorubicin treatment in mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:25–34. doi: 10.1007/s00210-009-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 25.O’Murchu B, Miller VM, Perrella MA, Burnett JC., Jr Increased production of nitric oxide in coronary arteries during congestive heart failure. J Clin Invest. 1994;93:165–171. doi: 10.1172/JCI116940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 27.Xi L, Jarrett NC, Hess ML, Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 1999;99:2157–2163. doi: 10.1161/01.cir.99.16.2157. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 30.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 31.Xi L, Tekin D, Gursoy E, Salloum F, Levasseur JE, Kukreja RC. Evidence that NOS2 acts as a trigger and mediator of late preconditioning induced by acute systemic hypoxia. Am J Physiol Heart Circ Physiol. 2002;283:H5–12. doi: 10.1152/ajpheart.00920.2001. [DOI] [PubMed] [Google Scholar]
- 32.Zhao T, Xi L, Chelliah J, Levasseur JE, Kukreja RC. Inducible nitric oxide synthase mediates delayed myocardial protection induced by activation of adenosine A1 receptors: evidence from gene-knockout mice. Circulation. 2000;102:902–907. doi: 10.1161/01.cir.102.8.902. [DOI] [PubMed] [Google Scholar]
- 33.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–1610. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 34.de Nigris F, Rienzo M, Schiano C, Fiorito C, Casamassimi A, Napoli C. Prominent cardioprotective effects of third generation beta blocker nebivolol against anthracycline-induced cardiotoxicity using the model of isolated perfused rat heart. Eur J Cancer. 2008;44:334–340. doi: 10.1016/j.ejca.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Riad A, Bien S, Westermann D, Becher PM, Loya K, Landmesser U, Kroemer HK, Schultheiss HP, Tschope C. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009;69:695–699. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- 36.Atar S, Ye Y, Lin Y, Freeberg SY, Nishi SP, Rosanio S, Huang MH, Uretsky BF, Perez-Polo JR, Birnbaum Y. Atorvastatin-induced cardioprotection is mediated by increasing inducible nitric oxide synthase and consequent S-nitrosylation of cyclooxygenase-2. Am J Physiol Heart Circ Physiol. 2006;290:H1960–H1968. doi: 10.1152/ajpheart.01137.2005. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda U, Shimpo M, Ikeda M, Minota S, Shimada K. Lipophilic statins augment inducible nitric oxide synthase expression in cytokine-stimulated cardiac myocytes. J Cardiovasc Pharmacol. 2001;38:69–77. doi: 10.1097/00005344-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 40.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 41.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 45.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchiya K, Kanematsu Y, Yoshizumi M, Ohnishi H, Kirima K, Izawa Y, Shikishima M, Ishida T, Kondo S, Kagami S, Takiguchi Y, Tamaki T. Nitrite is an alternative source of NO in vivo. Am J Physiol Heart Circ Physiol. 2005;288:H2163–H2170. doi: 10.1152/ajpheart.00525.2004. [DOI] [PubMed] [Google Scholar]
- 48.Gilchrist M, Winyard PG, Benjamin N. Dietary nitrate - good or bad? Nitric Oxide. 2010;22:104–109. doi: 10.1016/j.niox.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, Macallister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petersson J, Carlstrom M, Schreiber O, Phillipson M, Christoffersson G, Jagare A, Roos S, Jansson EA, Persson AEG, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, King SB, Laurienti PJ, Rejeski WJ, Burdette JH, Kim-Shapiro DB, Miller GD. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide. 2010;22:136–140. doi: 10.1016/j.niox.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Carlstrom M, Persson AE, Larsson E, Hezel M, Scheffer PG, Teerlink T, Weitzberg E, Lundberg JO. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc Res. 2011;89:574–585. doi: 10.1093/cvr/cvq366. [DOI] [PubMed] [Google Scholar]
- 54.Richardson G, Hicks SL, O’Byrne S, Frost MT, Moore K, Benjamin N, McKnight GM. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7:24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 55.Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci U S A. 2010;107:17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ, Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 57.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 58.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, VanBruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clementi ME, Giardina B, Di Stasio E, Mordente A, Misiti F. Doxorubicin-derived metabolites induce release of cytochrome C and inhibition of respiration on cardiac isolated mitochondria. Anticancer Res. 2003;23:2445–2450. [PubMed] [Google Scholar]
- 60.Solem LE, Heller LJ, Wallace KB. Dose-dependent increase in sensitivity to calcium-induced mitochondrial dysfunction and cardiomyocyte cell injury by doxorubicin. J Mol Cell Cardiol. 1996;28:1023–1032. doi: 10.1006/jmcc.1996.0095. [DOI] [PubMed] [Google Scholar]
- 61.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I Anthracycline radical formation by NADH dehydrogenase. J Biol Chem. 1986;261:3060–3067. [PubMed] [Google Scholar]
- 62.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–3074. [PubMed] [Google Scholar]
- 63.Nohl H, Gille L, Staniek K. The exogenous NADH dehydrogenase of heart mitochondria is the key enzyme responsible for selective cardiotoxicity of anthracyclines. Z Naturforsch C. 1998;53:279–285. doi: 10.1515/znc-1998-3-419. [DOI] [PubMed] [Google Scholar]
- 64.Bergh J. Best use of adjuvant systemic therapies II, chemotherapy aspects: dose of chemotherapy-cytotoxicity, duration and responsiveness. Breast. 2003;12:529–537. doi: 10.1016/s0960-9776(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 65.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23:15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- 66.van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 67.Chaiswing L, Cole MP, Ittarat W, Szweda LI, St Clair DK, Oberley TD. Manganese superoxide dismutase and inducible nitric oxide synthase modify early oxidative events in acute adriamycin-induced mitochondrial toxicity. Mol Cancer Ther. 2005;4:1056–1064. doi: 10.1158/1535-7163.MCT-04-0322. [DOI] [PubMed] [Google Scholar]
- 68.Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, Healey JH. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–2458. doi: 10.1200/JCO.1998.16.7.2452. [DOI] [PubMed] [Google Scholar]
- 69.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 71.Stanley BA, Sivakumaran V, Shi S, McDonald I, Lloyd D, Watson WH, Aon MA, Paolocci N. Thioredoxin reductase-2 is essential for keeping low levels of H2O2 emission from isolated heart mitochondria. J Biol Chem. 2011;286:33669–33677. doi: 10.1074/jbc.M111.284612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Witte AB, Anestal K, Jerremalm E, Ehrsson H, Arner ES. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med. 2005;39:696–703. doi: 10.1016/j.freeradbiomed.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 73.Marouga R, David S, Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal Bioanal Chem. 2005;382:669–678. doi: 10.1007/s00216-005-3126-3. [DOI] [PubMed] [Google Scholar]
- 74.Moore JC, Fu J, Chan YC, Lin D, Tran H, Tse HF, Li RA. Distinct cardiogenic preferences of two human embryonic stem cell (hESC) lines are imprinted in their proteomes in the pluripotent state. Biochem Biophys Res Commun. 2008;372:553–558. doi: 10.1016/j.bbrc.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 76.Kang SW, Rhee SG, Chang TS, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plaisant F, Clippe A, Vander SD, Knoops B, Gressens P. Recombinant peroxiredoxin 5 protects against excitotoxic brain lesions in newborn mice. Free Radic Biol Med. 2003;34:862–872. doi: 10.1016/s0891-5849(02)01440-5. [DOI] [PubMed] [Google Scholar]
- 78.Banmeyer I, Marchand C, Clippe A, Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS Lett. 2005;579:2327–2333. doi: 10.1016/j.febslet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 79.Trujillo M, Clippe A, Manta B, Ferrer-Sueta G, Smeets A, Declercq JP, Knoops B, Radi R. Pre-steady state kinetic characterization of human peroxiredoxin 5: taking advantage of Trp84 fluorescence increase upon oxidation. Arch Biochem Biophys. 2007;467:95–106. doi: 10.1016/j.abb.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Abbas K, Breton J, Picot CR, Quesniaux V, Bouton C, Drapier JC. Signaling events leading to peroxiredoxin 5 up-regulation in immunostimulated macrophages. Free Radic Biol Med. 2009;47:794–802. doi: 10.1016/j.freeradbiomed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 81.Perlman DH, Bauer SM, Ashrafian H, Bryan NS, Garcia-Saura MF, Lim CC, Fernandez BO, Infusini G, McComb ME, Costello CE, Feelisch M. Mechanistic insights into nitrite-induced cardioprotection using an integrated metabolomic/proteomic approach. Circ Res. 2009;104:796–804. doi: 10.1161/CIRCRESAHA.108.187005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 83.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 84.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, Dimenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 85.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic Biol Med. 2010;48:342–347. doi: 10.1016/j.freeradbiomed.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 86.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Daiber A, Gori T, Munzel T. Inorganic nitrate therapy improves doxorubicin-induced cardiomyopathy: a new window for an affordable cardiovascular therapy for everyone? J Am Coll Cardiol. 2011;57:2190–2193. doi: 10.1016/j.jacc.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 88.Powlson DS, Addisott TM, Benjamin N, Cassman KG, de Kok TM, van Grinsven H, L’hirondel JL, Avery AA, van Kessel C. When does nitrate become a risk for humans? J Environ Quality. 2008;37:291–295. doi: 10.2134/jeq2007.0177. [DOI] [PubMed] [Google Scholar]
- 89.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: A review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60:131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Milkowski A, Garg HK, Coughlin JR, Bryan NS. Nutritional epidemiology in the context of nitric oxide biology: A risk-benefit evaluation for dietary nitrite and nitrate. Nitric Oxide. 2010;22:110–119. doi: 10.1016/j.niox.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 91.McKnight GM, Duncan CW, Leifert C, Golden MH. Dietary nitrate in man: friend or foe? Br J Nutr. 1999;81:349–358. doi: 10.1017/s000711459900063x. [DOI] [PubMed] [Google Scholar]
- 92.Maekawa A, Ogiu T, Onodera H, Furuta K, Matsuoka C, Ohno Y, Odashima S. Carcinogenicity studies of sodium nitrite and sodium nitrate in F344 rats. Food Chem Toxicol. 1982;20:25–33. doi: 10.1016/s0278-6915(82)80005-7. [DOI] [PubMed] [Google Scholar]
- 93.Forman D, Aldabbagh S, Doll R. Nitrates, nitrites and gastric cancer in Great Britain. Nature. 1985;313:620–625. doi: 10.1038/313620a0. [DOI] [PubMed] [Google Scholar]
- 94.Aldabbagh S, Forman D, Bryson D, Stratton I, Doll R. Mortality of nitrate fertilizer workers. Brit J Indust Med. 1986;43:507–515. doi: 10.1136/oem.43.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ayebo A, Kross BC, Vlad M, Sinca A. Infant methemoglobinemia in the Transylvania region of Romania. Int J Occup Environ Health. 1997;3:20–29. doi: 10.1179/oeh.1997.3.1.20. [DOI] [PubMed] [Google Scholar]
- 96.Chow WA, Synold TW, Tetef ML, Longmate J, Frankel P, Lawrence J, Al Khadimi Z, Leong L, Lim D, Margolin K, Morgan RJ, Raschko J, Shibata S, Somlo G, Twardowski P, Yen Y, Doroshow JH. Feasibility and pharmacokinetic study of infusional dexrazoxane and dose-intensive doxorubicin administered concurrently over 96 h for the treatment of advanced malignancies. Cancer Chemother Pharmacol. 2004;54:241–248. doi: 10.1007/s00280-004-0803-4. [DOI] [PubMed] [Google Scholar]
- 97.Burrows FA, Hickey PR. Perioperative complications in patients with anthracycline chemotherapeutic agents. Can Anaesth Soc J. 1985;32:149–157. doi: 10.1007/BF03010041. [DOI] [PubMed] [Google Scholar]
- 98.Rossi F, Filippelli W, Russo S, Filippelli A, Berrino L. Cardiotoxicity of doxorubicin - Effects of drugs inhibiting the release of vasoactive substances. Pharmacol Toxicol. 1994;75:99–107. doi: 10.1111/j.1600-0773.1994.tb00330.x. [DOI] [PubMed] [Google Scholar]